Gut Microbiota Fermentation of Digested Almond–Psyllium–Flax Seed-Based Artisan Bread Promotes Mediterranean Diet-Resembling Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and In Vitro Digestion of Bread

2.2. In Vitro Human Gut Simulator (HGS) Experiments
| Medium Component | WM * | YEM + Bread * | YEM * |
|---|---|---|---|
| Digested UF bread | |||
| Pellet (dry residue) † | - | 27.0 | - |
| Digest supernatant ‡ | - | 34.3 | - |
| Carbohydrates | |||
| Arabinogalactan | 1.8 | - | - |
| Guar gum | 0.9 | - | - |
| Inulin | 0.9 | - | - |
| Pectin | 1.8 | - | - |
| Starch | 4.4 | - | - |
| Xylan | 0.9 | - | - |
| Cellobiose | 0.9 | - | - |
| Glucose | 0.5 | - | - |
| Fructose | 0.5 | - | - |
| Proteins | |||
| Peptone | 3.3 | - | - |
| Casein | 2.0 | - | - |
| Lipids | |||
| Mono + diacylglycerides | 5.4 | - | - |
| Mucin | 4.0 | - | - |
| Yeast extract | 3.0 | 3.0 | 3.0 |
| Vitamins | 1.0 | 1.0 | 1.0 |
| Salts, other components | 14.1 | 14.1 | 14.1 |
| Bile salts | 0.2 | 0.2 ** | 0.2 |
| Pancreatin | 0.1 | 0.1 ** | 0.1 |
2.3. Microbiota Analysis
2.4. Short-Chain Fatty Acid Measurements
2.5. Antioxidant Capacity Measurements
