Spatial Chromosome Organization and Adaptation of Escherichia coli under Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture and Heat Treatment
2.2. The 3C and RNA Sequencing
2.3. Fluorescence Imaging of Bacterial Nucleoid
2.4. Interaction Matrix and CID Analysis
2.5. Transcription Level and Correlation Analysis
2.6. Chromosome 3D Model Construction and Compactness Calculation
3. Results
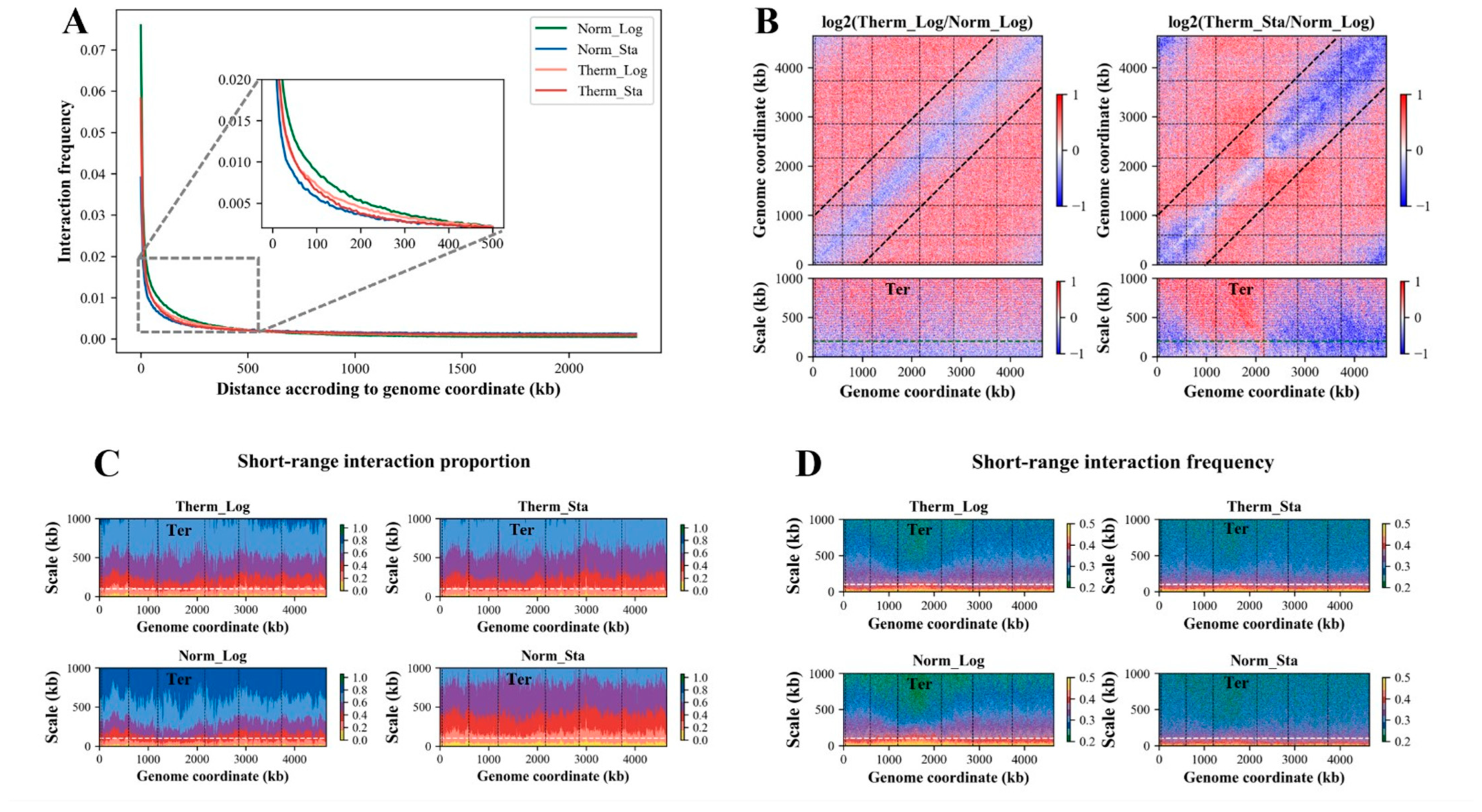
3.1. Chromosome Interaction Decreases with Linear Genomic Distance in All Conditions
3.2. The Long-Range (>200 kb) Interaction of Ter Macrodomain Increases under the Heat Stress
3.3. Short-Range (<100 kb) Interactions Are Significantly Reduced under Heat Stress
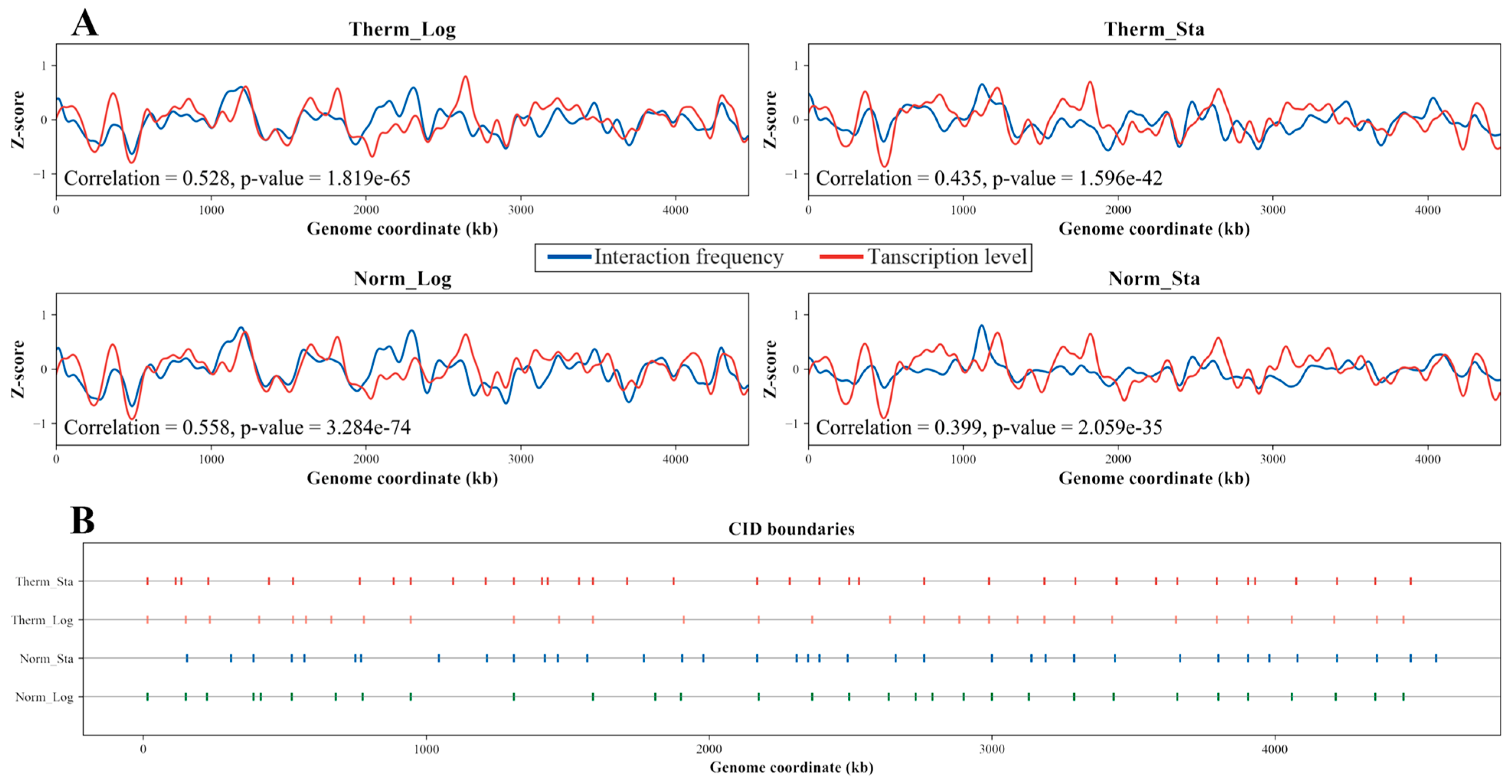
3.4. Transcription Level Positively Correlates with Interaction Frequency
3.5. Differential CID Boundary Genes Are Related with Cell Wall and Membrane
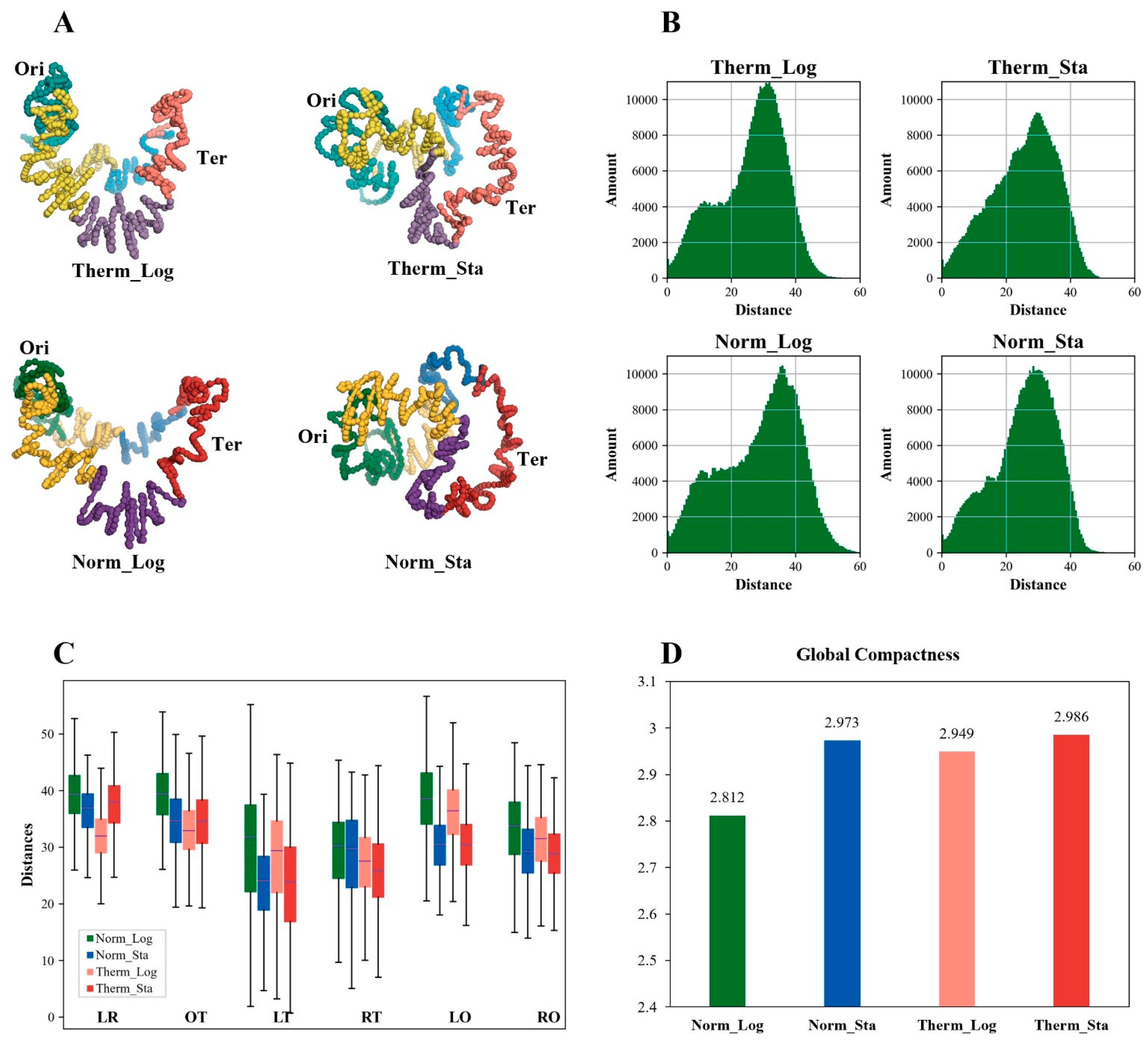
3.6. The Global Compactness of E. coli Chromosome Is Higher in Stationary Phase and High-Temperature Environment
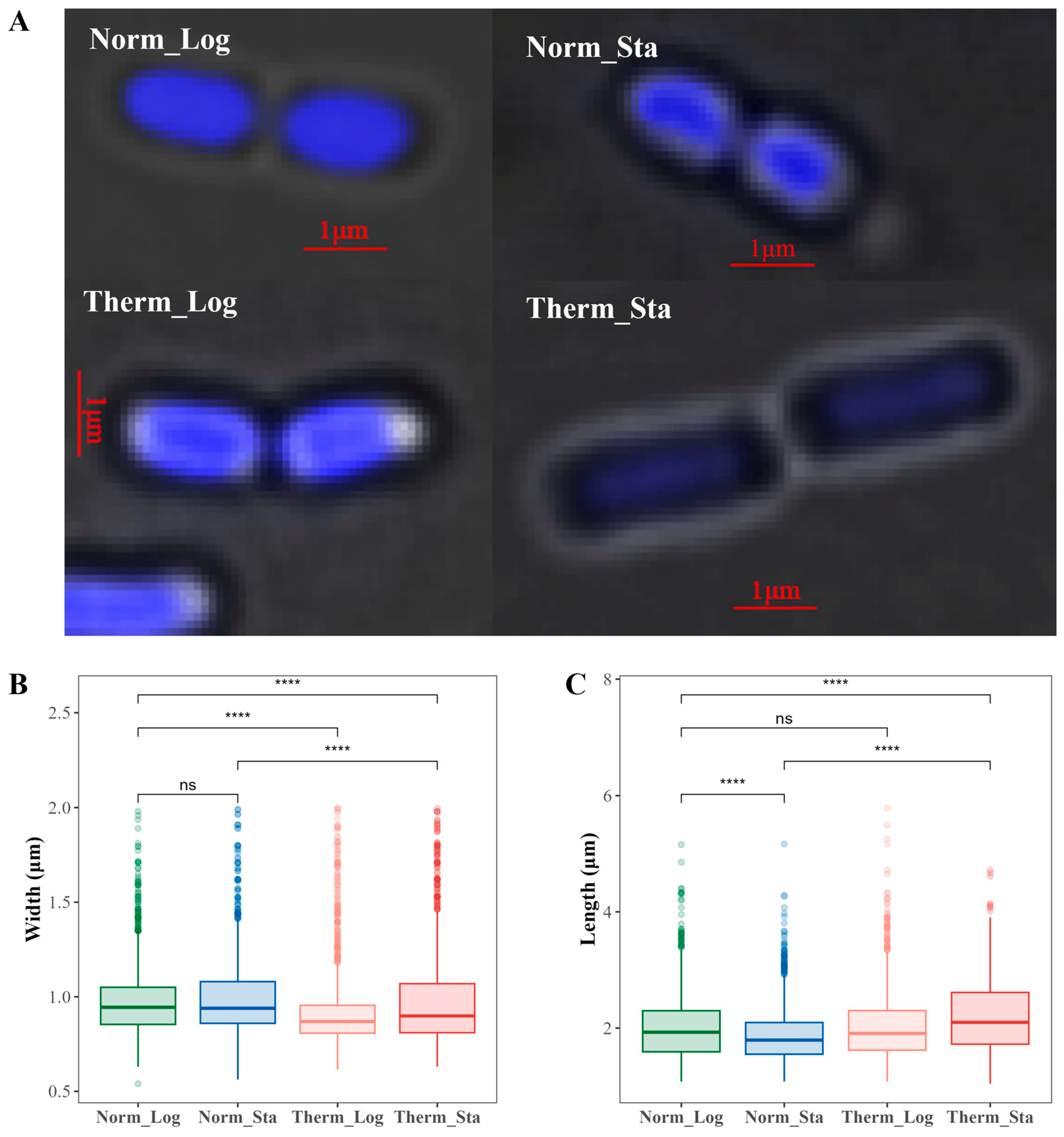
3.7. No Simple Relationship between Chromosome Global Compactness and the Nucleoid Size of E. coli Cell
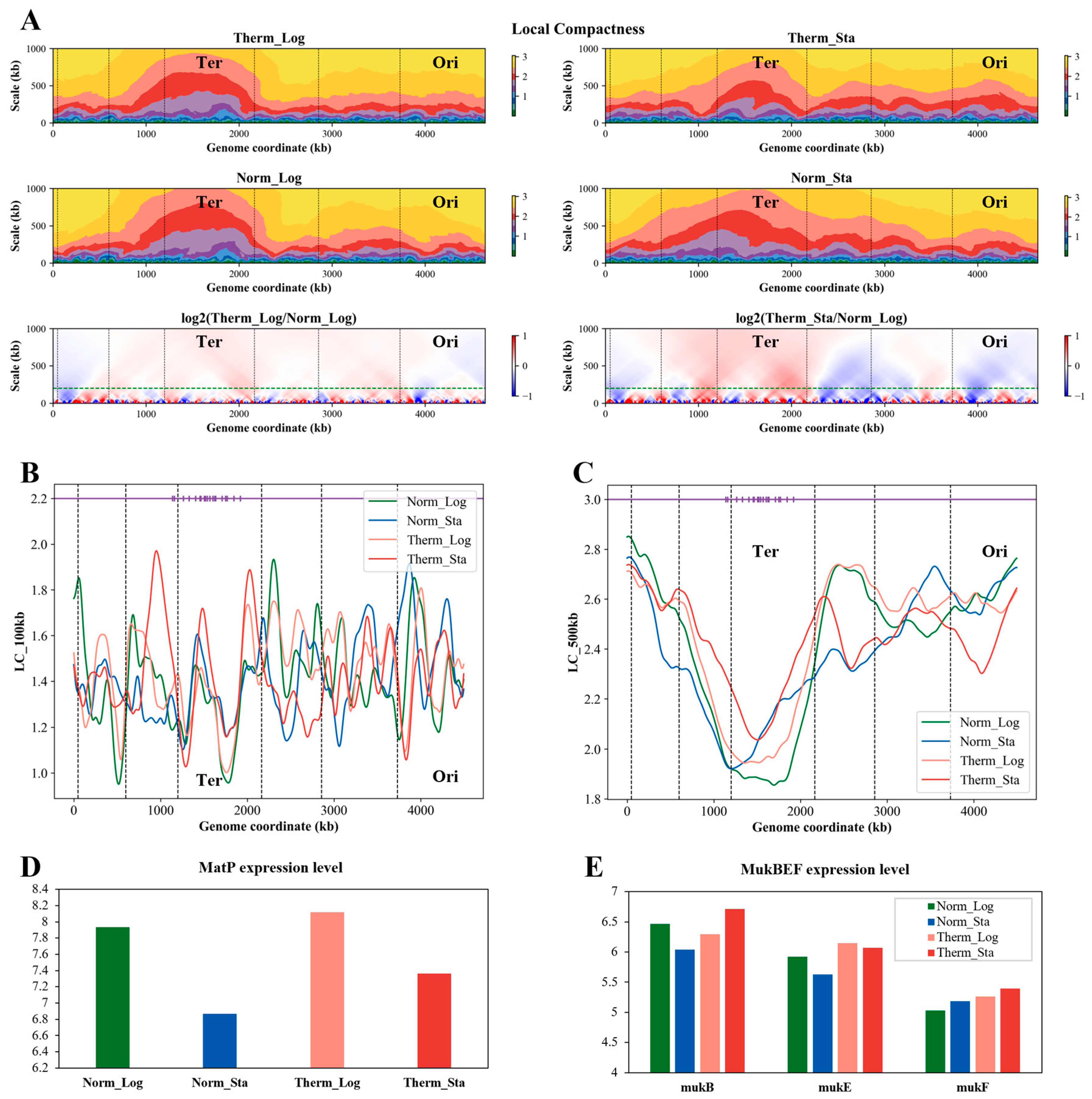
3.8. The Local Compactness of E. coli Chromosome Is Affected by Growth Condition
3.9. The Local Compactness Is Negatively Correlated with Transcription Level
