Leaf Health Status Regulates Endophytic Microbial Community Structure, Network Complexity, and Assembly Processes in the Leaves of the Rare and Endangered Plant Species Abies fanjingshanensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. DNA Extraction, PCR Amplification, Illumina Sequencing, and Data Processing
2.3. Statistical Analysis
3. Results
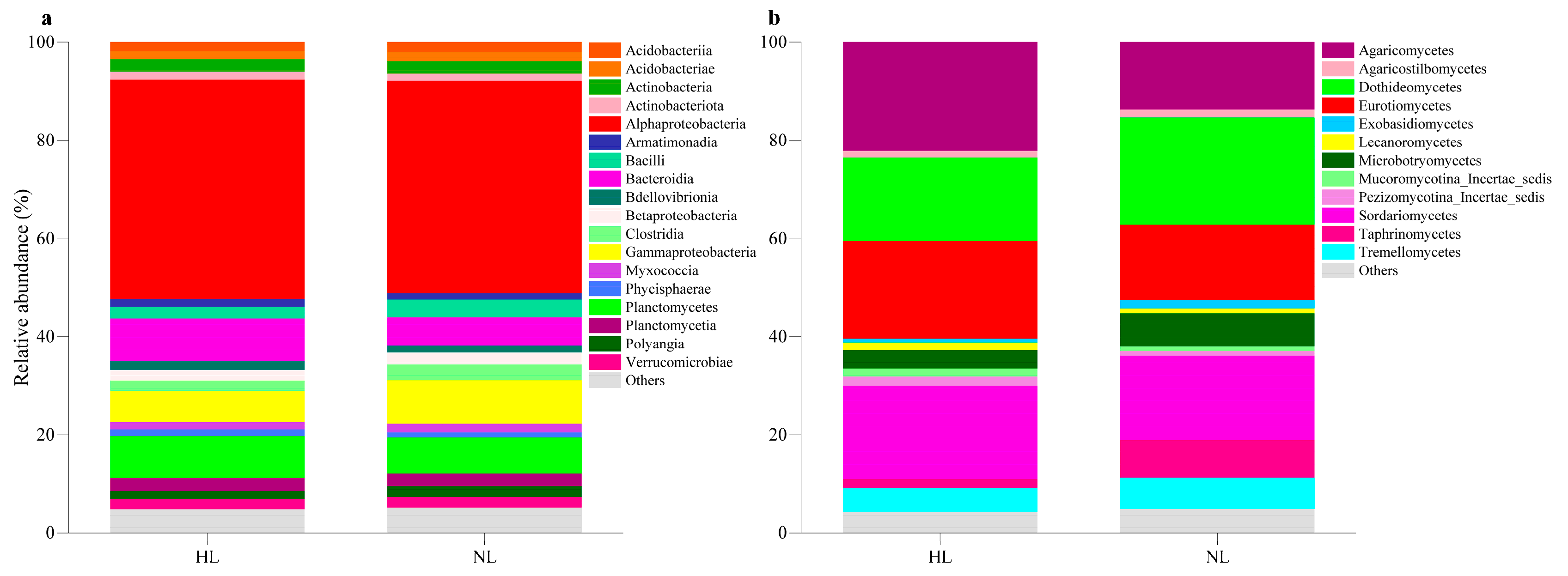
3.1. Composition of the Leaf Endophytic Microbial Community
3.2. Diversity of the Leaf Endophytic Microbial Community
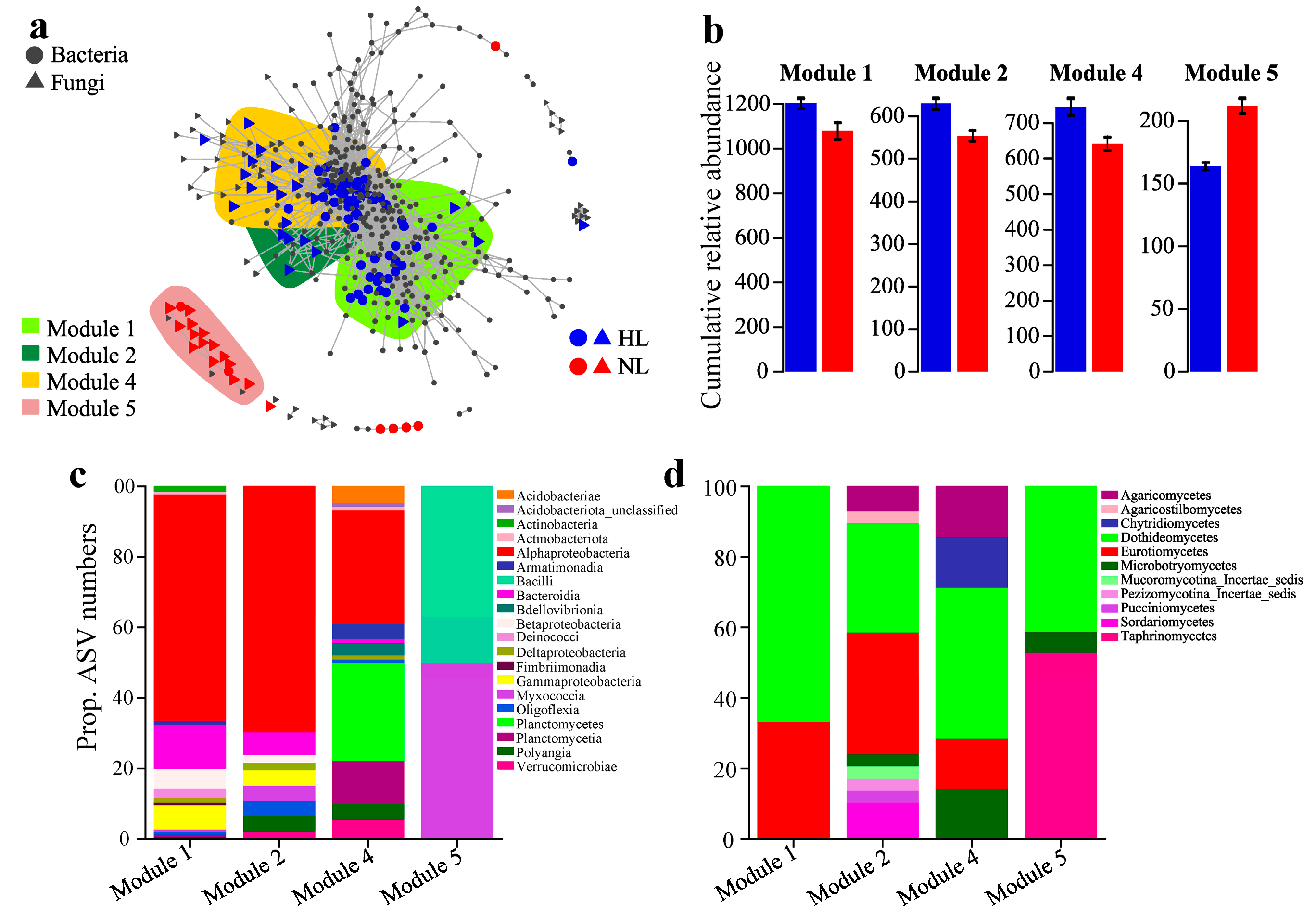
3.3. Co-Occurrence and Network Complexity of the Leaf Endophytic Microbial Community
3.4. Assembly Process of the Leaf Endophytic Microbial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bacon, C.W.; White, J. Microbial Endophytes; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Jia, Q.; Qu, J.; Mu, H.; Sun, H.; Wu, C. Foliar endophytic fungi: Diversity in species and functions in forest ecosystems. Symbiosis 2020, 80, 103–132. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Christian, N.; Herre, E.A.; Clay, K. Foliar endophytic fungi alter patterns of nitrogen uptake and distribution in Theobroma cacao. New Phytol. 2019, 222, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Mejía, L.C.; Herre, E.A.; Sparks, J.P.; Winter, K.; García, M.N.; Van Bael, S.A.; Stitt, J.; Shi, Z.; Zhang, Y.; Guiltinan, M.J.; et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 2014, 5, 479. [Google Scholar] [CrossRef] [PubMed]
- González-Teuber, M.; Palma-Onetto, V.; Aguilera-Sammaritano, J.; Mithöfer, A. Roles of leaf functional traits in fungal endophyte colonization: Potential implications for host–pathogen interactions. J. Ecol. 2021, 109, 3972–3987. [Google Scholar] [CrossRef]
- Faticov, M.; Abdelfattah, A.; Roslin, T.; Vacher, C.; Hambäck, P.; Blanchet, F.G.; Lindahl, B.D.; Tack, A.J.M. Climate warming dominates over plant genotype in shaping the seasonal trajectory of foliar fungal communities on oak. New Phytol. 2021, 231, 1770–1783. [Google Scholar] [CrossRef]
- González-Teuber, M.; Vilo, C.; Guevara-Araya, M.J.; Salgado-Luarte, C.; Gianoli, E. Leaf resistance traits influence endophytic fungi colonization and community composition in a South American temperate rainforest. J. Ecol. 2020, 108, 1019–1029. [Google Scholar] [CrossRef]