2.6. Data Analyses
3. Results
3.1. Oro-Gastro-Intestinal Digestion of Artisan Bread
3.2. Fermentation of Artisan Bread in the Human Gut Simulator
3.3. Microbial Composition Differs among Supplied Media
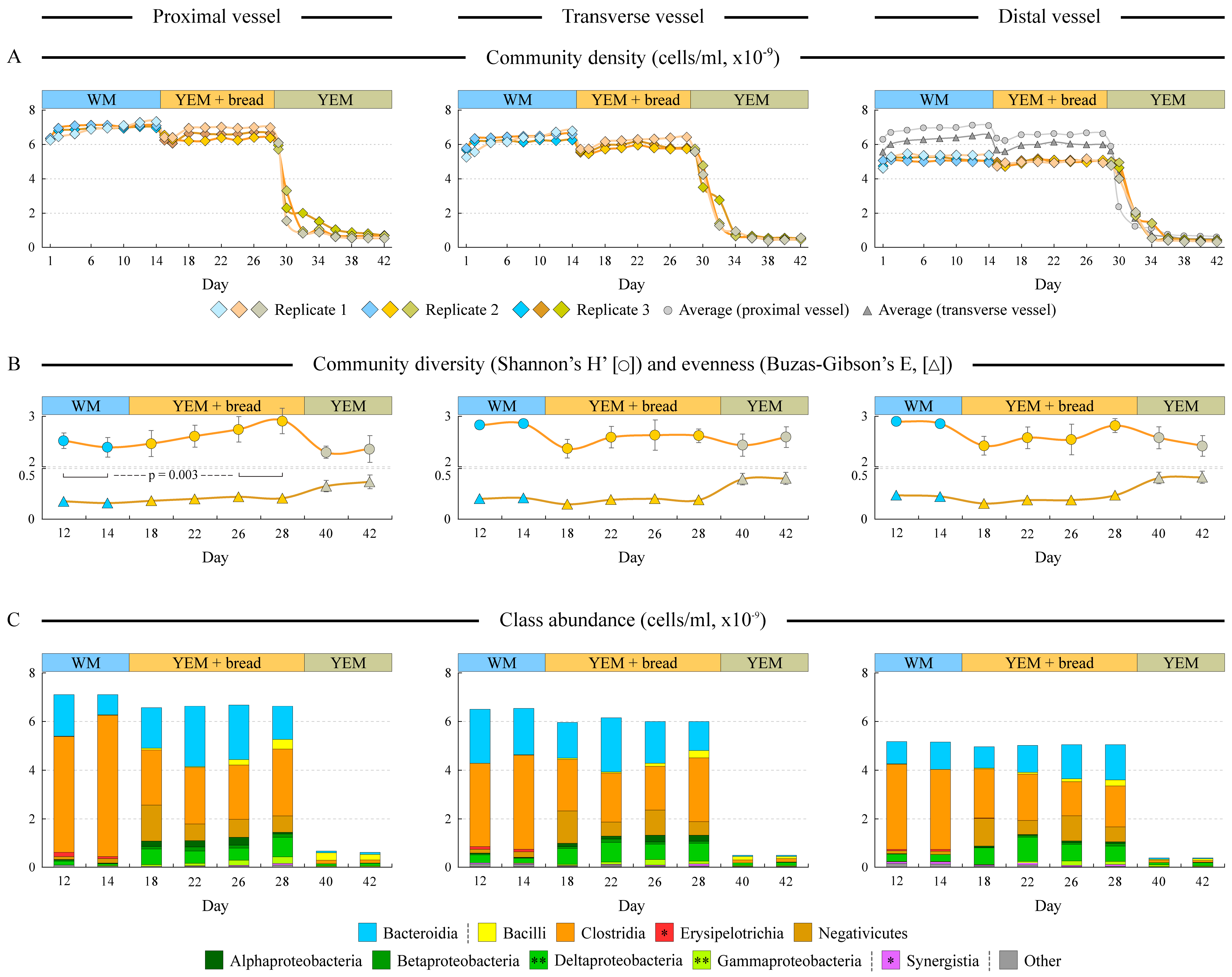
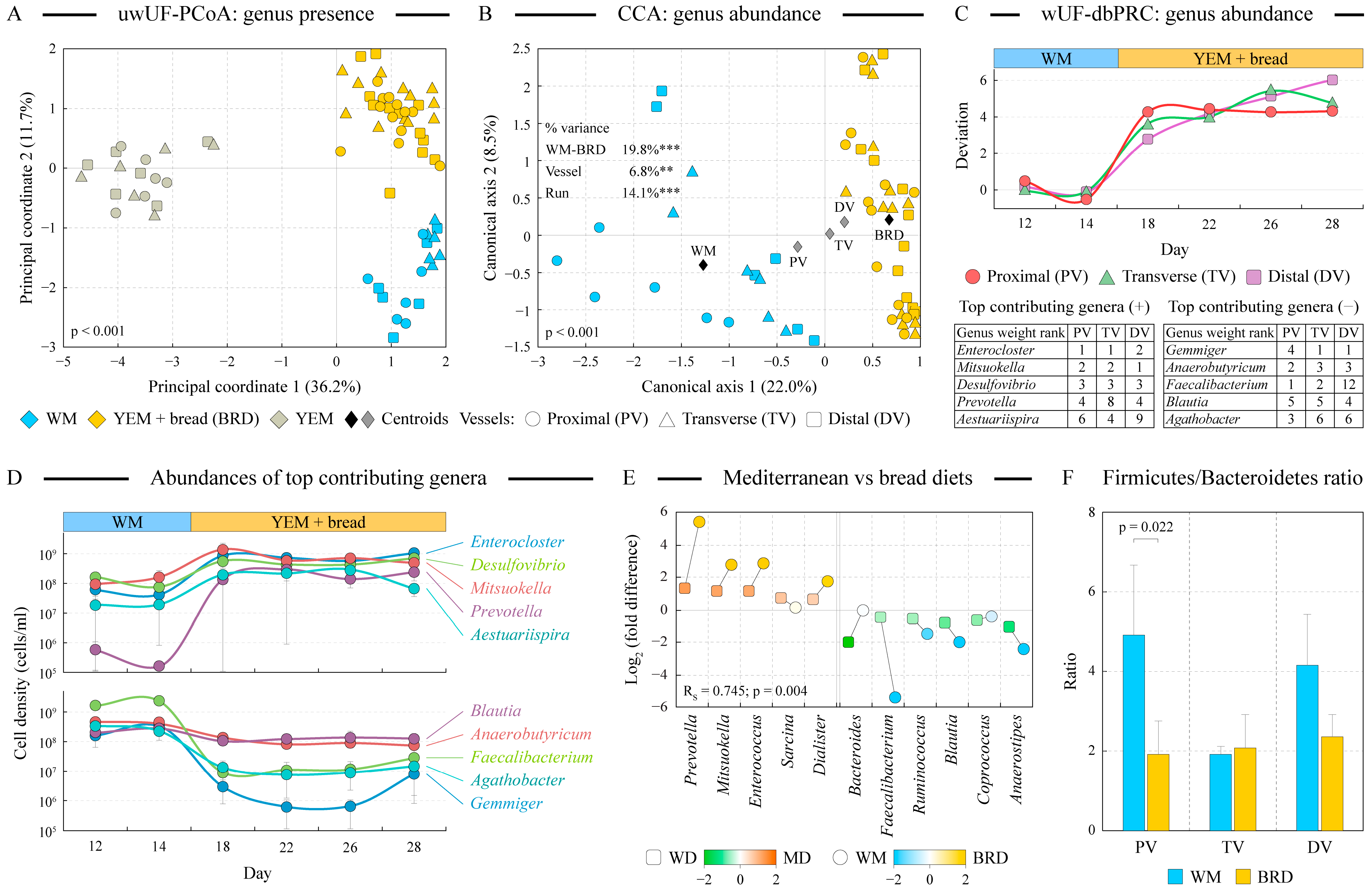
3.4. Fermentation of Artisan Bread Promotes Community Changes Analogous to the Effect of Mediterranean Diet Consumption
3.5. Firmicutes-to-Bacteroidetes Ratio Is Decreased upon the Switch from the Western to Digested Bread Medium

3.6. Composition of Fermentation End Products Differs between WM and BRD Media
3.7. Antioxidant Capacity of Artisan Bread-Based Medium Is Higher Than That of WM
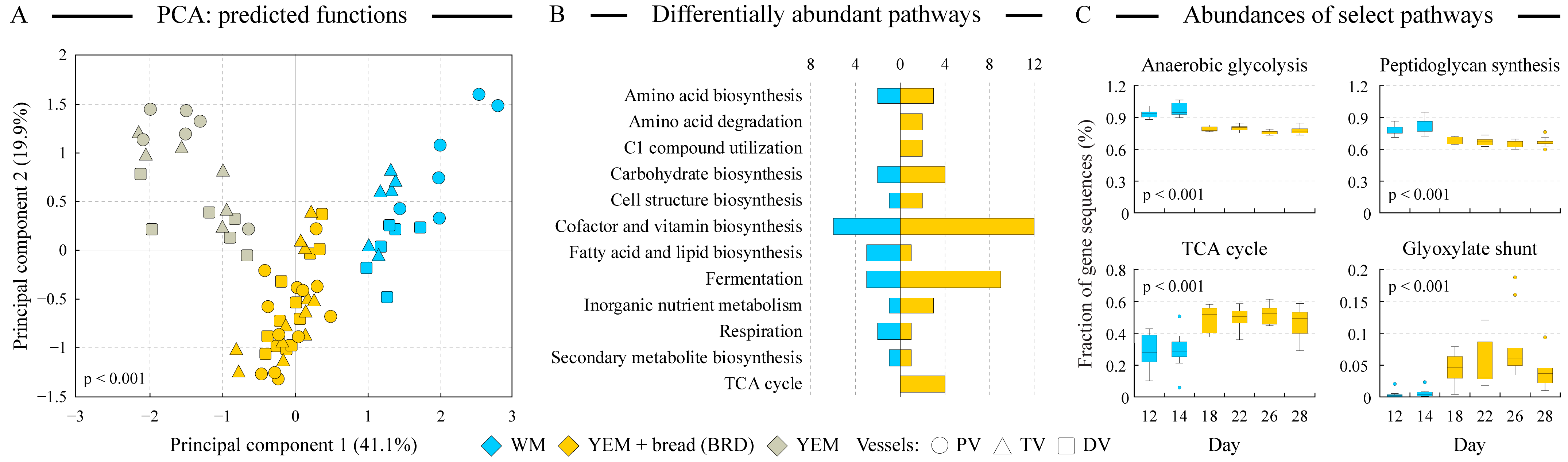
3.8. Functional Distinctions Separate WM and BRD Communities
4. Discussion
| Macronutrient | UF Bread * | White/Wheat Bread * | Western Diet † | Mediterranean Diet ‡ |
|---|---|---|---|---|
| Total carbs | 39.3% | 83.3% | 61% | 62% |
| Dietary fiber | 32.1% | 5.6% | 7–10% | 15% |
| Sugars | <3.6% | 11.1% | 18–22% | NG |
| Total protein | 21.4% | 11.1% | 20% | 21% |
| Total fats | 39.3% | 5.6% | 19% | 17% |
| Saturated fats | 3.6% | <3.6% | 9% | 4% |
