The Marine Fish Gut Microbiome as a Source of Novel Bacteriocins
Abstract
:1. Introduction
2. The Diversity of the Marine Fish Gut Microbiome
| Fish Species | Sample | Predominant Phyla | Ref. |
|---|---|---|---|
| Gadus morhua (Atlantic Cod) | Intestinal contents | Pseudomonadota, Bacteroidota, Bacillota | [38] |
| Siganus fuscescens (Mottled spinefoot rabbitfish) | Intestinal contents | Pseudomonadota, Bacillota, Bacteroidota, Fusobacteriota, Mycoplasmatota, Cyanobacteriota | [39] |
| Various White Sea (arctic) fish | Posterior intestine | Pseudomonadota, Bacillota, Actinomycetota, Bacteroidota, Mycoplasmatota, Fusobacteriota | [40] |
| Various Mediterranean fish | Midgut | Pseudomonadota, Bacillota, Bacteroidota, Actinobacteriota, Patescibacteria, Fusobacteriota, Planctomycetota, and Dependentiae | [41] |
| Coastal fish of Hong Kong | Gastrointestinal contents | Pseudomonadota, Bacillota, Mycoplasmatota | [29] |
| Various deep-sea fish of Atlantic Ocean | Intestinal contents | Pseudomonadota, Bacteroidota, Bacillota, Actinomycetota, Ascomycota, Basidiomycota, Euryarchaeota, Spirochaetes | [30] |
| Centroscyllium fabricii (Black dogfish shark) | Gut contents | Actinomycetota, Pseudomonadota, Acidobacteriota (Acidobacteria), Bacillota, Chloroflexota | [42] |
| Benthobatis moresbyi (Dark Blind Ray) | Gut contents | Actinomycetota, Pseudomonadota, Acidobacteriota, Chloroflexota, Bacillota | [37] |
| Fish | Sample | Abundant Genera | Abundant Families | Ref. |
|---|---|---|---|---|
| Centroscyllium fabricii (Black dogfish shark) | Gut contents | Acinetobacter, Thalassobacillus, Alteromonas, Leeuwenhoekiella, Corynebacterium, Pseudonocardia, Pseudomonas | NR | [42] |
| Benthobatis moresbyi (Dark Blind Ray) | Gut contents | Acinetobacter | Moraxellaceae, Koribacteraceae, Nitrospiraceae | [37] |
| White Sea (arctic) fish | Posterior intestine | Streptococcus, Sphingomonas, Micrococcus, Chthoniobacter, Pseudomonas, Corynebacterium, Staphylococcus, Acinetobacter, Propionibacterium, Vibrio, Photobacterium, Bacillus | Moraxellaceae, Vibrionaceae, Pseudomonadaceae, Propionibacteriaceae, Corynebacteriaceae, Micrococcaceae | [40] |
| Various Mediterranean fish | Midgut | Pseudoalteromonas, Bradyrhizobium, Diaphorobacter, Mycoplasma, Clostridium, Thaumasiovibrio, Microbulbifer | Xanthobacteraceae, Comamonadaceae, Pseudoalteromonadaceae, Clostridiaceae, Vibrionaceae, Propionibacteriaceae, Staphylococcaceae, Mycoplasmataceae, Flavobacteriaceae, and Peptostreptococcaceae | [41] |
| Various Antarctic fish | Gastrointestinal contents | Rhodococcus, Thermus, Acinetobacter, Propionibacterium, Streptococcus, and Mycoplasma | NR | [32] |
| Coastal fish of Hong Kong | Gastrointestinal contents | Clostridium, Photobacterium, Ralstonia, Acinetobacter, Thermus, Ralstonia, | NR | [29] |
3. Bacteriocins from Marine Fish Gut Microbiota
3.1. Bacteriocins from LAB
3.2. Bacteriocins from Bacilli
3.3. Bacteriocins from Actinobacteria
4. Applications of Marine Fish-Derived Bacteriocins
5. Challenges, Metagenomics and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Greenaway, S.F.; Sullivan, K.D.; Umfress, S.H.; Beittel, A.B.; Wagner, K.D. Revised depth of the Challenger Deep from submersible transects; including a general method for precise, pressure-derived depths in the ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2021, 178, 103644. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration USDoC. Layers of the Ocean. Available online: https://www.noaa.gov/jetstream/ocean/layers-of-ocean (accessed on 28 March 2023).
- Somero, G.N. Biochemical ecology of deep-sea animals. Experientia 1992, 48, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Approved Marine Drugs. 2023. Available online: https://www.marinepharmacology.org/approved (accessed on 14 August 2023).
