Nisin Inhibition of Gram-Negative Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strain Collection and Growth Standards
2.3. MIC of Gram-Negative Bacteria
2.4. Data Quantification
2.5. Figure and Table Creation
3. Results
3.1. Inhibitory Activity of Nisin against Gram-Negative Bacteria
3.2. Genera with High Levels of Nisin Sensitivity
3.3. Genera with Moderate Levels of Nisin Sensitivity
3.4. Genera with Low Levels of Nisin Sensitivity
3.5. Genera with No Detected Nisin Sensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allel, K.; Day, L.; Hamilton, A.; Lin, L.; Furuya-Kanamori, L.; Moore, C.E.; Van Boeckel, T.; Laxminarayan, R.; Yakob, L. Global Antimicrobial-Resistance Drivers: An Ecological Country-Level Study at the Human–Animal Interface. Lancet Planet. Health 2023, 7, e291–e303. [Google Scholar] [CrossRef]
- Kim, D.-W.; Cha, C.-J. Antibiotic Resistome from the One-Health Perspective: Understanding and Controlling Antimicrobial Resistance Transmission. Exp. Mol. Med. 2021, 53, 301–309. [Google Scholar] [CrossRef]
- Delannoy-Bruno, O.; Desai, C.; Raman, A.S.; Chen, R.Y.; Hibberd, M.C.; Cheng, J.; Han, N.; Castillo, J.J.; Couture, G.; Lebrilla, C.B.; et al. Evaluating Microbiome-Directed Fibre Snacks in Gnotobiotic Mice and Humans. Nature 2021, 595, 91–95. [Google Scholar] [CrossRef]
- Mitchell, M.; Thornton, L.; Riley, M.A. Identifying More Targeted Antimicrobials Active against Selected Bacterial Phytopathogens. J. Appl. Microbiol. 2022, 132, 4388–4399. [Google Scholar] [CrossRef]
- Thomas, V.M.; Brown, R.M.; Ashcraft, D.S.; Pankey, G.A. Synergistic Effect between Nisin and Polymyxin B against Pandrug-Resistant and Extensively Drug-Resistant Acinetobacter Baumannii. Int. J. Antimicrob. Agents 2019, 53, 663–668. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Riley, M.A.; Wertz, J.E. Bacteriocins: Evolution, Ecology, and Application. Annu. Rev. Microbiol. 2002, 56, 117–137. [Google Scholar] [CrossRef]
- Choi, G.-H.; Holzapfel, W.H.; Todorov, S.D. Diversity of the Bacteriocins, Their Classification and Potential Applications in Combat of Antibiotic Resistant and Clinically Relevant Pathogens. Crit. Rev. Microbiol. 2023, 49, 578–597. [Google Scholar] [CrossRef]
- Fernández, L.; Delgado, S.; Herrero, H.; Maldonado, A.; Rodríguez, J.M. The Bacteriocin Nisin, an Effective Agent for the Treatment of Staphylococcal Mastitis During Lactation. J. Hum. Lact. 2008, 24, 311–316. [Google Scholar] [CrossRef]
- Zhu, H.; Han, L.; Ni, Y.; Yu, Z.; Wang, D.; Zhou, J.; Li, B.; Zhang, W.; He, K. In Vitro and In Vivo Antibacterial Effects of Nisin Against Streptococcus Suis. Probiotics Antimicrob. Proteins 2021, 13, 598–610. [Google Scholar] [CrossRef]
- Campion, A.; Casey, P.G.; Field, D.; Cotter, P.D.; Hill, C.; Ross, R.P. In Vivo Activity of Nisin A and Nisin V against Listeria Monocytogenesin Mice. BMC Microbiol. 2013, 13, 23. [Google Scholar] [CrossRef]
- O’ Connor, P.M.; O’ Shea, E.F.; Cotter, P.D.; Hill, C.; Ross, R.P. The Potency of the Broad Spectrum Bacteriocin, Bactofencin A, against Staphylococci Is Highly Dependent on Primary Structure, N-Terminal Charge and Disulphide Formation. Sci. Rep. 2018, 8, 11833. [Google Scholar] [CrossRef]
- Ghapanvari, P.; Taheri, M.; Jalilian, F.A.; Dehbashi, S.; Dezfuli, A.A.Z.; Arabestani, M.R. The Effect of Nisin on the Biofilm Production, Antimicrobial Susceptibility and Biofilm Formation of Staphylococcus Aureus and Pseudomonas Aeruginosa. Eur. J. Med. Res. 2022, 27, 173. [Google Scholar] [CrossRef]