- Qin, D.; You, C.; Lan, W.; Wang, Y.; Yu, B.; Peng, Y.; Xu, J.; Dong, J. Microbial assemblages of Schisandraceae plants and the correlations between endophytic species and the accumulation of secondary metabolites. Plant Soil 2023, 483, 85–107. [Google Scholar] [CrossRef]
- Christian, N.; Basurto, B.E.; Toussaint, A.; Xu, X.; Ainsworth, E.A.; Busby, P.E.; Heath, K.D. Elevated carbon dioxide reduces a common soybean leaf endophyte. Glob. Chang. Biol. 2021, 27, 4154–4168. [Google Scholar] [CrossRef]
- Yao, H.; Sun, X.; He, C.; Maitra, P.; Li, X.-C.; Guo, L.-D. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, J.; Zhang, H.; Ji, G.; Zeng, L.; Li, Y.; Yu, C.; Fernando, W.G.D.; Chen, W. Bacterial Blight Induced Shifts in Endophytic Microbiome of Rice Leaves and the Enrichment of Specific Bacterial Strains with Pathogen Antagonism. Front. Plant Sci. 2020, 11, 963. [Google Scholar] [CrossRef]
- Humphrey, P.T.; Whiteman, N.K. Insect herbivory reshapes a native leaf microbiome. Nat. Ecol. Evol. 2020, 4, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Han, X.; Zhao, D.; Wei, K.; Yuan, Y.; Li, Y.; Liu, M.; Zhang, C.-S. Exploring Biocontrol Agents from Microbial Keystone Taxa Associated to Suppressive Soil: A New Attempt for a Biocontrol Strategy. Front. Plant Sci. 2021, 12, 655673. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, T.; Huang, Q.; Guo, H.; Zhang, H.; Xu, Q.; Shen, Q.; Ling, N. Core species impact plant health by enhancing soil microbial cooperation and network complexity during community coalescence. Soil Biol. Biochem. 2024, 188, 109231. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Ding, H.; Zhao, J.; Cai, Z.; Dai, C.; Huang, X. The fungal community outperforms the bacterial community in predicting plant health status. Appl. Microbiol. Biotechnol. 2021, 105, 6499–6513. [Google Scholar] [CrossRef]
- Bulgari, D.; Casati, P.; Quaglino, F.; Bianco, P.A. Endophytic bacterial community of grapevine leaves influenced by sampling date and phytoplasma infection process. BMC Microbiol. 2014, 14, 198. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Moya-Laraño, J.; Poveda, J.; Lino-Neto, T.; Baptista, P. Deciphering plant health status: The link between secondary metabolites, fungal community and disease incidence in olive tree. Front. Plant Sci. 2023, 14, 1048762. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.-X.; Lü, P.-P.; Wang, Y.-L.; Li, Z.-F.; Zhang, Y.; Gan, H.-Y.; Li, X.-C.; Mandal, D.; Cai, J.; et al. Community Assembly of Fungi and Bacteria along Soil-Plant Continuum Differs in a Zoige Wetland. Microbiol. Spectr. 