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dekker, J.; Rippe, K.; Dekker, M.; Kleckner, N. Capturing chromosome conformation. Science 2002, 295, 1306–1311. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, M.S.; Zhou, W.; Xiao, J. Differential roles of positive and negative supercoiling in organizing the E. coli genome. Nucleic Acids Res. 2024, 52, 724–737. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, C.; Wang, J.; Chu, Y.; Xiao, J.; Kang, Y.; Zhang, Z. Combined Study of Gene Expression and Chromosome Three-Dimensional Structure in Escherichia coli during Growth Process. Curr. Microbiol. 2024, 81, 122. [Google Scholar] [CrossRef]
- Yáñez-Cuna, F.O.; Koszul, R. Insights in bacterial genome folding. Curr. Opin. Struct. Biol. 2023, 82, 102679. [Google Scholar] [CrossRef]
- Niki, H.; Yamaichi, Y.; Hiraga, S. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev. 2000, 14, 212–223. [Google Scholar] [CrossRef]
- Espeli, O.; Mercier, R.; Boccard, F. DNA dynamics vary according to macrodomain topography in the E. coli chromosome. Mol. Microbiol. 2008, 68, 1418–1427. [Google Scholar] [CrossRef]
- Valens, M.; Penaud, S.; Rossignol, M.; Cornet, F.; Boccard, F. Macrodomain organization of the Escherichia coli chromosome. EMBO J. 2004, 23, 4330–4341. [Google Scholar] [CrossRef]
- Wu, F.; Japaridze, A.; Zheng, X.; Wiktor, J.; Kerssemakers, J.W.J.; Dekker, C. Direct imaging of the circular chromosome in a live bacterium. Nat. Commun. 2019, 10, 2194. [Google Scholar] [CrossRef]
- Lioy, V.S.; Cournac, A.; Marbouty, M.; Duigou, S.; Mozziconacci, J.; Espeli, O.; Boccard, F.; Koszul, R. Multiscale Structuring of the E. coli Chromosome by Nucleoid-Associated and Condensin Proteins. Cell 2018, 172, 771–783.e18. [Google Scholar] [CrossRef]
