Exploring the Agricultural Applications of Microbial Melanin
Abstract
:1. Introduction
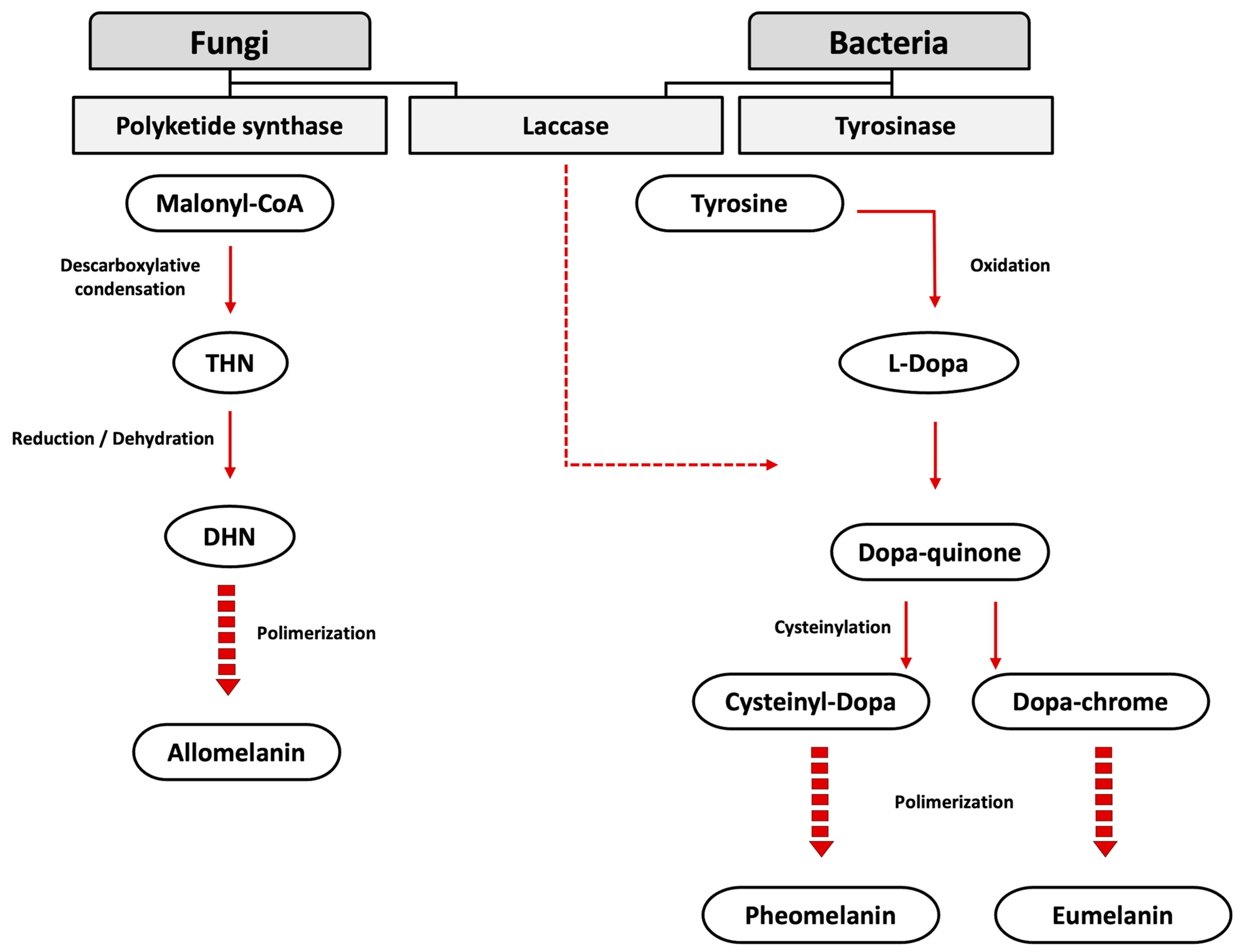
2. Microbial Biosynthesis of Melanin
2.1. Enzymes Involved in the Microbial Biosynthesis of Melanin
2.1.1. Polyketide Synthase
2.1.2. Tyrosinase
2.1.3. Laccase
3. Agricultural Applications of Melanin
3.1. Biological Control of Phytopathogenic Fungi and Bacteria
3.2. Biopesticide
3.3. Plant Growth Promotion
3.4. Bioremediation of Soils
4. Disadvantages and Limitations of Using Melanin in Agriculture
5. Prospects of Melanin in Agriculture
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Three Key Challenges Facing Agriculture and How to Start Solving Them. Available online: https://www.oecd.org/agriculture/key-challenges-agriculture-how-solve/ (accessed on 10 April 2024).