2022, 10, e0226022. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, X.; Zhang, J.; Cai, Z. Highly connected taxa located in the microbial network are prevalent in the rhizosphere soil of healthy plant. Biol. Fertil. Soils 2019, 55, 299–312. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Nelson, M.; Helgason, T.; Dytham, C.; Fitter, A.H. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J. 2010, 4, 337–345. [Google Scholar] [CrossRef]
- Chave, J. Neutral theory and community ecology. Ecol. Lett. 2004, 7, 241–253. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef]
- Taniguchi, T.; Isobe, K.; Imada, S.; Eltayeb, M.M.; Akaji, Y.; Nakayama, M.; Allen, M.F.; Aronson, E.L. Root endophytic bacterial and fungal communities in a natural hot desert are differentially regulated in dry and wet seasons by stochastic processes and functional traits. Sci. Total. Environ. 2023, 899, 165524. [Google Scholar] [CrossRef]
- Ku, Y.; Han, X.; Lei, Y.; Zhang, M.; Zhao, Z. Different sensitivities and assembly mechanisms of the root-associated microbial communities of Robinia pseudoacacia to spatial variation at the regional scale. Plant Soil 2023, 486, 621–637. [Google Scholar] [CrossRef]
- Fu, F.; Li, Y.; Zhang, B.; Zhu, S.; Guo, L.; Li, J.; Zhang, Y.; Li, J. Differences in soil microbial community structure and assembly processes under warming and cooling conditions in an alpine forest ecosystem. Sci. Total. Environ. 2024, 907, 167809. [Google Scholar] [CrossRef]
- Zhou, S.Y.D.; Lie, Z.; Liu, X.; Zhu, Y.G.; Peñuelas, J.; Neilson, R.; Su, X.; Liu, Z.; Chu, G.; Meng, Z.; et al. Distinct patterns of soil bacterial and fungal community assemblages in subtropical forest ecosystems under warming. Glob. Chang. Biol. 2023, 29, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Chen, Z.; Wu, Q.; Huang, K.; Ke, Q.; Zhu, L.; Lu, S.; Tang, Y.; Li, H.; et al. Ecological niche differences regulate the assembly of bacterial community in endophytic and rhizosphere of Eucalyptus. For. Ecol. Manag. 2022, 524, 120521. [Google Scholar] [CrossRef]
- Guo, J.; Ling, N.; Li, Y.; Li, K.; Ning, H.; Shen, Q.; Guo, S.; Vandenkoornhuyse, P. Seed-borne, endospheric and rhizospheric core microbiota as predictors of plant functional traits across rice cultivars are dominated by deterministic processes. New Phytol. 2021, 230, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Huang, W. The discovery of Abies fanjingshanensis forests and their scientifical significance. Guizhou Sci. 2001, 19, 1–9. [Google Scholar]