- Cagliero, C.; Grand, R.S.; Jones, M.B.; Jin, D.J.; O’Sullivan, J.M. Genome conformation capture reveals that the Escherichia coli chromosome is organized by replication and transcription. Nucleic Acids Res. 2013, 41, 6058–6071. [Google Scholar] [CrossRef]
- Japaridze, A.; van Wee, R.; Gogou, C.; Kerssemakers, J.W.J.; van den Berg, D.F.; Dekker, C. MukBEF-dependent chromosomal organization in widened Escherichia coli. Front. Microbiol. 2023, 14, 1107093. [Google Scholar] [CrossRef]
- Le, T.B.; Imakaev, M.V.; Mirny, L.A.; Laub, M.T. High-resolution mapping of the spatial organization of a bacterial chromosome. Science 2013, 342, 731–734. [Google Scholar] [CrossRef]
- Postow, L.; Hardy, C.D.; Arsuaga, J.; Cozzarelli, N.R. Topological domain structure of the Escherichia coli chromosome. Genes Dev. 2004, 18, 1766–1779. [Google Scholar] [CrossRef]
- Azam, T.A.; Ishihama, A. Twelve Species of the Nucleoid-associated Protein from Escherichia coli. J. Biol. Chem. 1999, 274, 33105–33113. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.W.; Chen, C.; Xie, X.S.; Zhuang, X. Chromosome organization by a nucleoid- associated protein in live bacteria. Science 2011, 333, 1445–1449. [Google Scholar] [CrossRef]
- Campos, M.; Govers, S.K.; Irnov, I.; Dobihal, G.S.; Cornet, F.; Jacobs-Wagner, C. Genomewide phenotypic analysis of growth, cell morphogenesis, and cell cycle events in Escherichia coli. Mol. Syst. Biol. 2018, 14, e7573. [Google Scholar] [CrossRef]