- Rayas-González, J.; Hernández.Abreu, E.; Valencia-Cantero, E.; López-Bucio, J. Microbial resources for improved crop productivity. In The Handbook of Microbial Bioresources, 1st ed.; Gupta, V., Sharma, G., Tuohy, M., Gaur, R., Eds.; CABI: Wallingford, UK, 2016; pp. 1–13. [Google Scholar]
- Muñoz-Torres, P.; Márquez, S.L.; Sepúlveda-Chavera, G.; Cárdenas-Ninasivincha, S.; Arismendi-Macuer, M.; Huanca-Mamani, W.; Aguilar, Y.; Quezada, A.; Bugueño, F. Isolation and Identification of Bacteria from Three Geothermal Sites of the Atacama Desert and Their Plant-Beneficial Characteristics. Microorganisms 2023, 11, 2635. [Google Scholar] [CrossRef] [PubMed]
- Tran-Ly, A.; Reyes, C.; Schwarze, F.; Ribera, J. Microbial production of melanin and its various application. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef] [PubMed]
- Pralea, I.; Moldovan, R.; Petrache, M.; Ilies, M.; Hedhes, S.; Ielciu, I.; Moldovan, M.; Ene, M.; Radu, M.; Uifălean, A.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin pigments and much more-types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Eisenman, H.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent advances and progress on melanin: From source to application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef] [PubMed]
- Choi, K. Bioprocess of microbial melanin production and isolation. Front. Bioeng. Biotechnol. 2021, 9, 765110. [Google Scholar] [CrossRef] [PubMed]
- Dadachova, E.; Casadevall, A. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008, 11, 525–531. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Melanoma Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Nosanchuk, J.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Nimse, S.; Elze Mathew, D.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.; Kumar, P.; Shinde, P. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef] [PubMed]
- Meraj, F. Microbial Melanin: Recent Developments and Challenges in the Production, Extraction, Purification and Application of Microbial Melanin. Annu. Res. Rev. Biol. 2023, 38, 24–35. [Google Scholar]
- El-Naggar, N.; Saber, W. Natural melanin: Current trends, and future approaches, with especial reference to microbial source. Polymers 2022, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Martínez, A.; Gosset, G. Production of melanins with recombinant microorganisms. Front. Bioeng. Biotechnol. 2019, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Plonka, P.; Grabacka, M. Melanin synthesis in microorganisms–biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Noel, J. Structure–Function Analyses of Plant Type III Polyketide Synthases. Methods Enzymol. 2012, 515, 317–335. [Google Scholar] [PubMed]
- Yu, F.; Chiu, C.; Lee, Y.; Lee, S.; Chou, C.; You, B.; Hsieh, D.; Lee, M.; Lee, M.; Bostock, R. Polyketide synthase gene expression in relation to chloromonilicin and melanin production in Monilinia fructicola. Phytopathology 2020, 110, 1465–1475. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Zhang, D.D.; Geng, Q.; Li, J.; Sheng, R.; Xue, H.; Zhu, H.; Kong, Z.; Dai, X.; et al. A polyketide synthase from Verticillium dahliae modulates melanin biosynthesis and hyphal growth to promote virulence. BMC Biol. 2022, 20, 125. [Google Scholar] [CrossRef]
- Woo, P.; Tam, E.; Chong, K.; Cai, J.; Tung, E.; Ngan, A.; Lau, S.; Yuen, K. High diversity of polyketide synthase genes and the melanin biosynthesis gene cluster in Penicillium marneffei. FEBS J. 2010, 277, 3750–3758. [Google Scholar] [CrossRef]
- Faccio, G.; Kruus, K.; Saloheimo, M.; Thony-Meyer, L. Bacterial tyrosinases and their application. Process Biochem. 2012, 47, 1749–1760. [Google Scholar] [CrossRef]