| Unsaturated fats | 35.7% | <3.6% | 10% | 13% |
- Bread digest supported a more diverse microbial community in the proximal vessel.
- Community structure alterations included increases in fiber degraders such as Prevotella and Mitsuokella on the BRD medium but also increases in potentially detrimental Enterocloster, Desulfovibrio, Bilophila, and Escherichia/Shigella as well as a reduction of total Faecalibacterium, the genus previously shown to possess potent anti-inflammatory properties [51]. Substantially higher fiber content of the BRD medium not only supported fiber degraders listed above but also increased the prevalence of encoded fermentation pathways in the community.
- The Firmicutes-to-Bacteroidetes ratio was reduced on the BRD medium in the proximal and distal vessels. A higher F-to-B ratio was previously found in some obese subjects [46]; thus, lowering this ratio on the bread digest medium might have beneficial effects on the host.
- Genomes of microbial communities grown on the bread digest medium were predicted to encode more functions in the pathways of the TCA cycle, glyoxylate bypass, and fatty acid degradation (see Figure 5B,C), all indicative of a higher acetyl-CoA utilization, consistent with the higher lipid content of the BRD medium. This was consistent with the higher abundance of Bilophila, a previously recognized “lipophilic” genus, in the BRD-grown cultures.
- Pathways of the inorganic nutrient metabolism were also more prevalent in the BRD-maintained communities, consistent with the increase of sulfate-reducing Desulfovibrio in BRD medium and high sulfur content of flax seeds.
- While the overall production of short-chain fatty acids was not statistically different between WM and BRD-grown communities, we observed a shift from the production of butyrate towards the release of propionate and acetate. This was in agreement with the reduction of the abundance of several butyrate producers (e.g., Faecalibacterium, Blautia, Anaerobutiricum) and an increase in propionate producers such as Enterocloster and Mitsuokella in the UF bread-grown communities. Both butyrate and propionate have been separately associated with protection from obesity and metabolic syndrome as well as eliciting a number of other health-associated benefits [50].