- Lioy, V.S.; Junier, I.; Boccard, F. Multiscale Dynamic Structuring of Bacterial Chromosomes. Annu. Rev. Microbiol. 2021, 75, 541–561. [Google Scholar] [CrossRef]
- Fisher, G.L.; Bolla, J.R.; Rajasekar, K.V.; Makela, J.; Baker, R.; Zhou, M.; Prince, J.P.; Stracy, M.; Robinson, C.V.; Arciszewska, L.K.; et al. Competitive binding of MatP and topoisomerase IV to the MukB hinge domain. Elife 2021, 10, e70444. [Google Scholar] [CrossRef]
- Maruyama, H.; Prieto, E.I.; Nambu, T.; Mashimo, C.; Kashiwagi, K.; Okinaga, T.; Atomi, H.; Takeyasu, K. Different Proteins Mediate Step-Wise Chromosome Architectures in Thermoplasma acidophilum and Pyrobaculum calidifontis. Front. Microbiol. 2020, 11, 1247. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, D.; Yang, H. Metabolic Responses of “Big Six” Escherichia coli in Wheat Flour to Thermal Treatment Revealed by Nuclear Magnetic Resonance Spectroscopy. Appl. Environ. Microbiol. 2022, 88, e0009822. [Google Scholar] [CrossRef]
- Xiang, Y.; Surovtsev, I.V.; Chang, Y.; Govers, S.K.; Parry, B.R.; Liu, J.; Jacobs-Wagner, C. Interconnecting solvent quality, transcription, and chromosome folding in Escherichia coli. Cell 2021, 84, 3626–3642.e14. [Google Scholar] [CrossRef]
- Neidhardt, F.C.; VanBogelen, R.A.; Vaughn, V. The Genetics and Regulation of Heat-Shock Proteins. Annu. Rev. Genet. 1984, 18, 295–329. [Google Scholar] [CrossRef]