- Yehia, H.M.; Alkhuriji, A.F.; Savvaidis, I.; Al-MASOUD, A.H. Bactericidal Effect of Nisin and Reuterin on Methicillin-Resistant Staphylococcus Aureus (MRSA) and S. Aureus ATCC 25937. Food Sci. Technol. 2022, 42, e105321. [Google Scholar] [CrossRef]
- De Araújo, R.A.; Neiva, J.N.M.; Pompeu, R.C.F.F.; Cândido, M.J.D.; Rogério, M.C.P.; Lucas, R.C.; Maranhão, S.R.; Fontinele, R.G.; Egito, A.S. Feeding Behavior and Physiological Parameters of Rearing Goats Fed Diets Containing Detoxified Castor Cake. Semina Ciênc. Agrár. 2018, 39, 2247–2259. [Google Scholar] [CrossRef]
- Khelissa, S.; Chihib, N.-E.; Gharsallaoui, A. Conditions of Nisin Production by Lactococcus Lactis Subsp. Lactis and Its Main Uses as a Food Preservative. Arch. Microbiol. 2021, 203, 465–480. [Google Scholar] [CrossRef]
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the Antimicrobial Activity of Nisin-Based Lantibiotics against Gram-Negative Pathogens. Appl. Environ. Microbiol. 2018, 84, e00052-18. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to Promote Abundance of Akkermansia Muciniphila, an Emerging Probiotics in the Gut, Evidence from Dietary Intervention Studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Lubelski, J.; Rink, R.; Khusainov, R.; Moll, G.N.; Kuipers, O.P. Biosynthesis, Immunity, Regulation, Mode of Action and Engineering of the Model Lantibiotic Nisin. Cell. Mol. Life Sci. 2008, 65, 455–476. [Google Scholar] [CrossRef]
- Hasper, H.E.; Kramer, N.E.; Smith, J.L.; Hillman, J.D.; Zachariah, C.; Kuipers, O.P.; de Kruijff, B.; Breukink, E. An Alternative Bactericidal Mechanism of Action for Lantibiotic Peptides That Target Lipid II. Science 2006, 313, 1636–1637. [Google Scholar] [CrossRef]
- Prince, A.; Sandhu, P.; Ror, P.; Dash, E.; Sharma, S.; Arakha, M.; Jha, S.; Akhter, Y.; Saleem, M. Lipid-II Independent Antimicrobial Mechanism of Nisin Depends on Its Crowding And Degree of Oligomerization. Sci. Rep. 2016, 6, 37908. [Google Scholar] [CrossRef]
- Vukomanović, M.; Žunič, V.; Kunej, Š.; Jančar, B.; Jeverica, S.; Podlipec, R.; Suvorov, D. Nano-Engineering the Antimicrobial Spectrum of Lantibiotics: Activity of Nisin against Gram Negative Bacteria. Sci. Rep. 2017, 7, 4324. [Google Scholar] [CrossRef]
- Helander, I.M.; Mattila-Sandholm, T. Permeability Barrier of the Gram-Negative Bacterial Outer Membrane with Special Reference to Nisin. Int. J. Food Microbiol. 2000, 60, 153–161. [Google Scholar] [CrossRef]
- Kuwano, K.; Tanaka, N.; Shimizu, T.; Nagatoshi, K.; Nou, S.; Sonomoto, K. Dual Antibacterial Mechanisms of Nisin Z against Gram-Positive and Gram-Negative Bacteria. Int. J. Antimicrob. Agents 2005, 26, 396–402. [Google Scholar] [CrossRef]
- Sangcharoen, N.; Klaypradit, W.; Wilaipun, P. Antimicrobial Activity of Microencapsulated Nisin with Ascorbic Acid and Ethylenediaminetetraacetic Acid Prepared Using Double Emulsion and Freeze-Drying Technique against Salmonella Enteritidis ATCC 13076 in Culture Broth and Minced Fish. Agric. Nat. Resour. 2023, 57, 65–76. [Google Scholar]
- Araújo, M.K.; Gumiela, A.M.; Bordin, K.; Luciano, F.B.; Macedo, R.E.F.d. Combination of Garlic Essential Oil, Allyl Isothiocyanate, and Nisin Z as Bio-Preservatives in Fresh Sausage. Meat Sci. 2018, 143, 177–183. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- CDC. The Biggest Antibiotic-Resistant Threats in the U.S. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html (accessed on 13 May 2024).
