Role of Nedd4L in Macrophage Pro-Inflammatory Polarization Induced by Influenza A Virus and Lipopolysaccharide Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. IAV
2.2. Animal Source and Establishment of Model
2.3. Cell Culture, Transfection, and siRNA Silencing
2.4. Establishment of Cell Model
2.5. Pulmonary Function
2.6. Lung Index and Wet-to-Dry Weight Ratio
2.7. Hematoxylin and Eosin Staining of Mouse Lung Tissues
2.8. Enzyme-Linked Immunosorbent for Mouse Serum and Cell Culture Supernatants
2.9. Immunohistochemistry for Nedd4L Protein Expression in Mouse Lung Tissues
2.10. Immunofluorescence for CD86 and CD206 Protein Expression in Mouse Lung Tissues
2.11. Real-Time Quantitative PCR for Nedd4L and Inflammatory Cytokine MRNA Expression in Mouse Lung Tissues and Macrophages
2.12. Western Blotting for Nedd4L Protein Expression in Cells
2.13. Statistical Analysis
3. Results
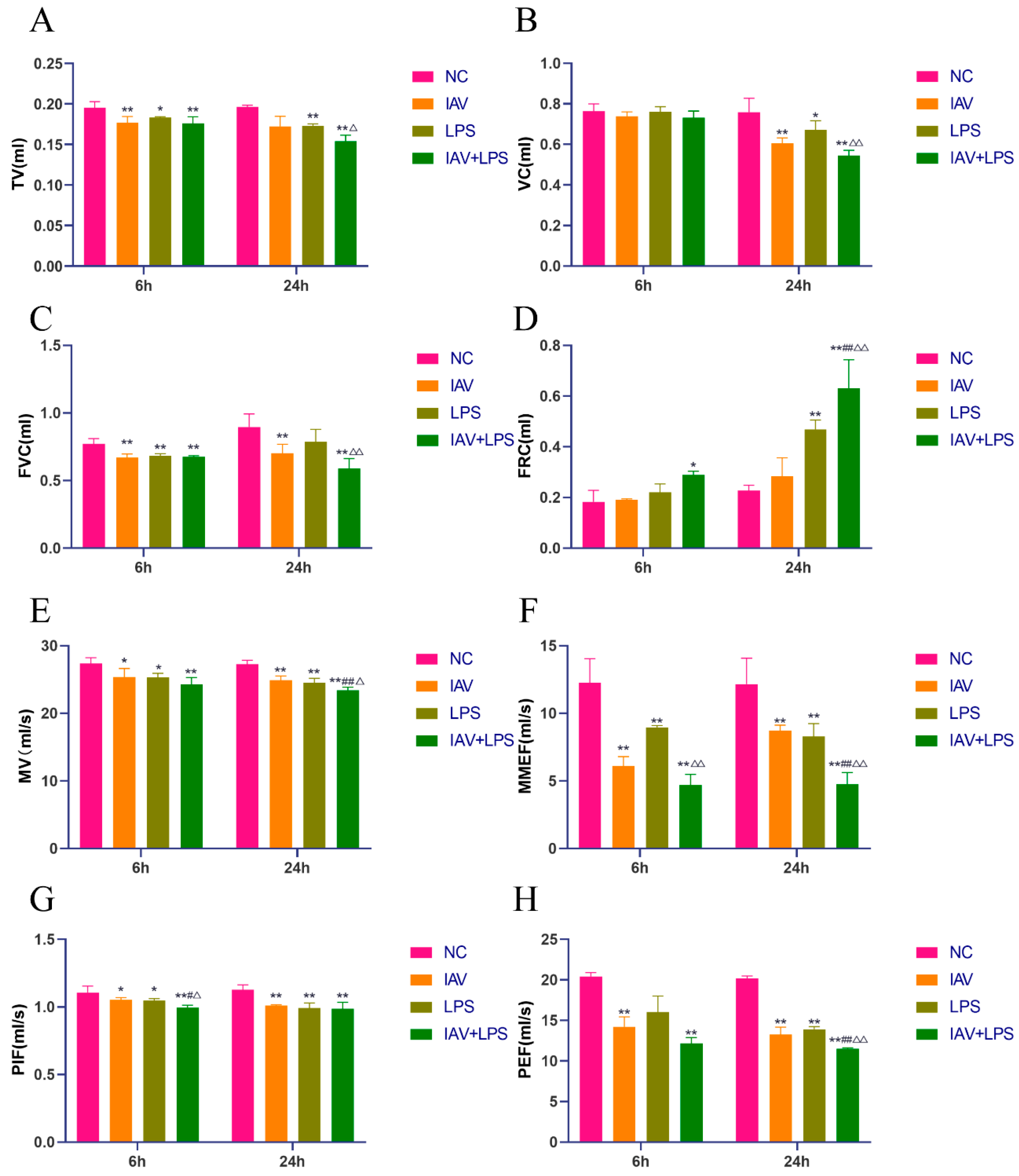
3.1. Impact of Combined IAV and LPS Stimulation on Mouse Pulmonary Function
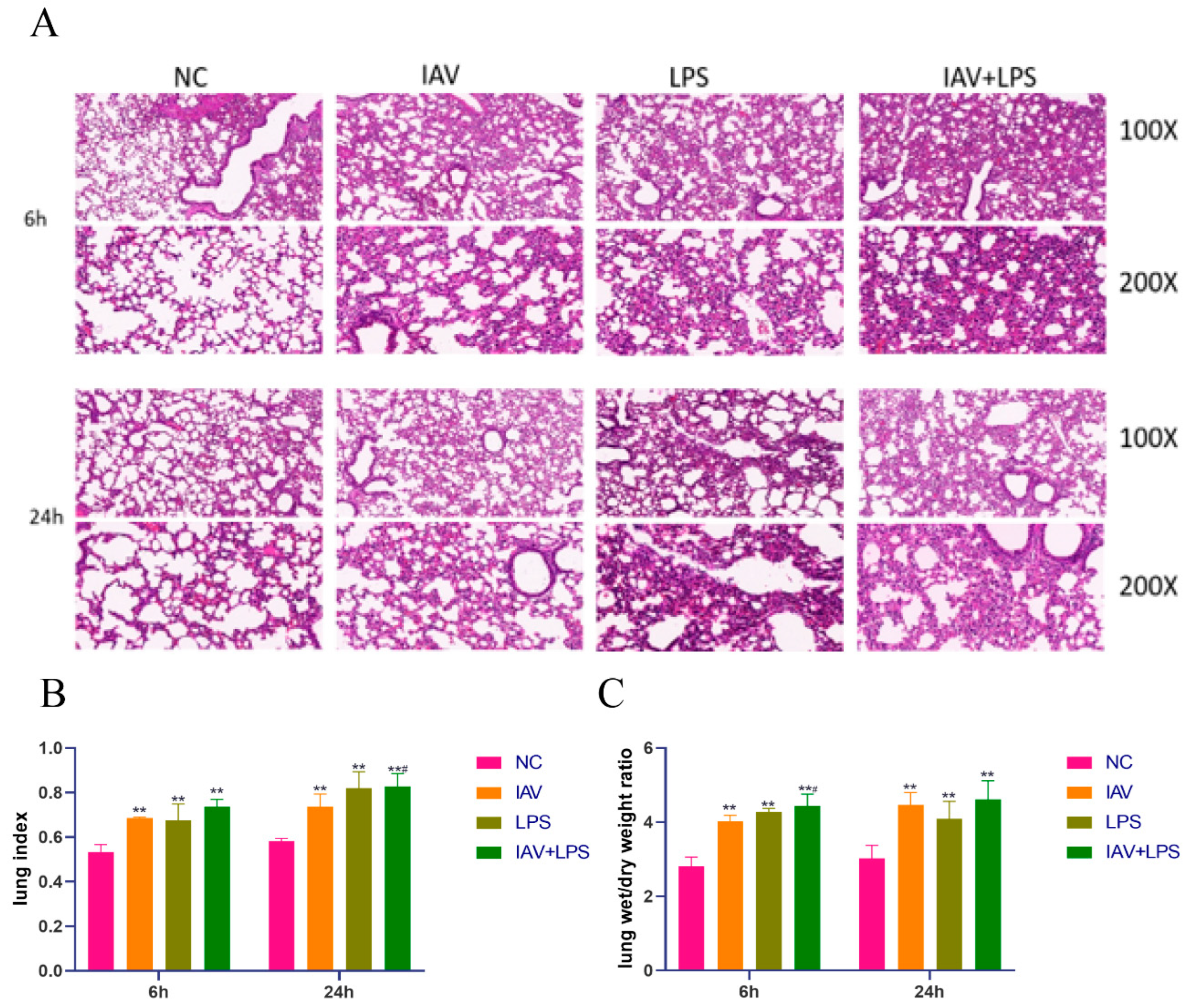
3.2. Damage and Edema in Mouse Lung Tissue Due to Combined IAV and LPS Stimulation
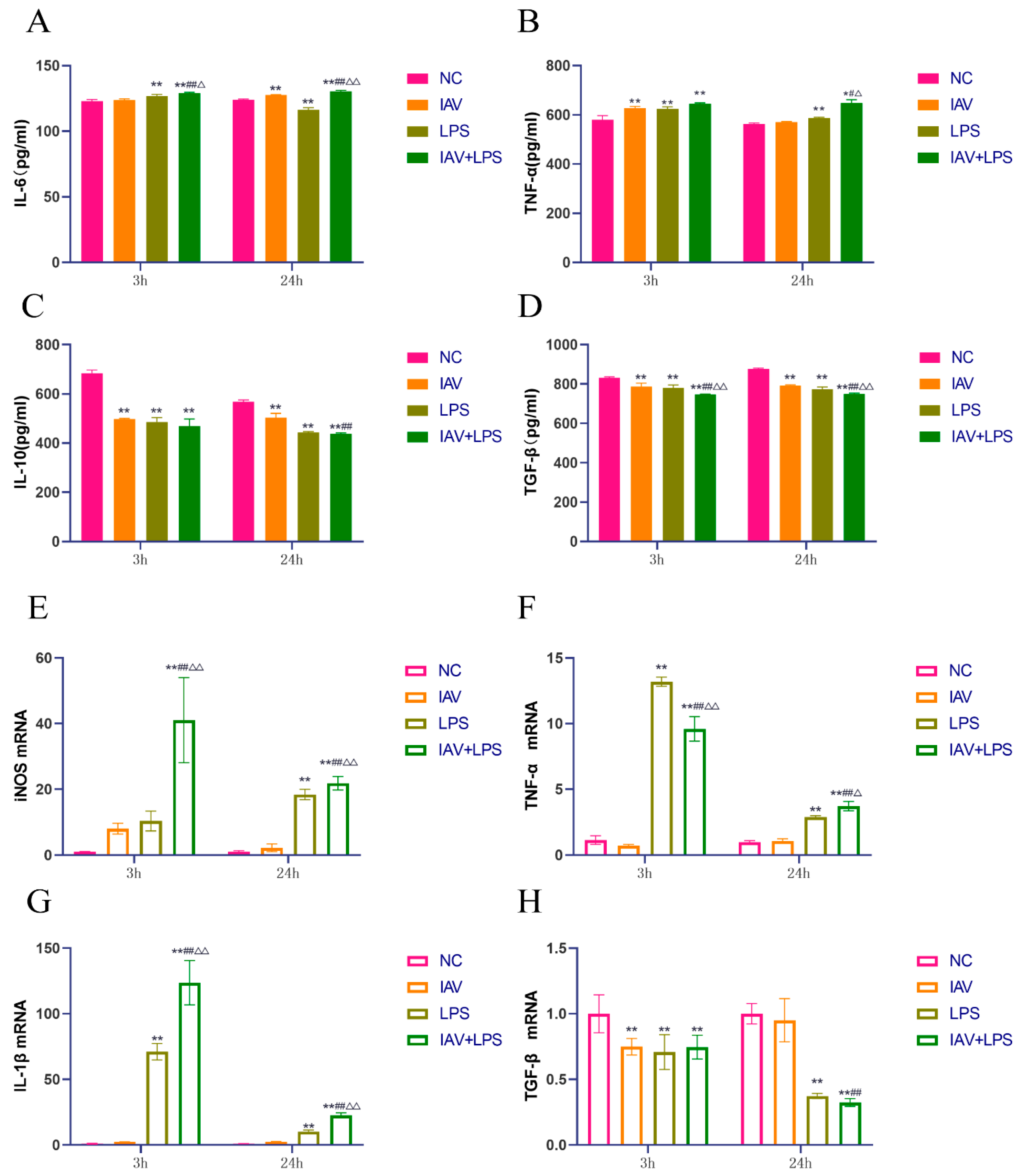
3.3. Effects of Combined IAV and LPS Stimulation on Serum Inflammatory Cytokines and Lung Tissue Inflammatory Cytokine mRNA Expression in Mice