- Tilly, K.; McKittrick, N.; Zylicz, M.; Georgopoulos, C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell 1983, 34, 641–646. [Google Scholar] [CrossRef]
- Ma, A.; Chui, L. Identification of heat resistant Escherichia coli by qPCR for the locus of heat resistance. J. Microbiol. Methods 2017, 133, 87–89. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Rodrigues, L.R. Potential Applications of the Escherichia coli Heat Shock Response in Synthetic Biology. Trends Biotechnol. 2018, 36, 186–198. [Google Scholar] [CrossRef]
- Yura, T. Regulation of the heat shock response in Escherichia coli: History and perspectives. Genes Genet. Syst. 2019, 94, 103–108. [Google Scholar] [CrossRef]
- Kai, Z.; Mingzhu, L.; Burgess, R.R. The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J. Biol. Chem. 2005, 280, 17758–17768. [Google Scholar]
- Xiong, H.; Yang, Y.; Hu, X.P.; He, Y.M.; Ma, B.G. Sequence determinants of prokaryotic gene expression level under heat stress. Gene 2014, 551, 92–102. [Google Scholar] [CrossRef]
- Miyazaki, R.; Yura, T.; Suzuki, T.; Dohmae, N.; Mori, H.; Akiyama, Y. A Novel SRP Recognition Sequence in the Homeostatic Control Region of Heat Shock Transcription Factor σ32. Sci. Rep. 2016, 6, 24147. [Google Scholar] [CrossRef]
- Eltzov, E.; Yehuda, A.; Marks, R.S. Creation of a new portable biosensor for water toxicity determination. Sens. Actuators B Chem. 2015, 221, 1044–1054. [Google Scholar] [CrossRef]
- Le, T.B.; Laub, M.T. Transcription rate and transcript length drive formation of chromosomal interaction domain boundaries. EMBO J. 2016, 35, 1582–1595. [Google Scholar] [CrossRef]