- Bertani, G. Studies on Lysogenesis. I. The Mode of Phage Liberation by Lysogenic Escherichia Coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Konstantinidis, T.; Tsigalou, C.; Karvelas, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Effects of Antibiotics upon the Gut Microbiome: A Review of the Literature. Biomedicines 2020, 8, 502. [Google Scholar] [CrossRef]
- Lanne, A.B.M.; Goode, A.; Prattley, C.; Kumari, D.; Drasbek, M.R.; Williams, P.; Conde-Álvarez, R.; Moriyón, I.; Bonev, B.B. Molecular Recognition of Lipopolysaccharide by the Lantibiotic Nisin. Biochim. Biophys. Acta BBA—Biomembr. 2019, 1861, 83–92. [Google Scholar] [CrossRef]
- Field, D.; Fernandez de Ullivarri, M.; Ross, R.P.; Hill, C. After a Century of Nisin Research—Where Are We Now? FEMS Microbiol. Rev. 2023, 47, fuad023. [Google Scholar] [CrossRef]
- Askari, P.; Yousefi, M.; Foadoddini, M.; Neshani, A.; Aganj, M.; Lotfi, N.; Movaqar, A.; Ghazvini, K.; Namaei, M.H. Antimicrobial Peptides as a Promising Therapeutic Strategy for Neisseria Infections. Curr. Microbiol. 2022, 79, 102. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of Nisin Resistance in Gram-Positive Bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Kim, T.-S.; Hur, J.-W.; Yu, M.-A.; Cheigh, C.-I.; Kim, K.-N.; Hwang, J.-K.; Pyun, Y.-R. Antagonism of Helicobacter Pylori by Bacteriocins of Lactic Acid Bacteria. J. Food Prot. 2003, 66, 3–12. [Google Scholar] [CrossRef]
- Rollema, H.S.; Kuipers, O.P.; Both, P.; de Vos, W.M.; Siezen, R.J. Improvement of Solubility and Stability of the Antimicrobial Peptide Nisin by Protein Engineering. Appl. Environ. Microbiol. 1995, 61, 2873–2878. [Google Scholar] [CrossRef]
- Ding, S.-Z. Global Whole Family Based-Helicobacter Pylori Eradication Strategy to Prevent Its Related Diseases and Gastric Cancer. World J. Gastroenterol. 2020, 26, 995–1004. [Google Scholar] [CrossRef]
- Georgopoulos, S.; Papastergiou, V. An Update on Current and Advancing Pharmacotherapy Options for the Treatment of H. Pylori Infection. Expert Opin. Pharmacother. 2021, 22, 729–741. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Schistosomes, Liver Flukes and Helicobacter Pylori; International Agency for Research on Cance: Lyon, France, 1994; Volume 61. [Google Scholar] [CrossRef]
- Salcedo, J.A.; Al-Kawas, F. Treatment of Helicobacter Pylori Infection. Arch. Intern. Med. 1998, 158, 842–851. [Google Scholar] [CrossRef]
- Bury-Moné, S.; Kaakoush, N.O.; Asencio, C.; Mégraud, F.; Thibonnier, M.; De Reuse, H.; Mendz, G.L. Is Helicobacter Pylori a True Microaerophile? Helicobacter 2006, 11, 296–303. [Google Scholar] [CrossRef]