- Collaborators, A.R. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2014–2015: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis, Antiviral, and Anthelmintic Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2020, 18, 5. [Google Scholar]
- Mayer, A.M.S.; Guerrero, A.J.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine Pharmacology in 2016–2017: Marine Compounds with Antibacterial, Antidiabetic, Antifungal, Anti-Inflammatory, Antiprotozoal, Antituberculosis and Antiviral Activities; Affecting the Immune and Nervous Systems, and Other Miscellaneous Mechanisms of Action. Mar. Drugs 2021, 19, 49. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Pierce, M.L.; Howe, K.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Nakamura, F.; Fusetani, N. Marine pharmacology in 2018: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Pharmacol. Res. 2022, 183, 106391. [Google Scholar] [CrossRef]
- Halloran, K.; Underwood, M.A. Probiotic mechanisms of action. Early Hum. Dev. 2019, 135, 58–65. [Google Scholar] [CrossRef]
- Wang, L.; Ravichandran, V.; Yin, Y.; Yin, J.; Zhang, Y. Natural Products from Mammalian Gut Microbiota. Trends Biotechnol. 2019, 37, 492–504. [Google Scholar] [CrossRef]
- Wanka, K.M.; Damerau, T.; Costas, B.; Krueger, A.; Schulz, C.; Wuertz, S. Isolation and characterization of native probiotics for fish farming. BMC Microbiol. 2018, 18, 119. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Ou, W.; Yu, G.; Zhang, Y.; Mai, K. Recent progress in the understanding of the gut microbiota of marine fishes. Mar. Life Sci. Technol. 2021, 3, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Montalbán-López, M.; Scott, T.A.; Ramesh, S.; Rahman, I.R.; van Heel, A.J.; Viel, J.H.; Bandarian, V.; Dittmann, E.; Genilloud, O.; Goto, Y.; et al. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 2021, 38, 130–239. [Google Scholar] [CrossRef] [PubMed]
- Nissen-Meyer, J.; Rogne, P.; Oppegard, C.; Haugen, S.H.; Kristiansen, E.P. Structure-Function Relationships of the Non-Lanthionine-Containing Peptide (class II) Bacteriocins Produced by Gram-Positive Bacteria. Curr. Pharm. Biotechnol. 2009, 10, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.C.; Sit, C.S.; Clayton, E.; O’Connor, P.M.; Whittal, R.M.; Zheng, J.; Vederas, J.C.; Ross, R.P.; Hill, C. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc. Natl. Acad. Sci. USA 2010, 107, 9352–9357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The Gut Microbiota of Marine Fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
- Ward, N.L.; Steven, B.; Penn, K.; Methé, B.A.; Detrich, W.H. Characterization of the intestinal microbiota of two Antarctic notothenioid fish species. Extremophiles 2009, 13, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Troussellier, M.; Escalas, A.; Bouvier, T.; Mouillot, D. Sustaining Rare Marine Microorganisms: Macroorganisms As Repositories and Dispersal Agents of Microbial Diversity. Front. Microbiol. 2017, 8, 947. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Ringø, E.; Merrifield, D. The Gut Microbiota of Fish. Aquac. Nutr. Gut Health Probiotics Prebiotics 2014. [Google Scholar] [CrossRef]