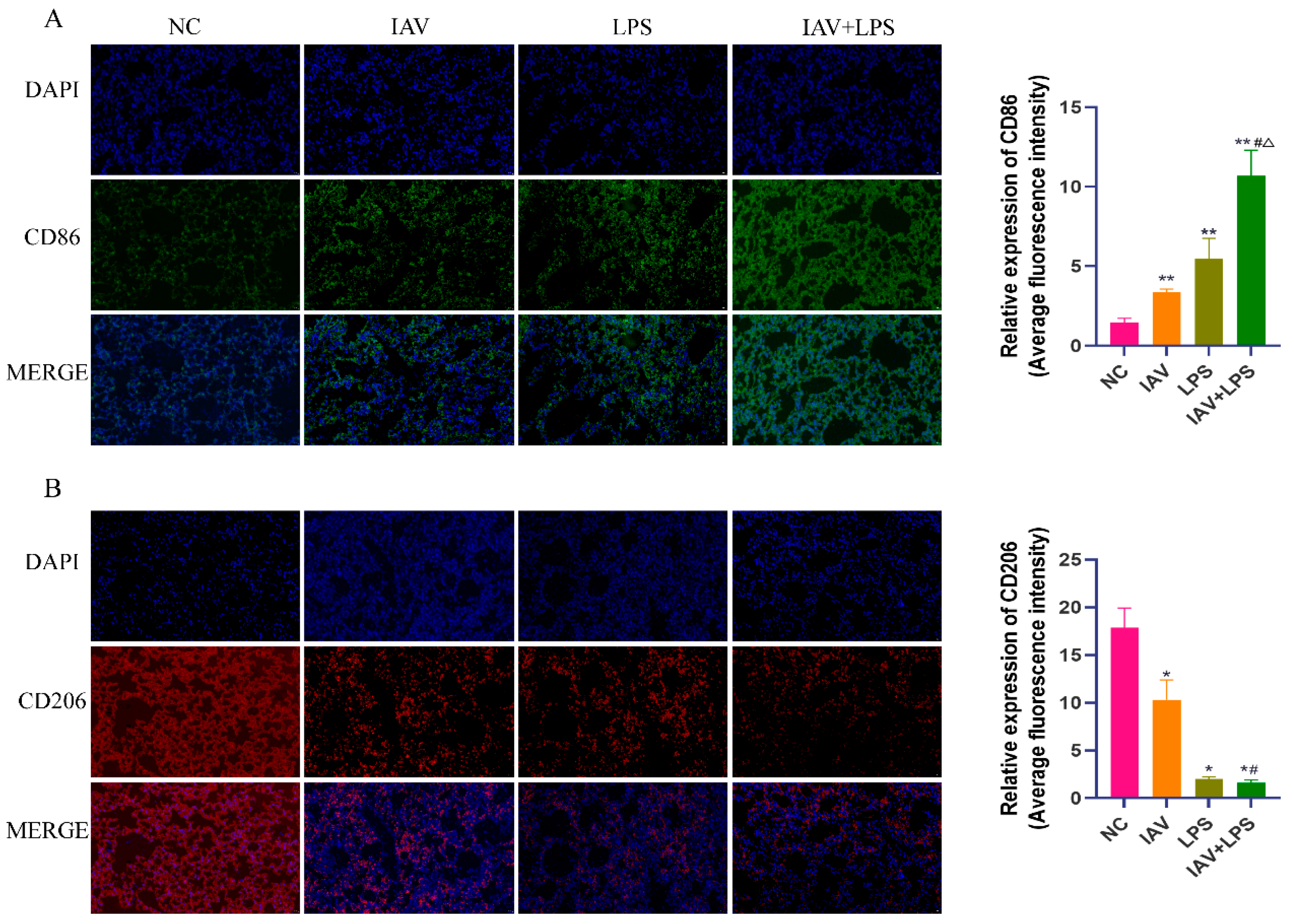
3.4. Impact of Combined IAV and LPS Stimulation on Macrophage Phenotypes in Lung Tissue
3.5. Impact of Combined IAV and LPS Stimulation on Macrophage Inflammatory Cytokine Expression
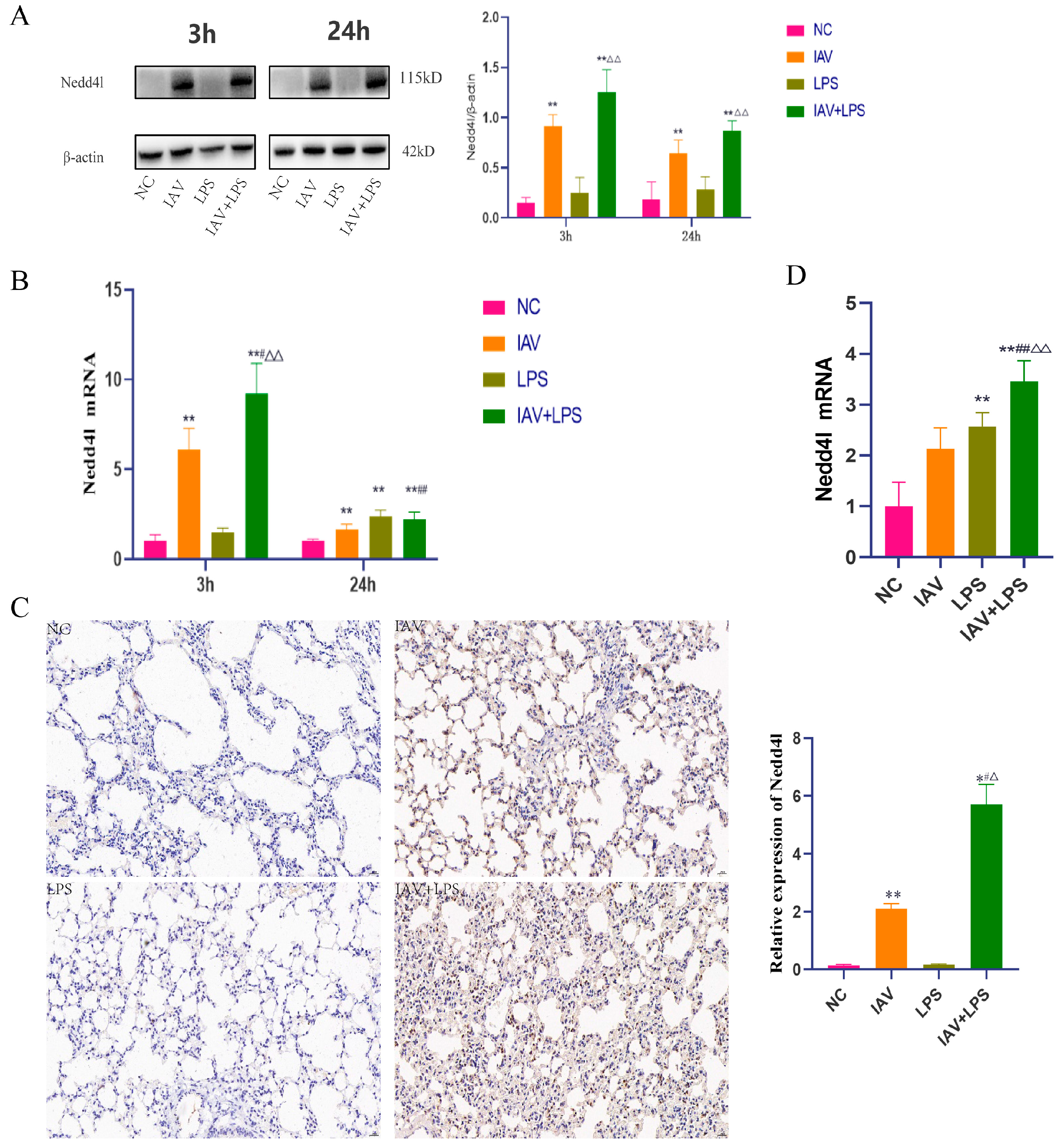
3.6. Impact of Combined IAV and LPS Stimulation on Nedd4L
3.7. Impact of Nedd4L Silencing on Cytokine Expression in Macrophages Stimulated with Combined IAV and LPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janke, B.H. Influenza A virus infections in swine: Pathogenesis and diagnosis. Vet. Pathol. 2014, 51, 410–426. [Google Scholar] [CrossRef]
- Taylor, K.Y.; Agu, I.; José, I.; Mäntynen, S.; Campbell, A.J.; Mattson, C.; Chou, T.-W.; Zhou, B.; Gresham, D.; Ghedin, E.; et al. Influenza A virus reassortment is strain dependent. PLoS Pathog. 2023, 19, e1011155. [Google Scholar] [CrossRef]
- Tao, H.; Li, L.; White, M.C.; Steel, J.; Lowen, A.C. Influenza A Virus Coinfection through Transmission Can Support High Levels of Reassortment. J. Virol. 2015, 89, 8453–8461. [Google Scholar] [CrossRef] [PubMed]
- Cawcutt, K.; Kalil, A.C. Pneumonia with bacterial and viral coinfection. Curr. Opin. Crit. Care 2017, 23, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Putker, F.; Bos, M.P.; Tommassen, J. Transport