- Cournac, A.; Marie-Nelly, H.; Marbouty, M.; Koszul, R.; Mozziconacci, J. Normalization of a chromosomal contact map. BMC Genom. 2012, 13, 436. [Google Scholar] [CrossRef]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef]
- Hua, K.J.; Ma, B.G. EVR: Reconstruction of bacterial chromosome 3D structure models using error- vector resultant algorithm. BMC Genom. 2019, 20, 738. [Google Scholar] [CrossRef]
- Mercier, R.; Petit, M.-A.; Schbath, S.; Robin, S.; El Karoui, M.; Boccard, F.; Espéli, O. The MatP/matS Site-Specific System Organizes the Terminus Region of the E. coli Chromosome into a Macrodomain. Cell 2008, 135, 475–485. [Google Scholar] [CrossRef]
- Monterroso, B.; Zorrilla, S.; Sobrinos-Sanguino, M.; Angel Robles-Ramos, M.; Alfonso, C.; Soderstrom, B.; Meiresonne, N.Y.; Verheul, J.; den Blaauwen, T.; Rivas, G. The Bacterial DNA Binding Protein MatP Involved in Linking the Nucleoid Terminal Domain to the Divisome at Midcell Interacts with Lipid Membranes. mBio 2019, 10, e00376-19. [Google Scholar] [CrossRef]
- Talukder, A.; Ishihama, A. Growth phase dependent changes in the structure and protein composition of nucleoid in Escherichia coli. Sci. China Life Sci. 2015, 58, 902–911. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Suh, D.H.; Lee, C.H.; Yoo, S.M.; Lee, S.Y.; Yoon, S.H. Heat-responsive and time-resolved transcriptome and metabolome analyses of Escherichia coli uncover thermo-tolerant mechanisms. Sci. Rep. 2020, 10, 17715. [Google Scholar] [CrossRef]