- Yano, Y.; Nakayama, A.; Yoshida, K. Population Sizes and Growth Pressure Responses of Intestinal Microfloras of Deep-Sea Fish Retrieved from the Abyssal Zone. Appl. Environ. Microbiol. 1995, 61, 4480–4483. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, K.; Tabor, P.S.; Colwell, R.R. Species composition and barotolerance of gut microflora of deep-sea benthic macrofauna collected at various depths in the atlantic ocean. Appl. Environ. Microbiol. 1980, 40, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Huang, Q.; Sham, R.C.; Deng, Y.; Mao, Y.; Wang, C.; Zhang, T.; Leung, K.M.Y. Diversity of gut microbiomes in marine fishes is shaped by host-related factors. Mol. Ecol. 2020, 29, 5019–5034. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.W.J. An Investigation into Antimicrobial Production in the Lactobacillus Genus and the Fish Microbiome. Ph.D. Thesis, University College Cork, Cork, Ireland, 2019. [Google Scholar]
- Andlid, T.; Juárez, R.-V.; Gustafsson, L. Yeast colonizing the intestine of rainbow trout (Salmo gairdneri) and turbot (Scophtalmus maximus). Microb. Ecol. 1995, 30, 321–334. [Google Scholar] [CrossRef]
- Song, W.; Li, L.; Huang, H.; Jiang, K.; Zhang, F.; Chen, X.; Zhao, M.; Ma, L. The Gut Microbial Community of Antarctic Fish Detected by 16S rRNA Gene Sequence Analysis. BioMed Res. Int. 2016, 2016, 3241529. [Google Scholar] [CrossRef]
- van der Maarel, M.J.E.C.; Sprenger, W.; Haanstra, R.; Forney, L.J. Detection of methanogenic archaea in seawater particles and the digestive tract of a marine fish species. FEMS Microbiol. Lett. 1999, 173, 189–194. [Google Scholar] [CrossRef]
- Vuillemin, A.; Wankel, S.D.; Coskun, Ö.K.; Magritsch, T.; Vargas, S.; Estes, E.R.; Spivack, A.J.; Smith, D.C.; Pockalny, R.; Murray, R.W. Archaea dominate oxic subseafloor communities over multimillion-year time scales. Sci. Adv. 2019, 5, eaaw4108. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Di Giallonardo, F.; Wille, M.; Ortiz-Baez, A.S.; Costa, V.A.; Ghaly, T.; Mifsud, J.C.O.; Turnbull, O.M.H.; Bellwood, D.R.; Williamson, J.E.; et al. Virome composition in marine fish revealed by meta-transcriptomics. Virus Evol. 2021, 7, veab005. [Google Scholar] [CrossRef] [PubMed]
- Johny, T.K.; Saidumohamed, B.E.; Sasidharan, R.S.; Bhat, S.G. Inferences of gut bacterial diversity from next-generation sequencing of 16S rDNA in deep sea blind ray—Benthobatis moresbyi. Ecol. Genet. Genom. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Star, B.; Haverkamp, T.H.; Jentoft, S.; Jakobsen, K.S. Next generation sequencing shows high variation of the intestinal microbial species composition in Atlantic cod caught at a single location. BMC Microbiol. 2013, 13, 248. [Google Scholar] [CrossRef]
- Jones, J.; DiBattista, J.D.; Stat, M.; Bunce, M.; Boyce, M.C.; Fairclough, D.V.; Travers, M.J.; Huggett, M.J. The Microbiome of the Gastrointestinal Tract of a Range-Shifting Marine Herbivorous Fish. Front. Microbiol. Orig. Res. 2018, 9, 2000. [Google Scholar] [CrossRef]
- Burtseva, O.; Kublanovskaya, A.; Fedorenko, T.; Lobakova, E.; Chekanov, K. Gut microbiome of the White Sea fish revealed by 16S rRNA metabarcoding. Aquaculture 2021, 533, 736175. [Google Scholar] [CrossRef]