- Zaidi, K.; Ali, A.; Ali, S.; Naaz, I. Microbial tyrosinases: Promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014, 2014, 854687. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, C. Extracellular and intracellular polyphenol oxidases cause opposite effects on sensitivity of Streptomyces to phenolics: A case of double-edged sword. PLoS ONE 2009, 4, e7462. [Google Scholar] [CrossRef] [PubMed]
- Pavan, M.E.; López, N.I.; Pettinari, M.J. Melanin biosynthesis in bacteria, regulation and production perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Hong, M.; Choi, S.; Kim, Y.; Cho, S. Purification and characterization of a highly stable tyrosinase from Thermomicrobium roseum. Biotechnol. Appl. Biochem. 2000, 31, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Blanco, J.; Fernández-López, M.; Olivares, J. Melanin production by Rhizobium meliloti GR4 is linked to nonsymbiotic plasmid pRmeGR4b: Cloning, sequencing, and expression of the tyrosinase gene mepA. J. Bacteriol. 1993, 175, 5403–5410. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, T.; Huang, Y.; Ou, J.; Shen, P. A heat inducible tyrosinase with distinct properties from Bacillus thuringiensis. Lett. Appl. Microbiol. 2004, 39, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Shuster, V.; Fishman, A. Isolation, cloning and characterization of a tyrosinase with improved activity in organic solvents from Bacillus megaterium. J. Mol. Microbiol. Biotechnol. 2009, 17, 188–200. [Google Scholar] [PubMed]
- Gamal Shalaby, A.; Ragab, T.; Helal, M.; Esawy, M. Optimization of Bacillus licheniformis MAL tyrosinase: In vitro anticancer activity for brown and black eumelanin. Heliyon 2019, 5, e01657. [Google Scholar] [CrossRef]
- Goudenège, D.; Labreuche, Y.; Krin, E.; Ansquer, D.; Mangenot, S.; Calteau, A.; Médigue, C.; Mazel, D.; Polz, M.; Le Roux, F. Comparative genomics of pathogenic lineages of Vibrio nigripulchritudo identifies virulence-associated traits. ISME J. 2013, 7, 1985–1996. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, K.; Streibek, M.; Jahn, B.; Haase, G.; Brakhage, A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sowinski, S.; Martinez-Drets, G.; Okon, T. Laccase activity in melanin-producing strains of Sinorhizobium meliloti. FEMS Microbiol. Lett. 2002, 209, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hullo, M.; Moszer, I.; Danchin, A.; Martin-Verstraete, I. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 2001, 183, 5426–5430. [Google Scholar] [CrossRef] [PubMed]
- Drewnowska, J.; Zambrzycka, M.; Kalska-Szostko, B.; Fiedoruk, K.; Swiecicka, I. Melanin-like pigment synthesis by soil Bacillus weihenstephanensis isolates from northeastern Poland. PLoS ONE 2015, 10, e0125428. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.; Doyle, E.; Brooks, S.; O’Connor, K. Biochemical characterization of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6. Enzym. Microb. Technol. 2007, 40, 1435–1441. [Google Scholar] [CrossRef]
- Sanchez-Amat, A.; Solano, F. A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochem. Biophys. Res. Commun. 1997, 240, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Dalfard, A.; Khajeh, K.; Soudi, M.; Naderi-Manesh, H.; Ranjbar, B.; Sajedi, R. Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium. Enzym. Microb. Technol. 2006, 39, 1409–1416. [Google Scholar] [CrossRef]
- Lü, Z.; Kang, X.; Xiang, Z.; He, N. Laccase gene Sh-lac is involved in the growth and melanin biosynthesis of Scleromitrula shiraiana. Phytopathology 2017, 107, 353–361. [Google Scholar] [CrossRef]
- Eisenman, H.; Mues, M.; Weber, S.; Frases, S.; Chaskes, S.; Gerfen, G.; Casadevall, A. Cryptococcus neoformans laccase catalyzes melanin synthesis from D-and L-DOPA. Microbiology 2007, 153, 3954–3962. [Google Scholar] [CrossRef]