- Chi, H.; Holo, H. Synergistic Antimicrobial Activity Between the Broad Spectrum Bacteriocin Garvicin KS and Nisin, Farnesol and Polymyxin B Against Gram-Positive and Gram-Negative Bacteria. Curr. Microbiol. 2018, 75, 272–277. [Google Scholar] [CrossRef]
- Kenny, M.A.; Pollock, H.M.; Minshew, B.H.; Casillas, E.; Schoenknecht, F.D. Cation Components of Mueller-Hinton Agar Affecting Testing of Pseudomonas Aeruginosa Susceptibility to Gentamicin. Antimicrob. Agents Chemother. 1980, 17, 55–62. [Google Scholar] [CrossRef]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ştefan, M.G.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An Overview of Antimicrobial, Toxicity, and Biosafety Assessment by in Vivo Models. Front. Microbiol. 2021, 12, 630695. [Google Scholar] [CrossRef]
- Xin, X.-F.; Kvitko, B.; He, S.Y. Pseudomonas Syringae: What It Takes to Be a Pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Vanneste, J.L.; Voyle, M.D.; Yu, J.; Cornish, D.A.; Boyd, R.J.; Mclaren, G.F. Copper and Streptomycin Resistance in Pseudomonas Strains Isolated from Pipfruit and Stone Fruit Orchards in New Zealand. In Pseudomonas syringae Pathovars and Related Pathogens—Identification, Epidemiology and Genomics; Fatmi, M., Collmer, A., Iacobellis, N.S., Mansfield, J.W., Murillo, J., Schaad, N.W., Ullrich, M., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 81–90. [Google Scholar] [CrossRef]
- Sundin, G.W.; Bender, C.L. Ecological and Genetic Analysis of Copper and Streptomycin Resistance in Pseudomonas Syringae Pv. Syringae. Appl. Environ. Microbiol. 1993, 59, 1018–1024. [Google Scholar] [CrossRef]
- Reidl, J.; Klose, K.E. Vibrio Cholerae and Cholera: Out of the Water and into the Host. FEMS Microbiol. Rev. 2002, 26, 125–139. [Google Scholar] [CrossRef]
- Freitag, A.; Ellett, A.; Burkart, H.; Jacobs, J. Estimating the Economic Burden of Vibrio Parahaemolyticus in Washington State Oyster Aquaculture: Implications for the Future. J. Shellfish Res. 2022, 40, 555–564. [Google Scholar] [CrossRef]
- Tang, K.; Bondad-Reantaso, M. Impacts of Acute Hepatopancreatic Necrosis Disease on Commercial Shrimp Aquaculture: -EN- -FR- Effets de La Maladie de Nécrose Hépatopancréatique Aiguë Sur La Production Commerciale de Crevettes d’élevage -ES- Repercusiones de La Enfermedad de La Necrosis Hepatopancreática Aguda En La Producción Camaronícola Industrial. Rev. Sci. Tech. OIE 2019, 38, 477–490. [Google Scholar] [CrossRef]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating Antimicrobial Resistance in the Global Shrimp Industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef]