- Kormas, K.; Nikouli, E.; Kousteni, V.; Damalas, D. Midgut bacterial microbiota of 12 fish species from a marine protected area in the Aegean Sea (Greece). Microb. Ecol. 2023, 86, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Johny, T.K.; Saidumohamed, B.E.; Sasidharan, R.S.; Bhat, S.G. Metabarcoding data of bacterial diversity of the deep sea shark, Centroscyllium fabricii. Data Brief 2018, 21, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.W.J.; O’Connor, P.M.; O’Sullivan, O.; Rea, M.C.; Hill, C.; Ross, P.R. Formicin—A novel broad-spectrum two-component lantibiotic produced by Bacillus paralicheniformis APC 1576. Microbiology 2016, 162, 1662–1671. [Google Scholar] [CrossRef]
- Heo, W.-S.; Kim, E.-Y.; Kim, Y.-R.; Hossain, M.T.; Kong, I.-S. Salt effect of nisin Z isolated from a marine fish on the growth inhibition of Streptococcus iniae, a pathogen of streptococcosis. Biotechnol. Lett. 2012, 34, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Sequeiros, C.; Garcés, M.E.; Vallejo, M.; Marguet, E.R.; Olivera, N.L. Potential aquaculture probiont Lactococcus lactis TW34 produces nisin Z and inhibits the fish pathogen Lactococcus garvieae. Arch. Microbiol. 2015, 197, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Pilet, M.-F.; Dousset, X.; Barré, R.; Novel, G.; Desmazeaud, M.; Piard, J.-C. Evidence for Two Bacteriocins Produced by Carnobacterium piscicola and Carnobacterium divergens Isolated from Fish and Active Against Listeria monocytogenes. J. Food Prot. 1995, 58, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Bhugaloo-Vial, P.; Dousset, X.; Metivier, A.; Sorokine, O.; Anglade, P.; Boyaval, P.; Marion, D. Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium piscicola V1 that display significantly different levels of specific inhibitory activity. Appl. Environ. Microbiol. 1996, 62, 4410–4416. [Google Scholar] [CrossRef] [PubMed]
- Metivier, A.; Pilet, M.-F.; Dousset, X.; Sorokine, O.; Anglade, P.; Zagorec, M.; Piard, J.-C.; Marlon, D.; Cenatiempo, Y.; Fremaux, C. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: Primary structure and genomic organization. Microbiology 1998, 144, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Schelegueda, L.I.; Vallejo, M.; Gliemmo, M.F.; Marguet, E.R.; Campos, C.A. Synergistic antimicrobial action and potential application for fish preservation of a bacteriocin produced by Enterococcus mundtii isolated from Odontesthes platensis. LWT—Food Sci. Technol. 2015, 64, 794–801. [Google Scholar] [CrossRef]
- Shastry, R.P.; Arunrenganathan, R.R.; Rai, V.R. Characterization of probiotic Enterococcus lactis RS5 and purification of antibiofilm enterocin. Biocatal. Agric. Biotechnol. 2021, 31, 101897. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Q.; Wu, Y.; Chen, Z.; Liu, Y.; Fang, Z.; Deng, Q. Purification, characterization and structural identification of a novel bacteriocin produced by marine original Enterococcus durans YQ-6, and its inhibition of Listeria monocytogenes. LWT 2023, 173, 114329. [Google Scholar] [CrossRef]
- Bindiya, E.S.; Tina, K.J.; Raghul, S.S.; Bhat, S.G. Characterization of Deep Sea Fish Gut Bacteria with Antagonistic Potential, from Centroscyllium fabricii (Deep Sea Shark). Probiotics Antimicrob Proteins 2015, 7, 157–163. [Google Scholar] [CrossRef]
- Bindiya, E.S.; Tina, K.J.; Sasidharan, R.S.; Bhat, S.G. BaCf3: Highly thermostable bacteriocin from Bacillus amyloliquefaciens BTSS3 antagonistic on food-borne pathogens. 3 Biotech 2019, 9, 136. [Google Scholar] [CrossRef]
- Ringø, E.; Hoseinifar, S.H.; Ghosh, K.; Doan, H.V.; Beck, B.R.; Song, S.K. Lactic acid bacteria in finfish—An update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef] [PubMed]