- Nagai, M.; Kawata, M.; Watanabe, H.; Ogawa, M.; Saito, K.; Takesawa, T.; Kanda, K.; Sato, T. Important role of fungal intracellular laccase for melanin synthesis: Purification and characterization of an intracellular laccase from Lentinula edodes fruit bodies. Microbiology 2003, 149, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Sapmak, A.; Boyce, K.; Andrianopoulos, A.; Vanittanakom, N. The pbrB gene encodes a laccase required for DHN-melanin synthesis in conidia of Talaromyces (Penicillium) marneffei. PLoS ONE 2015, 10, e0122728. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.; Abdelrazak, A.; Elsayed, A.; El-Shishtawy, H.; Osman, Y. Optimizing production of a biopesticide protectant by black yeast. Egypt. J. Biol. Pest Control 2018, 28, 72. [Google Scholar] [CrossRef]
- Styczynski, M.; Rogowska, A.; Nyabayo, C.; Decewicz, P.; Romaniuk, F.; Paczkowski, C.; Szakiel, A.; Suessmuth, R.; Dziewit, L. Heterologous production and characterization of pyomelanin of antartic Pseudomonas sp. ANT_H4: A metabolite protecting against UV and free radicals, interacting with iron from minerals and exhibiting priming properties toward plant hairy roots. Microb. Cell Fact. 2022, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Zerrad, A.; Anissi, J.; Ghanam, J.; Sendide, K.; El Hassouni, M. Antioxidant and antimicrobial activities of melanin produced by a Pseudomonas balearica strain. J. Biotechnol. Lettt. 2014, 5, 87–94. [Google Scholar]
- Sosa, M.F.; Sobrero, P.; Valverde, C.; Agaras, B. A black-pigmented pseudomonad isolate with antibacterial activity against phyllospheric pathogens. Rhizosphere 2020, 15, 100207. [Google Scholar] [CrossRef]
- Gurikar, C.; Naik, M.K.; Sreenivasa, M.Y. Azotobacter: PGPR activities with special reference to effect of pesticides and biodegradation. In Microbial Inoculants in Sustainable Agricultural Productivity, 1st ed.; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; Volume 1, pp. 229–244. [Google Scholar]
- Xu, C.; Li, J.; Yang, L.; Shi, F.; Yang, L.; Ye, M. Antibacterial activity and membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control 2017, 73, 1445–1451. [Google Scholar] [CrossRef]
- Wan, X.; Liu, H.M.; Liao, Y.; Su, Y.; Geng, J.; Yang, M.Y.; Chen, X.D.; Shen, P. Isolation of a novel strain of Aeromonas media producing high levels of DOPA-melanin and assessment of the photoprotective role of the melanin in bioinsecticide applications. J. Appl. Microbiol. 2007, 103, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Yu, Z.; Fang, B.; He, W.; Wang, Y.; Shen, P. Melanin pigment formation and increased UV resistance in Bacillus thuringiensis following high temperature induction. Syst. Appl. Microbiol. 2004, 27, 286–289. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, M.; Ji, D.; Wu, I.; Chou, C.; Chen, C. Protection from ultraviolet irradiation by melanin of mosquitocidal activity of Bacillus thuringiensis var. israelensis. J. Invertebr. Pathol. 1993, 62, 131–136. [Google Scholar] [CrossRef]
- Garude, N.; Jaybhaye, P.; Budukale, R. Isolation, characterization and screening of microbial melanin for its role in protection of plant growth promoting bacteria. J. Adv. Zool. 2023, 44, 188–193. [Google Scholar] [CrossRef]