- Joyce, G.; Robertson, B.D.; Williams, K.J. A modified agar pad method for mycobacterial live-cell imaging. BMC Res. Notes 2011, 4, 73. [Google Scholar] [CrossRef]
- Ali Azam, T.; Iwata, A.; Nishimura, A.; Ueda, S.; Ishihama, A. Growth Phase-Dependent Variation in Protein Composition of the Escherichia coli Nucleoid. J. Bacteriol. 1999, 181, 6361–6370. [Google Scholar] [CrossRef]
- Chintakayala, K.; Singh, S.S.; Rossiter, A.E.; Shahapure, R.; Dame, R.T.; Grainger, D.C. E. coli Fis protein insulates the cbpA gene from uncontrolled transcription. PLoS Genet. 2013, 9, e1003152. [Google Scholar] [CrossRef]
- Lim, C.J.; Whang, Y.R.; Kenney, L.J.; Yan, J. Gene silencing H-NS paralogue StpA forms a rigid protein filament along DNA that blocks DNA accessibility. Nucleic Acids Res. 2012, 40, 3316–3328. [Google Scholar] [CrossRef]
- Zhang, A.; Belfort, M. Nucleotide sequence of a newly-identified Escherichia coli gene, stpA, encoding an H-NS-like protein. Nucleic Acids Res. 1992, 20, 6735. [Google Scholar] [CrossRef]
- Wolf, T.; Janzen, W.; Blum, C.; Schnetz, K. Differential dependence of StpA on H-NS in autoregulation of stpA and in regulation of bgl. J. Bacteriol. 2006, 188, 6728–6738. [Google Scholar] [CrossRef]
- Sonnenfield, J.M.; Burns, C.M.; Higgins, C.F.; Hinton, J.C. The nucleoid-associated protein StpA binds curved DNA, has a greater DNA-binding affinity than H-NS and is present in significant levels in hns mutants. Biochimie 2001, 83, 243–249. [Google Scholar] [CrossRef]