- Duffes, F.; Leroi, F.; Boyaval, P.; Dousset, X. Inhibition of Listeria monocytogenes by Carnobacterium spp. strains in a simulated cold smoked fish system stored at 4 °C. Int. J. Food Microbiol. 1999, 47, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Mulders, J.W.; Boerrigter, I.J.; Rollema, H.S.; Siezen, R.J.; de Vos, W.M. Identification and characterization of the lantibiotic nisin, Z.; a natural nisin variant. Eur. J. Biochem. 1991, 201, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Breukink, E.; van Kraaij, C.; Demel, R.A.; Siezen, R.J.; Kuipers, O.P.; de Kruijff, B. The C-Terminal Region of Nisin Is Responsible for the Initial Interaction of Nisin with the Target Membrane. Biochemistry 1997, 36, 6968–6976. [Google Scholar] [CrossRef] [PubMed]
- Saidumohamed, B.E.; Johny, T.K.; Raveendran, A.T.; Sheela, U.B.; Sreeranganathan, M.; Sasidharan, R.S.; Bhat, S.G. 3D Structure Elucidation and Appraisal of Mode of Action of a Bacteriocin BaCf3 with Anticancer Potential Produced by Marine Bacillus amyloliquefaciens BTSS3. Re:GEN Open 2022, 2, 45–56. [Google Scholar] [CrossRef]
- Singh, P.K.; Chittpurna; Ashish; Sharma, V.; Patil, P.B.; Korpole, S. Identification, purification and characterization of laterosporulin, a novel bacteriocin produced by Brevibacillus sp. strain GI-9. PLoS ONE 2012, 7, e31498. [Google Scholar]
- An, J.; Zhu, W.; Liu, Y.; Zhang, X.; Sun, L.; Hong, Y.; Xu, C.; Xu, D.; Liu, H. Purification and characterization of a novel bacteriocin CAMT2 produced by Bacillus amyloliquefaciens isolated from marine fish Epinephelus areolatus. Food Control 2015, 51, 278–282. [Google Scholar] [CrossRef]
- Saidumohamed, B.E.; Baburaj, A.P.; Johny, T.K.; Sheela, U.B.; Sreeranganathan, M.; Bhat, S.G. A magainin-2 like bacteriocin BpSl14 with anticancer action from fish gut Bacillus safensis SDG14. Anal. Biochem. 2021, 627, 114261. [Google Scholar] [CrossRef] [PubMed]
- Emam, A.M.; Dunlap, C.A. Genomic and phenotypic characterization of Bacillus velezensis AMB-y1; a potential probiotic to control pathogens in aquaculture. Antonie Leeuwenhoek 2020, 113, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhang, Z.; Zhao, F.; Liu, H.; Yu, L.; Zha, J.; Wang, G. Probiotic potential of Bacillus velezensis JW: Antimicrobial activity against fish pathogenic bacteria and immune enhancement effects on Carassius auratus. Fish Shellfish Immunol. 2018, 78, 322–330. [Google Scholar] [CrossRef]
- Wu, J.; Xu, G.; Jin, Y.; Sun, C.; Zhou, L.; Lin, G.; Xu, R.; Wei, L.; Fei, H.; Wang, D.; et al. Isolation and characterization of Bacillus sp. GFP-2, a novel Bacillus strain with antimicrobial activities, from Whitespotted bamboo shark intestine. AMB Express 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Valliappan, K.; Sun, W.; Li, Z. Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Appl. Microbiol. Biotechnol. 2014, 98, 7365–7377. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Nam, S.-J.; Locke, J.B.; Kauffman, C.A.; Beatty, D.S.; Paul, L.A.; Fenical, W. Anthracimycin, a Potent Anthrax Antibiotic from a Marine-Derived Actinomycete. Angew. Chem. Int. Ed. 2013, 52, 7822–7824. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, A.; Ayswarya, S.; Gopikrishnan, V.; Radhakrishnan, M. Bioactive Potential of Actinobacteria Isolated from the Gut of Marine Fishes; NISCAIR-CSIR: New Delhi, India, 2019. [Google Scholar]