- Tavares-Carreon, F.; De Anda-Mora, K.; Rojas-Barrera, I.C.; Andrade, A. Serratia Marcescens Antibiotic Resistance Mechanisms of an Opportunistic Pathogen: A Literature Review. PeerJ 2023, 11, e14399. [Google Scholar] [CrossRef]
- Field, D.; Baghou, I.; Rea, M.C.; Gardiner, G.E.; Ross, R.P.; Hill, C. Nisin in Combination with Cinnamaldehyde and EDTA to Control Growth of Escherichia Coli Strains of Swine Origin. Antibiotics 2017, 6, 35. [Google Scholar] [CrossRef]
- Delves-Broughton, J. The Use of EDTA to Enhance the Efficacy of Nisin towards Gram-Negative Bacteria. Int. Biodeterior. Biodegrad. 1993, 32, 87–97. [Google Scholar] [CrossRef]
- Khan, A.; Vu, K.D.; Riedl, B.; Lacroix, M. Optimization of the Antimicrobial Activity of Nisin, Na-EDTA and pH against Gram-Negative and Gram-Positive Bacteria. LWT—Food Sci. Technol. 2015, 61, 124–129. [Google Scholar] [CrossRef]
- Ranjan, K.P.; Ranjan, N. Citrobacter: An Emerging Health Care Associated Urinary Pathogen. Urol. Ann. 2013, 5, 313–314. [Google Scholar] [CrossRef]
- Liu, L.; Chen, D.; Liu, L.; Lan, R.; Hao, S.; Jin, W.; Sun, H.; Wang, Y.; Liang, Y.; Xu, J. Genetic Diversity, Multidrug Resistance, and Virulence of Citrobacter Freundii From Diarrheal Patients and Healthy Individuals. Front. Cell. Infect. Microbiol. 2018, 8, 233. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective. Front. Microbiol. 2017, 8, 1205. [Google Scholar] [CrossRef]
- Castanheira, M.; Mendes, R.E.; Jones, R.N. Update on Acinetobacter Species: Mechanisms of Antimicrobial Resistance and Contemporary in Vitro Activity of Minocycline and Other Treatment Options. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 59 (Suppl. 6), S367–S373. [Google Scholar] [CrossRef]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General Principles of Antimicrobial Therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef]
- Stevens, K.A.; Klapes, N.A.; Sheldon, B.W.; Klaenhammer, T.R. Antimicrobial Action of Nisin against Salmonella Typhimurium Lipopolysaccharide Mutants. Appl. Environ. Microbiol. 1992, 58, 1786–1788. [Google Scholar] [CrossRef]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arunachalam, S.; Kim, G.-S.; Murugesan, R. Pseudomonas Aeruginosa Biofilm Formation and Its Control. Biologics 2021, 1, 312–336. [Google Scholar] [CrossRef]