of lipopolysaccharide to the Gram-negative bacterial cell surface. FEMS Microbiol. Rev. 2015, 39, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Hunt, F. Patterns of LPS synthesis in gram negative bacteria. J. Theor. Biol. 1985, 115, 213–219. [Google Scholar] [CrossRef]
- Metzger, D.W.; Sun, K. Immune dysfunction and bacterial coinfections following influenza. J. Immunol. 2013, 191, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Barua, S.; Tripathi, B.N.; Rouse, B.T. Virological and Immunological Outcomes of Coinfections. Clin. Microbiol. Rev. 2018, 31, e00111-17. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Zeng, S.; Hu, W. Discovery of highly potent agents against influenza A virus. Eur. J. Med. Chem. 2011, 46, 52–57. [Google Scholar] [CrossRef]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef] [PubMed]
- Garbi, N.; Lambrecht, B.N. Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflugers Arch. 2017, 469, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; King, I.L.; Pernet, E. Alveolar macrophages and type I IFN in airway homeostasis and immunity. Trends Immunol. 2015, 36, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, N.; Jiang, Y.; Wang, H.; Xin, Z.; An, H.; Pan, H.; Ma, W.; Zhang, T.; Wang, X.; et al. E3 ubiquitin ligase NEDD4L negatively regulates inflammation by promoting ubiquitination of MEKK2. EMBO Rep. 2022, 23, e54603. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Ma, X.; Yuan, M.; Yi, Y.; Liu, G.; Wen, M.; Jiang, W.; Ji, R.; Zhu, L.; Tang, Z.; et al. E3 ligase Nedd4l promotes antiviral innate immunity by catalyzing K29-linked cysteine ubiquitination of TRAF3. Nat. Commun. 2021, 12, 1194. [Google Scholar] [CrossRef]
- Ishikawa, H.; Fukui, T.; Ino, S.; Sasaki, H.; Awano, N.; Kohda, C.; Tanaka, K. Influenza virus infection causes neutrophil dysfunction through reduced G-CSF production and an increased risk of secondary bacteria infection in the lung. Virology 2016, 499, 23–29. [Google Scholar] [CrossRef]
- Sumitomo, T.; Nakata, M.; Nagase, S.; Takahara, Y.; Honda-Ogawa, M.; Mori, Y.; Akamatsu, Y.; Yamaguchi, M.; Okamoto, S.; Kawabata, S. GP96 Drives Exacerbation of Secondary Bacterial Pneumonia following Influenza A Virus Infection. mBio 2021, 12, e0326920. [Google Scholar] [CrossRef]
- Robinson, K.M.; Lee, B.; Scheller, E.V.; Mandalapu, S.; I Enelow, R.; Kolls, J.K.; Alcorn, J.F. The role of IL-27 in susceptibility to post-influenza Staphylococcus aureus pneumonia. Respir. Res. 2015, 16, 10. [Google Scholar] [CrossRef]
- Bosch, A.A.; Biesbroek, G.; Trzcinski, K.; Sanders, E.A.; Bogaert, D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013, 9, e1003057. [Google Scholar] [CrossRef]