- Rassabina, A.; Khabibrakhmanova, V.; Babaev, V.; Daminova, A.; Minibayeva, F. Melanins from the lichens Lobaria pulmonaria and Lobaria retigera as eco-friendly adsorbents of synthetic dyes. Int. J. Mol. Sci. 2022, 23, 15605. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Recio, P.; Rodríguez-Ruiz, G.; Barja, I.; Gutiérrez, E.; García, L. Relationships between soil pollution by heavy metals and melanin-dependent coloration of a fossorial amphisbaenian reptile. Integr. Zool. 2022, 17, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Claus, H. Copper—Containing oxidases: Occurrence in soil microorganisms, properties, and applications. In Soil Heavy Metals. Soil Biology, 1st ed.; Sherameti, I., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 19, pp. 281–313. [Google Scholar]
- Singh, L. Biodegradation of synthetic dyes: A mycoremediation approach for degradation/decolourization of textile dyes and effluents. J. Appl. Biotechnol. Bioeng. 2017, 3, 430–435. [Google Scholar] [CrossRef]
- Castillo, J.M.; Casas, J.; Romero, E. Isolation of an endosulfan–degrading bacterium from a coffee farm soil: Persistence and inhibitory effect on its biological function. Sci. Total Environ, 2011; 412–413, 20–27. [Google Scholar]
- Gutiérrez-Bustos, D.; Hernández-Marín, A.; Corrales-Ramírez, L. Pseudomonas oryzihabitans: A microorganism of growing scientific interest. Nova 2009, 7, 11. [Google Scholar] [CrossRef]
- Turick, C.E.; Knox, A.S.; Leverette, C.L.; Kritzas, Y.G. In situ uranium stabilization by microbial metabolites. J. Environ. Radioact. 2008, 99, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Manirethan, V.; Raval, K.; Balakrishnan, M. Adsorptive removal of trivalent and pentavalent arsenic from aqueous solutions using iron and copper impregnated melanin extracted from the marine bacterium Pseudomonas stutzeri. Environ. Pollut. 2020, 257, 113576. [Google Scholar] [CrossRef] [PubMed]
- Manirethan, V.; Balakrishnan, M. Batch and continuous studies on the removal of heavy metals using biosynthesized melanin impregnated activated carbon. Environ. Technol. Innov. 2020, 20, 101085. [Google Scholar] [CrossRef]
- Nosanchuk, J.; Casadevall, A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006, 50, 3519–3528. [Google Scholar] [CrossRef]
- Kozlov, M.; Zvyagintsev, D.; Zvyagintsev, D. Effects of Melanin on Soil Microbial Communities. Eurasian Soil Sci. 2010, 43, 1370–1376. [Google Scholar]
- Zhao, G.; Kan, J.; Li, Y.; Zeng, C. Advances in microbial melanin production processes and its applications in agricultural and environmental engineering. J. Agric. Food Chem. 2017, 65, 6801–6812. [Google Scholar]
- Schrama, M.; Huijbregts, M. Toward more robust life cycle assessment of agricultural systems. Science 2015, 350, 526–527. [Google Scholar]
- Bell, A.; Wheeler, M.; Liu, J. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Kong, Q.; Yuan, J.; Gao, L.; Zhao, L.; Jiang, W. Effects of agricultural practices on environment and quality of agricultural products. Sci. China Earth Sci. 2014, 57, 1754–1764. [Google Scholar]
- Jacobson, E. Pathogenic roles for fungal melanins. Clin. Microbiol. Rev. 2000, 13, 708–717. [Google Scholar] [CrossRef]
- Zafari, D.; Ghobadi Nejad, S.; Ebrahimzadeh, M.; Ramezanian, A. Melanin in agriculture and food industry. Int. J. Nutr. Sci. 2017, 2, 101–106. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Torres, P.; Cárdenas-Ninasivincha, S.; Aguilar, Y. Exploring the Agricultural Applications of Microbial Melanin. Microorganisms 2024, 12, 1352. https://doi.org/10.3390/microorganisms12071352
Muñoz-Torres P, Cárdenas-Ninasivincha S, Aguilar Y. Exploring the Agricultural Applications of Microbial Melanin. Microorganisms. 2024; 12(7):1352. https://doi.org/10.3390/microorganisms12071352
Chicago/Turabian StyleMuñoz-Torres, Patricio, Steffany Cárdenas-Ninasivincha, and Yola Aguilar. 2024. "Exploring the Agricultural Applications of Microbial Melanin" Microorganisms 12, no. 7: 1352. https://doi.org/10.3390/microorganisms12071352