- Ruekit, S.; Srijan, A.; Serichantalergs, O.; Margulieux, K.R.; Mc Gann, P.; Mills, E.G.; Stribling, W.C.; Pimsawat, T.; Kormanee, R.; Nakornchai, S.; et al. Molecular Characterization of Multidrug-Resistant ESKAPEE Pathogens from Clinical Samples in Chonburi, Thailand (2017–2018). BMC Infect. Dis. 2022, 22, 695. [Google Scholar] [CrossRef]
- Shin, J.M.; Ateia, I.; Paulus, J.R.; Liu, H.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Antimicrobial Nisin Acts against Saliva Derived Multi-Species Biofilms without Cytotoxicity to Human Oral Cells. Front. Microbiol. 2015, 6, 617. [Google Scholar] [CrossRef]
- Field, D.; Seisling, N.; Cotter, P.D.; Ross, R.P.; Hill, C. Synergistic Nisin-Polymyxin Combinations for the Control of Pseudomonas Biofilm Formation. Front. Microbiol. 2016, 7, 1713. [Google Scholar] [CrossRef]
- Jayaraman, J.; Jones, W.T.; Harvey, D.; Hemara, L.M.; McCann, H.C.; Yoon, M.; Warring, S.L.; Fineran, P.C.; Mesarich, C.H.; Templeton, M.D. Variation at the Common Polysaccharide Antigen Locus Drives Lipopolysaccharide Diversity within the Pseudomonas Syringae Species Complex. Environ. Microbiol. 2020, 22, 5356–5372. [Google Scholar] [CrossRef]
- King, J.D.; Kocíncová, D.; Westman, E.L.; Lam, J.S. Review: Lipopolysaccharide Biosynthesis in Pseudomonas Aeruginosa. Innate Immun. 2009, 15, 261–312. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Drider, D.; Baah, J.; Teather, R. Nisin A and Polymyxin B as Synergistic Inhibitors of Gram-Positive and Gram-Negative Bacteria. Probiotics Antimicro. Prot. 2010, 2, 98–103. [Google Scholar] [CrossRef]
- Singh, A.P.; Prabha, V.; Rishi, P. Value Addition in the Efficacy of Conventional Antibiotics by Nisin against Salmonella. PLoS ONE 2013, 8, e76844. [Google Scholar] [CrossRef]
- Zhao, X.; Kuipers, O.P. Synthesis of Silver-Nisin Nanoparticles with Low Cytotoxicity as Antimicrobials against Biofilm-Forming Pathogens. Colloids Surf. B Biointerfaces 2021, 206, 111965. [Google Scholar] [CrossRef]
- Andre, C.; de Jesus Pimentel-Filho, N.; de Almeida Costa, P.M.; Vanetti, M.C.D. Changes in the Composition and Architecture of Staphylococcal Biofilm by Nisin. Braz. J. Microbiol. 2019, 50, 1083–1090. [Google Scholar] [CrossRef]
- Hage, M.; Chihib, N.-E.; Abdallah, M.; Khelissa, S.; Crocco, B.; Akoum, H.; Bentiss, F.; Jama, C. Nisin-Based Coatings for the Prevention of Biofilm Formation: Surface Characterization and Antimicrobial Assessments. Surf. Interfaces 2021, 27, 101564. [Google Scholar] [CrossRef]