- Vadivel, M.; Venugopal, G.; Angamuthu, V.; Manikkam, R.; Joseph, J.; Aruni, W. (Eds.) Exploration of Fish Gut Associated Actinobacteria for its Anti-Microbial and Anti-Quorum Sensing Properties. In International Seminar on Promoting Local Resources for Sustainable Agriculture and Development (ISPLRSAD 2020); Atlantis Press: Dordrecht, The Netherlands, 2021. [Google Scholar]
- Subramani, R.; Sipkema, D. Marine Rare Actinomycetes: A Promising Source of Structurally Diverse and Unique Novel Natural Products. Mar. Drugs 2019, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.M.; Wong, W.R.; Riener, R.M.; Schulze, C.J.; Linington, R.G. Examining the Fish Microbiome: Vertebrate-Derived Bacteria as an Environmental Niche for the Discovery of Unique Marine Natural Products. PLoS ONE 2012, 7, e35398. [Google Scholar] [CrossRef] [PubMed]
- Desriac, F.; Defer, D.; Bourgougnon, N.; Brillet, B.; Le Chevalier, P.; Fleury, Y. Bacteriocin as Weapons in the Marine Animal-Associated Bacteria Warfare: Inventory and Potential Applications as an Aquaculture Probiotic. Mar. Drugs 2010, 8, 1153–1177. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.K.; Jena, P.K.; Patel, A.K.; Seshadri, S. Bacteriocins and their applications for the treatment of bacterial diseases in aquaculture: A review. Aquac. Res. 2016, 47, 1013–1027. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in Action; Report No.: 978-92-5-132692-3 Contract No.: 63; FAO: Rome, Italy, 2020. [Google Scholar]
- Nguyen, T.L.; Park, C.-I.; Kim, D.-H. Improved growth rate and disease resistance in olive flounder, Paralichthys olivaceus, by probiotic Lactococcus lactis WFLU12 isolated from wild marine fish. Aquaculture 2017, 471, 113–120. [Google Scholar] [CrossRef]
- Deming, J.W.; Baross, J.A. Survival, Dormancy, and Nonculturable Cells in Extreme Deep-Sea Environments. In Nonculturable Microorganisms in the Environment; Colwell, R.R., Grimes, D.J., Eds.; Springer: Boston, MA, USA, 2000; pp. 147–197. [Google Scholar]
- Choi Eun, J.; Nam, S.-J.; Paul, L.; Beatty, D.; Kauffman Christopher, A.; Jensen, P.R.; Fenical, W. Previously Uncultured Marine Bacteria Linked to Novel Alkaloid Production. Chem. Biol. 2015, 22, 1270–1279. [Google Scholar] [CrossRef]
- López, R.; Monteón, V.; Chan, E.; Montejo, R.; Chan, M. Oxygen limitation favors the production of protein with antimicrobial activity in Pseudoalteromonas sp. Braz. J. Microbiol. [Publ. Braz. Soc. Microbiol.] 2012, 43, 1206–1212. [Google Scholar] [CrossRef]
- Nakayama, A.; Yano, Y.; Yoshida, K. New Method for Isolating Barophiles from Intestinal Contents of Deep-Sea Fishes Retrieved from the Abyssal Zone. Appl. Environ. Microbiol. 1994, 60, 4210–4212. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xiao, X.; Wang, P.; Wang, F.P. Screening and characterization of psychrotrophic, lipolytic bacteria from deep-sea sediments. J. Microbiol. Biotechnol. 2004, 14, 952–958. [Google Scholar]