- Peltola, V.T.; McCullers, J.A. Respiratory viruses predisposing to bacterial infections: Role of neuraminidase. Pediatr. Infect. Dis. J. 2004, 23 (Suppl. S1), S87–S97. [Google Scholar] [CrossRef]
- McCullers, J.A.; Bartmess, K.C. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 2003, 187, 1000–1009. [Google Scholar] [CrossRef]
- Wu, X.; Li, R.F.; Lin, Z.S.; Xiao, C.; Liu, B.; Mai, K.-L.; Zhou, H.-X.; Zeng, D.-Y.; Cheng, S.; Weng, Y.-C.; et al. Coinfection with influenza virus and non-typeable Haemophilus influenzae aggregates inflammatory lung injury and alters gut microbiota in COPD mice. Front. Microbiol. 2023, 14, 1137369. [Google Scholar] [CrossRef]
- Haney, J.; Vijayakrishnan, S.; Streetley, J.; Dee, K.; Goldfarb, D.M.; Clarke, M.; Mullin, M.; Carter, S.D.; Bhella, D.; Murcia, P.R.; et al. Coinfection by influenza A virus and respiratory syncytial virus produces hybrid virus particles. Nat. Microbiol. 2022, 7, 1879–1890. [Google Scholar] [CrossRef]
- Jie, F.; Wu, X.; Zhang, F.; Li, J.; Liu, Z.; He, Y.; Li, C.; Zhang, H.; Lin, Y.; Zhu, X.; et al. Influenza Virus Infection Increases Host Susceptibility To Secondary Infection with Pseudomonas aeruginosa, and This Is Attributed To Neutrophil Dysfunction through Reduced Myeloperoxidase Activity. Microbiol. Spectr. 2023, 11, e0365522. [Google Scholar] [CrossRef]