- Membrillo-Hernández, J.; Núñez-de la Mora, A.; del Rio-Albrechtsen, T.; Camacho-Carranza, R.; Gomez-Eichelmann, M.C. Thermally-induced cell lysis in Escherichia coli K12. J. Basic Microbiol. 1995, 35, 41–46. [Google Scholar] [CrossRef]
- Wang, X.T.; Xiao, S.D.; Ma, B.G. Molecular Responses to Heat Stress in Escherichia coli. In Stress: Genetics, Epigenetics and Genomics (Handbook of Stress, Volume 4); Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 289–296. [Google Scholar]
- Roncarati, D.; Scarlato, V. Regulation of heat-shock genes in bacteria: From signal sensing to gene expression output. FEMS Microbiol. Rev. 2017, 41, 549–574. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-T.; Ma, B.-G. Spatial Chromosome Organization and Adaptation of Escherichia coli under Heat Stress. Microorganisms 2024, 12, 1229. https://doi.org/10.3390/microorganisms12061229
Wang X-T, Ma B-G. Spatial Chromosome Organization and Adaptation of Escherichia coli under Heat Stress. Microorganisms. 2024; 12(6):1229. https://doi.org/10.3390/microorganisms12061229
Chicago/Turabian StyleWang, Xu-Ting, and Bin-Guang Ma. 2024. "Spatial Chromosome Organization and Adaptation of Escherichia coli under Heat Stress" Microorganisms 12, no. 6: 1229. https://doi.org/10.3390/microorganisms12061229
APA StyleWang, X.-T., & Ma, B.-G. (2024). Spatial Chromosome Organization and Adaptation of Escherichia coli under Heat Stress. Microorganisms, 12(6), 1229. https://doi.org/10.3390/microorganisms12061229