- Nichols, D.; Lewis, K.; Orjala, J.; Mo, S.; Ortenberg, R.; O’Connor, P.; Zhao, C.; Vouros, P.; Kaeberlein, T.; Epstein, S.S. Short Peptide Induces an “Uncultivable” Microorganism To Grow In Vitro. Appl. Environ. Microbiol. 2008, 74, 4889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Zeng, Z.; Xu, M.; Sun, F.; Yang, L.; Bi, X.; Lin, Y.; Gao, Y.; Hao, H.; et al. Advances in Metagenomics and Its Application in Environmental Microorganisms. Front. Microbiol. Rev. 2021, 12, 766364. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.; van Hijum, S.A.F.T.; Bijlsma, J.J.E.; Kok, J.; Kuipers, O.P. BAGEL: A web-based bacteriocin genome mining tool. Nucleic Acids Res. 2006, 34 (Suppl. S2), W273–W279. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.A.; Crossman, L.; Almeida, E.L.; Margassery, L.M.; Kennedy, J.; Dobson, A.D.W. Diverse and Abundant Secondary Metabolism Biosynthetic Gene Clusters in the Genomes of Marine Sponge Derived Streptomyces spp. Isolates. Mar. Drugs 2018, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, S.; Tang, Y.; Wang, H.; Bai, X.; Zhang, H. Genomic and AntiSMASH Analyses of Marine-Sponge-Derived Strain Aspergillus niger L14 Unveiling Its Vast Potential of Secondary Metabolites Biosynthesis. J. Fungi 2022, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Bech, P.K.; Lysdal, K.L.; Gram, L.; Bentzon-Tilla, M.; Strube, M.L. Marine Sediments Hold an Untapped Potential for Novel Taxonomic and Bioactive Bacterial Diversity. mSystems 2020, 5, e00782-20. [Google Scholar] [CrossRef]
- Xiao, Y.; Yan, F.; Cui, Y.; Du, J.; Hu, G.; Zhai, W.; Liu, R.; Zhang, Z.; Fang, J.; Chen, L.; et al. A symbiotic bacterium of Antarctic fish reveals environmental adaptability mechanisms and biosynthetic potential towards antibacterial and cytotoxic activities. Front. Microbiol. Orig. Res. 2023, 13, 1085063. [Google Scholar] [CrossRef]
- Tietz, J.I.; Schwalen, C.J.; Patel, P.S.; Maxson, T.; Blair, P.M.; Tai, H.-C.; Zakai, U.I.; Mitchell, D.A. A new genome-mining tool redefines the lasso peptide biosynthetic landscape. Nat. Chem. Biol. 2017, 13, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Merwin, N.J.; Mousa, W.K.; Dejong, C.A.; Skinnider, M.A.; Cannon, M.J.; Li, H.; Dial, K.; Gunabalasingam, M.; Johnston, C.; Magarvey, N.A. DeepRiPP integrates multiomics data to automate discovery of novel ribosomally synthesized natural products. Proc. Natl. Acad. Sci. USA 2020, 117, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; He, B.; Li, J.; Li, Y.-X. Challenges and advances in genome mining of ribosomally synthesized and post-translationally modified peptides (RiPPs). Synth. Syst. Biotechnol. 2020, 5, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.H.; Truman, A.W. Genome mining strategies for ribosomally synthesised and post-translationally modified peptides. Comput. Struct. Biotechnol. J. 2020, 18, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Johny, T.K.; Puthusseri, R.M.; Bhat, S.G. Metagenomic landscape of taxonomy, metabolic potential and resistome of Sardinella longiceps gut microbiome. Arch. Microbiol. 2021, 204, 87. [Google Scholar] [CrossRef]