- Li, X.; Tao, C.; Wang, Q.; Cui, G. Characteristics of geographic distribution of four critically endangered species of Abies in subtropical China and its relationship with climate. Chin. J. Plant Ecol. 2012, 36, 1154–1164. (In Chinese) [Google Scholar] [CrossRef]
- Xiang, Q. A preliminary survey on the distribution of rare and endangered plants of Abies in China. Guihaia 2001, 21, 113–117. (In Chinese) [Google Scholar]
- Liu, Y.; Luo, W.; Mu, G.; Wu, X.; Su, S.; Zhang, Z. C:N:P stoichiometric characteristics of the soil–vegetation system of three rare tree species growing on Mount Fanjing in Southwest China. Glob. Ecol. Conserv. 2021, 32, e01893. [Google Scholar] [CrossRef]
- Huang, W.; Tu, Y.; Yang, L. Scientific Survey of the Fanjingshan Mountain Preserve Guizhou Province, China; China Environmental Science Press: Beijing, China, 1982. (In Chinese) [Google Scholar]
- Carretero, R.; Bancal, M.O.; Miralles, D.J. Effect of leaf rust (Puccinia triticina) on photosynthesis and related processes of leaves in wheat crops grown at two contrasting sites and with different nitrogen levels. Eur. J. Agron. 2011, 35, 237–246. [Google Scholar] [CrossRef]
- Ranulfi, A.C.; Senesi, G.S.; Caetano, J.B.; Meyer, M.C.; Magalhães, A.B.; Villas-Boas, P.R.; Milori, D.M. Nutritional characterization of healthy and Aphelenchoides besseyi infected soybean leaves by laser-induced breakdown spectroscopy (LIBS). Microchem. J. 2018, 141, 118–126. [Google Scholar] [CrossRef]
- Lima, M.R.; Felgueiras, M.L.; Cunha, A.; Chicau, G.; Ferreres, F.; Dias, A.C. Differential phenolic production in leaves of Vitis vinifera cv. Alvarinho affected with esca disease. Plant Physiol. Biochem. 2017, 112, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, X.; Wei, J.-G.; Lou, J.-F.; Guo, L.-D. A new endophytic fungus Neofabraea illicii isolated from Illicium verum. Mycoscience 2014, 56, 332–339. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Rivers, A.R.; Weber, K.C.; Gardner, T.G.; Liu, S.; Armstrong, S.D. ITSxpress: Software to rapidly trim internally transcribed spacer sequences with quality scores for marker gene analysis. F1000Research 2018, 7, 1418. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. Version 2.6-4. 2022. Available online: https://cran.r-project.org/package=vegan (accessed on 30 January 2024).