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paliy, O.; Rajakaruna, S. Development of microbiota—Is the process continuing through adolescence? In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Oxford, UK, 2022; pp. 59–68. [Google Scholar]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Wilson, A.S.; Koller, K.R.; Ramaboli, M.C.; Nesengani, L.T.; Ocvirk, S.; Chen, C.; Flanagan, C.A.; Sapp, F.R.; Merritt, Z.T.; Bhatti, F.; et al. Diet and the human gut microbiome: An international review. Dig. Dis. Sci. 2020, 65, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, S.; Freedman, D.A.; Sehgal, A.R.; Bui, X.; Paliy, O. Diet quality and body mass indices show opposite associations with distal gut microbiota in a low-income cohort. J. Food Sci. Technol. 2019, 4, 846–851. [Google Scholar]
- Rajakaruna, S.; Pérez-Burillo, S.; Rufián-Henares, J.Á.; Paliy, O. Human gut microbiota fermentation of cooked eggplant, garlic, and onion supports distinct microbial communities. Food Funct. 2024, 15, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal th17 cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Sun, T.Y.; He, Y.; Gou, W.; Zuo, L.S.; Fu, Y.; Miao, Z.; Shuai, M.; Xu, F.; Xiao, C.; et al. Dietary fruit and vegetable intake, gut microbiota, and type 2 diabetes: Results from two large human cohort studies. BMC Med. 2020, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Ángel Rufián-Henares, J. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, S.; Pérez-Burillo, S.; Kramer, D.L.; Rufián-Henares, J.Á.; Paliy, O. Dietary melanoidins from biscuits and bread crust alter the structure and short-chain fatty acid production of human gut microbiota. Microorganisms 2022, 10, 1268. [Google Scholar] [CrossRef]
- Solhi, P.; Azadmard-Damirchi, S.; Hesari, J.; Hamishehkar, H. Effect of fortification with asparagus powder on the qualitative properties of processed cheese. Int. J. Dairy Technol. 2020, 73, 226–233. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Marette, A. Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv. Nutr. 2017, 8, 155S–164S. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Mehta, T.; Pastoriza, S.; Kramer, D.L.; Paliy, O.; Rufian-Henares, J.A. Potential probiotic salami with dietary fiber modulates antioxidant capacity, short chain fatty acid production and gut microbiota community structure. LWT 2019, 105, 355–362. [Google Scholar] [CrossRef]
- Arranz-Otaegui, A.; Gonzalez Carretero, L.; Ramsey, M.N.; Fuller, D.Q.; Richter, T. Archaeobotanical evidence reveals the origins of bread 14,400 years ago in northeastern jordan. Proc. Natl. Acad. Sci. USA 2018, 115, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Hazard, B.; Trafford, K.; Lovegrove, A.; Griffiths, S.; Uauy, C.; Shewry, P. Strategies to improve wheat for human health. Nature Food 2020, 1, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Koksel, H.; Cetiner, B.; Shamanin, V.P.; Tekin-Cakmak, Z.H.; Pototskaya, I.V.; Kahraman, K.; Sagdic, O.; Morgounov, A.I. Quality, nutritional properties, and glycemic index of colored whole wheat breads. Foods 2023, 12, 3376. [Google Scholar] [CrossRef] [PubMed]
- Rej, A.; Aziz, I.; Sanders, D.S. Breaking bread! Proc. Nutr. Soc. 2019, 78, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Mollakhalili-Meybodi, N.; Arab, M.; Nematollahi, A.; Mousavi Khaneghah, A. Prebiotic wheat bread: Technological, sensorial and nutritional perspectives and challenges. LWT 2021, 149, 111823. [Google Scholar] [CrossRef]