- Tong, Z.; Zhang, Y.; Ling, J.; Ma, J.; Huang, L.; Zhang, L. An In Vitro Study on the Effects of Nisin on the Antibacterial Activities of 18 Antibiotics against Enterococcus Faecalis. PLoS ONE 2014, 9, e89209. [Google Scholar] [CrossRef]

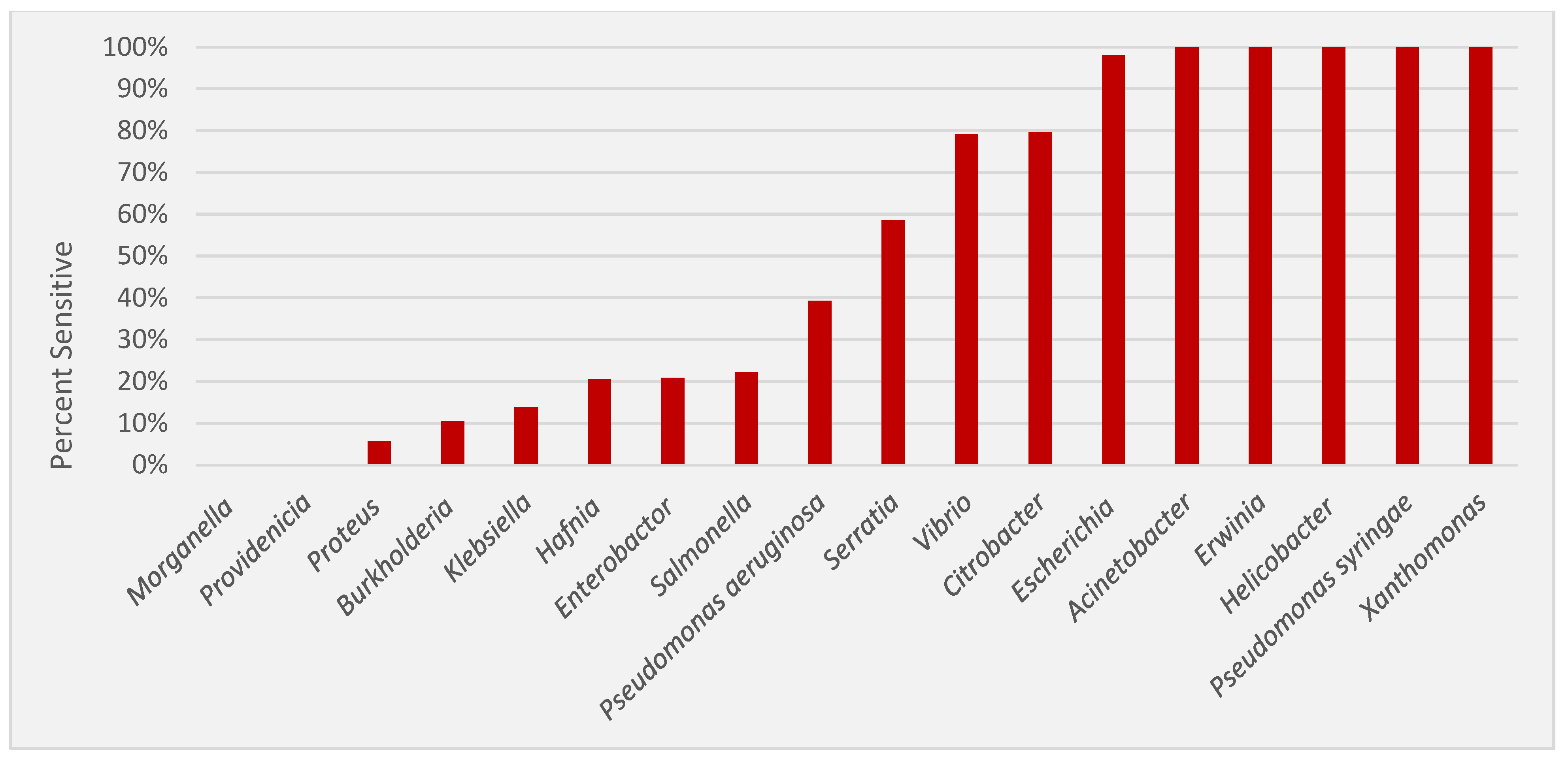

| Genus | # of Strains | # of Nisin Sensitive (%) * | MIC Range (μg/mL) | MIC Mean (μg/mL) | MIC Mean (nmol/mL) |
|---|---|---|---|---|---|
| Acinetobacter | 67 | 67 (100%) | 1.65–26.40 | 5.86 | 1.75 |
| Burkholderia | 19 | 2 (10.50%) | 423 | 423 | 126.12 |
| Citrobacter | 49 | 39 (79.60%) | 26.40–423 | 267.85 | 79.86 |
| Enterobacter | 48 | 10 (20.80%) | 6.60–423 | 296.76 | 88.48 |
| Erwinia | 21 | 21 (100%) | 6.60–211.50 | 55.66 | 16.60 |
| Escherichia | 51 | 50 (98%) | 26.40–423 | 171.81 | 51.22 |
| Hafnia | 39 | 8 (20.50%) | 105.70–423 | 347.46 | 103.59 |
| Helicobacter | 6 | 6 (100%) | 0.21–13.20 | 5.12 | 1.5265 |
| Klebsiella | 65 | 9 (13.80%) | 105.70–423 | 304.03 | 90.65 |
| Morganella | 13 | 0 (0.00%) | NS | NS | NS |
| Proteus | 35 | 2 (5.70%) | 105.70 | 105.70 | 31.51 |
| Providencia | 13 | 0 (0.00%) | NS | NS | NS |
| Pseudomonas | 44 | 38 (86.40%) | 6.60–423 | 92.50 | 27.58 |
| Salmonella | 9 | 3 (33.33%) | 211.50 | 211.50 | 63.06 |
| Serratia | 41 | 24 (58.50%) | 13.20–423 | 371.23 | 110.68 |
| Vibrio | 24 | 19 (79.20%) | 3.30–423 | 60.13 | 17.93 |
| Xanthomonas | 11 | 11 (100%) | 13.10–423 | 130.36 | 38.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charest, A.M.; Reed, E.; Bozorgzadeh, S.; Hernandez, L.; Getsey, N.V.; Smith, L.; Galperina, A.; Beauregard, H.E.; Charest, H.A.; Mitchell, M.; et al. Nisin Inhibition of Gram-Negative Bacteria. Microorganisms 2024, 12, 1230. https://doi.org/10.3390/microorganisms12061230
Charest AM, Reed E, Bozorgzadeh S, Hernandez L, Getsey NV, Smith L, Galperina A, Beauregard HE, Charest HA, Mitchell M, et al. Nisin Inhibition of Gram-Negative Bacteria. Microorganisms. 2024; 12(6):1230. https://doi.org/10.3390/microorganisms12061230
Chicago/Turabian StyleCharest, Adam M., Ethan Reed, Samantha Bozorgzadeh, Lorenzo Hernandez, Natalie V. Getsey, Liam Smith, Anastasia Galperina, Hadley E. Beauregard, Hailey A. Charest, Mathew Mitchell, and et al. 2024. "Nisin Inhibition of Gram-Negative Bacteria" Microorganisms 12, no. 6: 1230. https://doi.org/10.3390/microorganisms12061230
APA StyleCharest, A. M., Reed, E., Bozorgzadeh, S., Hernandez, L., Getsey, N. V., Smith, L., Galperina, A., Beauregard, H. E., Charest, H. A., Mitchell, M., & Riley, M. A. (2024). Nisin Inhibition of Gram-Negative Bacteria. Microorganisms, 12(6), 1230. https://doi.org/10.3390/microorganisms12061230