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; van der Heijden, M.G.; Wittwer, R.A.; Banerjee, S.; Walser, J.-C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 14. [Google Scholar]
- Harrell, F.E.; Dupont, C. Hmisc: Harrell Miscellaneous. R Package Version 5.1-1. 2023. Available online: https://cran.r-project.org/package=Hmisc (accessed on 15 February 2024).
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJ. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Zhang, J. spaa: SPecies Association Analysis. R Package Version 0.2.2. 2016. Available online: https://cran.r-project.org/package=spaa (accessed on 20 February 2024).
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Stephens, W.Z.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016, 10, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Hart, E.M.; Ellison, A.M. EcoSimR: Null model analysis for ecological data. R Package Version 0.1.0. 2015. Available online: http://github.com/gotellilab/EcoSimR (accessed on 20 February 2024).
- Yang, J.; Masoudi, A.; Li, H.; Gu, Y.; Wang, C.; Wang, M.; Yu, Z.; Liu, J. Microbial community structure and niche differentiation under different health statuses of Pinus bungeana in the Xiong’an New Area in China. Front. Microbiol. 2022, 13, 913349. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.-T.; Wang, W.-H.; Tsui, C.K.; Cai, L. Changes in Bacterial and Fungal Microbiomes Associated with Tomatoes of Healthy and Infected by Fusarium oxysporum f. sp. lycopersici. Microb. Ecol. 2021, 81, 1004–1017. [Google Scholar] [CrossRef]
- Choi, B.Y.; Lee, S.; Kim, J.; Park, H.; Kim, J.-H.; Kim, M.; Park, S.-J.; Kim, K.-T.; Ryu, H.; Shim, D. Comparison of Endophytic and Epiphytic Microbial Communities in Surviving and Dead Korean Fir (Abies koreana) Using Metagenomic Sequencing. Forests 2022, 13, 1932. [Google Scholar] [CrossRef]
- Li, Y.; Tao, S.; Liang, Y. Time-Course Responses of Apple Leaf Endophytes to the Infection of Gymnosporangium yamadae. J. Fungi 2024, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Wang, T.; Hao, Y.; Zhu, M.; Yu, S.; Ran, W.; Xue, C.; Ling, N.; Shen, Q. Characterizing differences in microbial community composition and function between Fusarium wilt diseased and healthy soils under watermelon cultivation. Plant Soil 2019, 438, 421–433. [Google Scholar] [CrossRef]
- Fan, Q.; Xie, K.; Cui, X.; Zhang, G.; Zheng, H.; Chang, S.; Hou, F. Microecosystem of yak rumen on the Qinghai-Tibetan Plateau is stable and is unaffected by soil or grass microbiota. Environ. Microbiol. 2022, 24, 5760–5773. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Frascella, A.; Santopolo, L.; Bazzicalupo, M.; Biondi, E.G.; Scotti, C.; Mengoni, A. Exploring the plant-associated bacterial communities in Medicago sativa L. BMC Microbiol. 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Danhorn, T.; Fuqua, C. Biofilm Formation by Plant-Associated Bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Moyes, A.B.; Kueppers, L.M.; Pett-Ridge, J.; Carper, D.L.; Vandehey, N.; O’Neil, J.; Frank, A.C. Evidence for foliar endophytic nitrogen fixation in a widely distributed subalpine conifer. New Phytol. 2016, 210, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, A.; Romero, D.; de Vicente, A. Plant protection and growth stimulation by microorganisms: Biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011, 22, 187–193. [Google Scholar] [CrossRef]
- Li, L.; Huang, D.; Hu, Y.; Rudling, N.M.; Canniffe, D.P.; Wang, F.; Wang, Y. Globally distributed Myxococcota with photosynthesis gene clusters illuminate the origin and evolution of a potentially chimeric lifestyle. Nat. Commun. 2023, 14, 6450. [Google Scholar] [CrossRef]
- Dong, C.; Wang, L.; Li, Q.; Shang, Q. Epiphytic and Endophytic Fungal Communities of Tomato Plants. Hortic. Plant J. 2021, 7, 38–48. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Q.; Sun, X.; Chen, D.; Insam, H.; Koide, R.T.; Zhang, S. Effects of mixed-species litter on bacterial and fungal lignocellulose degradation functions during litter decomposition. Soil Biol. Biochem. 2020, 141, 107690. [Google Scholar] [CrossRef]