- Collins, F.W.J.; Walsh, C.J.; Gomez-Sala, B.; Guijarro-García, E.; Stokes, D.; Jakobsdóttir, K.B.; Kristjánsson, K.; Burns, F.; Cotter, P.D.; Rea, M.C.; et al. The microbiome of deep-sea fish reveals new microbial species and a sparsity of antibiotic resistance genes. Gut Microbes 2021, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.J.; Lee, K.C.; Handley, K.M.; Angert, E.R.; White, W.L.; Clements, K.D. Substrate degradation pathways, conserved functions and community composition of the hindgut microbiota in the herbivorous marine fish Kyphosus sydneyanus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 272, 111283. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Liang, L.; Wang, Z.; Ai, P.; You, X.; Bian, C.; Shi, Q.; Dong, B. A Comparative Metagenomics Study on Gastrointestinal Microbiota in Amphibious Mudskippers and Other Vertebrate Animals. Animals 2019, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef]
- Collins, F.W.J.; Mesa-Pereira, B.; O’Connor, P.M.; Rea, M.C.; Hill, C.; Ross, P.R. Reincarnation of Bacteriocins from the Lactobacillus Pangenomic Graveyard. Front. Microbiol. 2018, 9, 1298. [Google Scholar] [CrossRef]

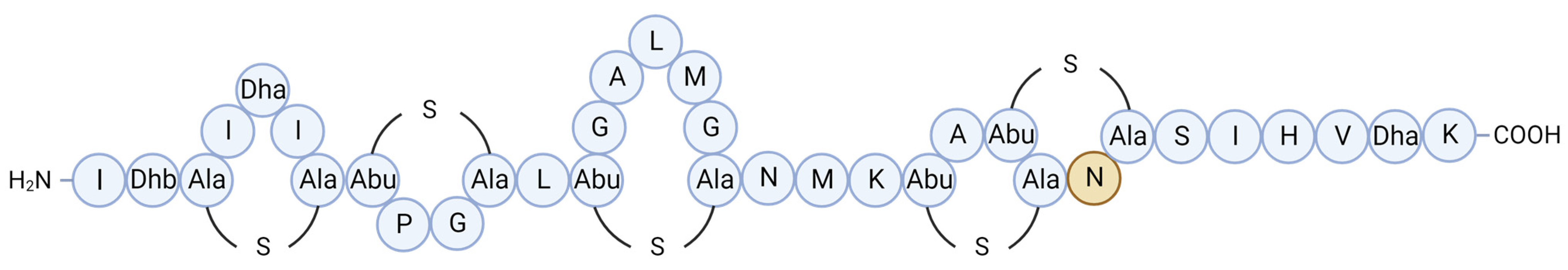
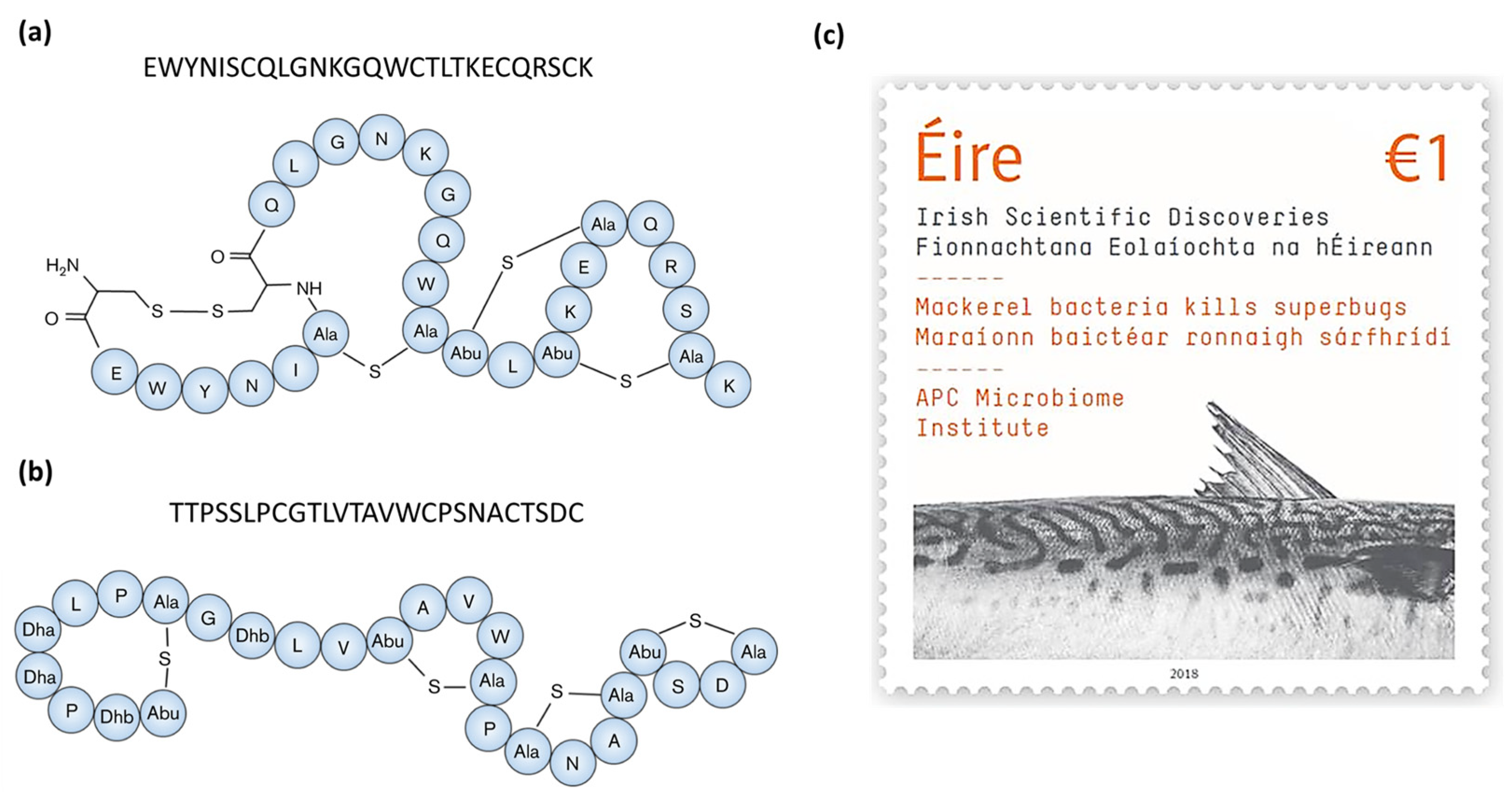

| Molecule | Producer | Host, Source | Susceptible Organism(s) | Ref. |
|---|---|---|---|---|
| Class I bacteriocins | ||||
| Formicin | Bacillus paralicheniformis APC 1576 | Scomber scombrus, intestine | Clostridia spp., Bacillus spp., Listeria spp., Enterococcus spp., Streptococcus mutans, M. luteus | [43] |
| Nisin Z | Lactococcus lactis subsp. lactis | Paralichthys olivaceus, intestine | Streptococcus iniae | [44] |
| Nisin Z | Lactococcus lactis TW34 | Odontesthes platensis, intestine | Lactococcus garvieae | [45] |
| Class IIa bacteriocins | ||||
| Piscicocins Vla, Vlb | Carnobacterium piscola V1 | salmon/trout, intestine | Listeria spp. | [46,47] |
| Divercin V41 | Carnobacterium divergens V41 | salmon or trout, intestine | Carnobacterium piscicola, Listeria spp. | [46,48] |
| Mundticin KS | Enterococcus mundtii Tw56 | Odontesthes platensis, intestine | Enterococcus spp., Listeria spp., M. luteus, Pseudomonas aeruginosa, Shewanella putrefaciens | [49] |
| Enterocin R5 | Enterococcus lactis RS5 | Sillago indica, gut | E. coli, S. enterica Typhimurium, S. aureus, P. aeruginosa. B. subtilis, B. cereus, Proteus vulgaris | [50] |
| Class IId bacteriocins | ||||
| CAMT6 | Enterococcus durans YQ-6 | Larimichthys polyactis, NR | S. aureus, Bacillus spp., S. haemolyticus, P. acnes, Salmonella paratyphi, V. parahaemolyticus, P. foulis, E. aerogenes, Fusarium sylvaticum, Aspergillus fumigatus | [51] |
| Other AMPs /bacteriocin-like inhibitory substances | ||||
| BaCf3 | Bacillus amyloliquefaciens BTSS3 | Centroscyllium fabricii, intestine | Bacillus spp., Clostridium perfringens, Salmonella Typhimurium, Proteus vulgaris | [52,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uniacke-Lowe, S.; Stanton, C.; Hill, C.; Ross, R.P. The Marine Fish Gut Microbiome as a Source of Novel Bacteriocins. Microorganisms 2024, 12, 1346. https://doi.org/10.3390/microorganisms12071346
Uniacke-Lowe S, Stanton C, Hill C, Ross RP. The Marine Fish Gut Microbiome as a Source of Novel Bacteriocins. Microorganisms. 2024; 12(7):1346. https://doi.org/10.3390/microorganisms12071346
Chicago/Turabian StyleUniacke-Lowe, Shona, Catherine Stanton, Colin Hill, and R. Paul Ross. 2024. "The Marine Fish Gut Microbiome as a Source of Novel Bacteriocins" Microorganisms 12, no. 7: 1346. https://doi.org/10.3390/microorganisms12071346
APA StyleUniacke-Lowe, S., Stanton, C., Hill, C., & Ross, R. P. (2024). The Marine Fish Gut Microbiome as a Source of Novel Bacteriocins. Microorganisms, 12(7), 1346. https://doi.org/10.3390/microorganisms12071346