- Korus, J.; Grzelak, K.; Achremowicz, K.; Sabat, R. Influence of prebiotic additions on the quality of gluten-free bread and on the content of inulin and fructooligosaccharides. Food Sci. Technol. Int. 2006, 12, 489–495. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Bertolino, M.; Barbosa-Pereira, L.; Ben Haj Kbaier, H.; Bouzouita, N.; Zeppa, G. Almond hull as a functional ingredient of bread: Effects on physico-chemical, nutritional, and consumer acceptability properties. Foods 2022, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Osorio, A.; Navajas-Porras, B.; Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Toledano-Marín, Á.; Pastoriza de la Cueva, S.; Paliy, O.; Rufián-Henares, J.Á. Cultivar and harvest time of almonds affect their antioxidant and nutritional profile through gut microbiota modifications. Antioxidants 2024, 13, 84. [Google Scholar] [CrossRef]
- Creedon, A.C.; Dimidi, E.; Hung, E.S.; Rossi, M.; Probert, C.; Grassby, T.; Miguens-Blanco, J.; Marchesi, J.R.; Scott, S.M.; Berry, S.E.; et al. The impact of almonds and almond processing on gastrointestinal physiology, luminal microbiology, and gastrointestinal symptoms: A randomized controlled trial and mastication study. Am. J. Clin. Nutr. 2022, 116, 1790–1804. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, S.; Li, H.; Bai, X.; Shan, S.; Gao, P.; Dong, X. The effects of psyllium husk on gut microbiota composition and function in chronically constipated women of reproductive age using 16s rrna gene sequencing analysis. Aging 2021, 13, 15366–15383. [Google Scholar] [CrossRef] [PubMed]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; de Vos, W.M.; Spiller, R. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef] [PubMed]
- Sawane, K.; Hosomi, K.; Park, J.; Ookoshi, K.; Nanri, H.; Nakagata, T.; Chen, Y.-A.; Mohsen, A.; Kawashima, H.; Mizuguchi, K.; et al. Identification of human gut microbiome associated with enterolignan production. Microorganisms 2022, 10, 2169. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.-H.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T.; et al. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol. Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufian-Henares, J.A.; Paliy, O. Dietary fatty acids sustain the growth of the human gut microbiota. Appl. Environ. Microbiol. 2018, 84, e01525-18. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. Infogest static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, R.; Inoue, K.Y.; Nishino, K.; Yamasaki, S. Intestinal and fecal ph in human health. Front. Microbiomes 2023, 2, e00047-17. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Rajakaruna, S.; Paliy, O. Growth of bifidobacterium species is inhibited by free fatty acids and bile salts but not by glycerides. AIMS Microbiol. 2022, 8, 53. [Google Scholar] [CrossRef]
- Polz, M.F.; Cavanaugh, C.M. Bias in template-to-product ratios in multitemplate pcr. Appl. Environ. Microbiol. 1998, 64, 3724–3730. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Lee, Y.; Chae, W.; Cho, J.-Y. An improved method to quantify short-chain fatty acids in biological samples using gas chromatography–mass spectrometry. Metabolites 2022, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Homer, D.; Rigsbee, L.; Khamis, H.J.; Michail, S.; Raymer, M.; Reo, N.V.; Paliy, O. The networks of human gut microbe-metabolite associations are different between health and irritable bowel syndrome. ISME J. 2015, 9, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Agans, R.; Paliy, O. Advantages of phylogenetic distance based constrained ordination analyses for the examination of microbial communities. Sci. Rep. 2017, 7, 6481. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. Picrust2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B Met. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Paliy, O.; Shankar, V. Application of multivariate statistical techniques in microbial ecology. Mol. Ecol. 2016, 25, 1032–1057. [Google Scholar] [CrossRef]