- U’ren, J.M.; Arnold, A.E. Diversity, taxonomic composition, and functional aspects of fungal communities in living, senesced, and fallen leaves at five sites across North America. PeerJ 2016, 4, e2768. [Google Scholar] [CrossRef] [PubMed]
- Bacigálová, K.; Lopandic, K.; Rodrigues, M.G.; Fonseca, A.; Herzberg, M.; Pinsker, W.; Prillinger, H. Phenotypic and genotypic identification and phylogenetic characterisation of Taphrina fungi on alder. Mycol. Prog. 2003, 2, 179–196. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, F.-M.; Kang, N.; Gong, J.-H.; Zhang, W.; Chen, Y.; Dai, C.-C. Rice carbohydrate dynamics regulate endophytic colonization of Diaporthe liquidambaris in response to external nitrogen. Fungal Ecol. 2019, 39, 213–224. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, R.; He, T.; Sun, Y.; Wu, M.; Xue, Y.; Meng, F.; Wang, J. Disentangling the impact of straw incorporation on soil microbial communities: Enhanced network complexity and ecological stochasticity. Sci. Total Environ. 2023, 863, 160918. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Jiang, Y.; Cheng, M.; Dini-Andreote, F.; Sui, Y.; Xu, Q.; Geisen, S.; Sun, B. Organism body size structures the soil microbial and nematode community assembly at a continental and global scale. Nat. Commun. 2020, 11, 6406. [Google Scholar] [CrossRef]
- Kaspari, M.; Stevenson, B.S.; Shik, J.; Kerekes, J.F. Scaling community structure: How bacteria, fungi, and ant taxocenes differentiate along a tropical forest floor. Ecology 2010, 91, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Pei, J.; Zhao, L.; Ahmad, B.; Huang, L.F. Fighting climate change: Soil bacteria communities and topography play a role in plant colonization of desert areas. Environ. Microbiol. 2021, 23, 6876–6894. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, L.; Yan, R.; Yang, Y.; Adams, J.M.; Liu, J. Abundant bacterial subcommunity is structured by a stochastic process in an agricultural system with P fertilizer inputs. Sci. Total. Environ. 2023, 871, 162178. [Google Scholar] [CrossRef]
- López-González, R.C.; Gómez-Cornelio, S.; De la Rosa-García, S.C.; Garrido, E.; Oropeza-Mariano, O.; Heil, M.; Partida-Martínez, L.P. The age of lima bean leaves influences the richness and diversity of the endophytic fungal community, but not the antagonistic effect of endophytes against Colletotrichum lindemuthianum. Fungal Ecol. 2017, 26, 1–10. [Google Scholar] [CrossRef]
- Frank, A.C.; Guzmán, J.P.S.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Ait Barka, E.d. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microb. 2005, 71, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Zimmerman, N.B.; Arnold, A.E. Observations on the Early Establishment of Foliar Endophytic Fungi in Leaf Discs and Living Leaves of a Model Woody Angiosperm, Populus trichocarpa (Salicaceae). J. Fungi 2018, 4, 58. [Google Scholar] [CrossRef]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zheng, R.; Wang, Z.; Li, H.; Shi, Y.; Pan, Z.; Liu, M. Leaf Health Status Regulates Endophytic Microbial Community Structure, Network Complexity, and Assembly Processes in the Leaves of the Rare and Endangered Plant Species Abies fanjingshanensis. Microorganisms 2024, 12, 1254. https://doi.org/10.3390/microorganisms12071254
Li L, Zheng R, Wang Z, Li H, Shi Y, Pan Z, Liu M. Leaf Health Status Regulates Endophytic Microbial Community Structure, Network Complexity, and Assembly Processes in the Leaves of the Rare and Endangered Plant Species Abies fanjingshanensis. Microorganisms. 2024; 12(7):1254. https://doi.org/10.3390/microorganisms12071254
Chicago/Turabian StyleLi, Long, Rong Zheng, Zuhua Wang, Haibo Li, Yongjia Shi, Zhongjie Pan, and Min Liu. 2024. "Leaf Health Status Regulates Endophytic Microbial Community Structure, Network Complexity, and Assembly Processes in the Leaves of the Rare and Endangered Plant Species Abies fanjingshanensis" Microorganisms 12, no. 7: 1254. https://doi.org/10.3390/microorganisms12071254
APA StyleLi, L., Zheng, R., Wang, Z., Li, H., Shi, Y., Pan, Z., & Liu, M. (2024). Leaf Health Status Regulates Endophytic Microbial Community Structure, Network Complexity, and Assembly Processes in the Leaves of the Rare and Endangered Plant Species Abies fanjingshanensis. Microorganisms, 12(7), 1254. https://doi.org/10.3390/microorganisms12071254