- Lee, B.; Robinson, K.M.; McHugh, K.J.; Scheller, E.V.; Mandalapu, S.; Chen, C.; Di, Y.P.; Clay, M.E.; Enelow, R.I.; Dubin, P.J.; et al. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L158–L167. [Google Scholar] [CrossRef]
- Nakamura, S.; Davis, K.M.; Weiser, J.N. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J. Clin. Investig. 2011, 121, 3657–3665. [Google Scholar] [CrossRef]
- McCullers, J.A.; Rehg, J.E. Lethal synergism between influenza virus and Streptococcus pneumoniae: Characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 2002, 186, 341–350. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef]
- Chesarino, N.M.; McMichael, T.M.; Yount, J.S. E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3. PLoS Pathog. 2015, 11, e1005095. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, H.; Wang, Q.; Li, J.; Zhang, W.; Chen, T.; Li, P. NEDD4L-induced ubiquitination mediating UBE2T degradation inhibits progression of lung adenocarcinoma via PI3K-AKT signaling. Cancer Cell Int. 2021, 21, 631. [Google Scholar] [CrossRef] [PubMed]
- Aragón, E.; Goerner, N.; Zaromytidou, A.I.; Xi, Q.; Escobedo, A.; Massagué, J.; Macias, M.J. A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev. 2011, 25, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Villeret, B.; Solhonne, B.; Straube, M.; Lemaire, F.; Cazes, A.; Garcia-Verdugo, I.; Sallenave, J.-M. Influenza A Virus Pre-Infection Exacerbates Pseudomonas aeruginosa-Mediated Lung Damage Through Increased MMP-9 Expression, Decreased Elafin Production and Tissue Resilience. Front. Immunol. 2020, 11, 117. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, M.; Zhao, C.; Lu, F.; Zhang, X.; Wang, X.; He, L.; Chen, B. Role of Nedd4L in Macrophage Pro-Inflammatory Polarization Induced by Influenza A Virus and Lipopolysaccharide Stimulation. Microorganisms 2024, 12, 1291. https://doi.org/10.3390/microorganisms12071291
Peng M, Zhao C, Lu F, Zhang X, Wang X, He L, Chen B. Role of Nedd4L in Macrophage Pro-Inflammatory Polarization Induced by Influenza A Virus and Lipopolysaccharide Stimulation. Microorganisms. 2024; 12(7):1291. https://doi.org/10.3390/microorganisms12071291
Chicago/Turabian StylePeng, Meihong, Cheng Zhao, Fangguo Lu, Xianggang Zhang, Xiaoqi Wang, Li He, and Bei Chen. 2024. "Role of Nedd4L in Macrophage Pro-Inflammatory Polarization Induced by Influenza A Virus and Lipopolysaccharide Stimulation" Microorganisms 12, no. 7: 1291. https://doi.org/10.3390/microorganisms12071291