- Shankar, V.; Gouda, M.; Moncivaiz, J.; Gordon, A.; Reo, N.V.; Hussein, L.; Paliy, O. Differences in gut metabolites and microbial composition and functions between egyptian and u.S. Children are consistent with their diets. mSystems 2017, 2, e00169-16. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Yun, Y.; Kim, H.-N.; Kim, S.E.; Heo, S.G.; Chang, Y.; Ryu, S.; Shin, H.; Kim, H.-L. Comparative analysis of gut microbiota associated with body mass index in a large korean cohort. BMC Microbiol. 2017, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Manimurugan, C.; Sujatha, M.; Rathnakumar, A.L.; Sandhanalakshmi, M.; Zanwar, A.A. Role of flaxseed (Linum usitatissimum L.) in disease prevention and treatment. Asian Pac. J. Trop. Biomed. 2023, 13, 277–286. [Google Scholar]
- Beam, A.; Clinger, E.; Hao, L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Molitoris, D.R.; Tomzynski, T.J.; Lawson, P.A.; Collins, M.D.; Finegold, S.M. Clostridium bolteae sp. Nov., isolated from human sources. Syst. Appl. Microbiol. 2003, 26, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The metacyc database of metabolic pathways and enzymes—A 2019 update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Pearson, T.A.; Wan, Y.; Hargrove, R.L.; Moriarty, K.; Fishell, V.; Etherton, T.D. High-monounsaturated fatty acid diets lower both plasma cholesterol and triacylglycerol concentrations. Am. J. Clin. Nutr. 1999, 70, 1009–1015. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Shively, C.A.; Appt, S.E.; Vitolins, M.Z.; Uberseder, B.; Michalson, K.T.; Silverstein-Metzler, M.G.; Register, T.C. Mediterranean versus western diet effects on caloric intake, obesity, metabolism, and hepatosteatosis in nonhuman primates. Obesity 2019, 27, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, M.; Arrieta, M.-C. The global human gut microbiome: Genes, lifestyles, and diet. Trends Mol. Med. 2023, 29, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Ngo, P.A.; Neurath, M.F.; López-Posadas, R. Impact of epithelial cell shedding on intestinal homeostasis. Int. J. Mol. Sci. 2022, 23, 4160. [Google Scholar] [CrossRef]
- Stone, W.L.; Krishnan, K.; Campbell, S.E.; Palau, V.E. The role of antioxidants and pro-oxidants in colon cancer. World J. Gastrointest. Oncol. 2014, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Digested after OGI Process * | Solids Remaining after OGI Digestion * |
|---|---|---|
| UF bread | 29.6% | 70.4% |
| Almond flour | 65.2% | 34.8% |
| Baking powder | 71.4% | 28.6% |
| Eggs | 98.6% | 1.4% |
| Flax seeds | 23.3% | 76.7% |
| Psyllium husks | <1% | 99.0% |
| White bread | 79.4% | 20.6% |
| Whole wheat bread | 76.6% | 23.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprague, K.L.; Rajakaruna, S.; Bandow, B.; Burchat, N.; Bottomley, M.; Sampath, H.; Paliy, O. Gut Microbiota Fermentation of Digested Almond–Psyllium–Flax Seed-Based Artisan Bread Promotes Mediterranean Diet-Resembling Microbial Community. Microorganisms 2024, 12, 1189. https://doi.org/10.3390/microorganisms12061189
Sprague KL, Rajakaruna S, Bandow B, Burchat N, Bottomley M, Sampath H, Paliy O. Gut Microbiota Fermentation of Digested Almond–Psyllium–Flax Seed-Based Artisan Bread Promotes Mediterranean Diet-Resembling Microbial Community. Microorganisms. 2024; 12(6):1189. https://doi.org/10.3390/microorganisms12061189
Chicago/Turabian StyleSprague, Kourtney L., Sumudu Rajakaruna, Brant Bandow, Natalie Burchat, Michael Bottomley, Harini Sampath, and Oleg Paliy. 2024. "Gut Microbiota Fermentation of Digested Almond–Psyllium–Flax Seed-Based Artisan Bread Promotes Mediterranean Diet-Resembling Microbial Community" Microorganisms 12, no. 6: 1189. https://doi.org/10.3390/microorganisms12061189
APA StyleSprague, K. L., Rajakaruna, S., Bandow, B., Burchat, N., Bottomley, M., Sampath, H., & Paliy, O. (2024). Gut Microbiota Fermentation of Digested Almond–Psyllium–Flax Seed-Based Artisan Bread Promotes Mediterranean Diet-Resembling Microbial Community. Microorganisms, 12(6), 1189. https://doi.org/10.3390/microorganisms12061189





