Effects of PEF on Cell and Transcriptomic of Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Bacterial Strains
2.2. Instruments and Equipment
2.3. Methods
2.3.1. Bacterial Suspension Preparation
2.3.2. Evaluation of the Effect of PEF on the Inactivation of E. coli
2.3.3. Field Emission Scanning Electron Microscopy and Transmission Electron Microscope
2.3.4. Leakage of Intracellular Material
2.3.5. Enzyme Activity Analysis
2.3.6. Transcriptomic Analysis
2.3.7. Quantitative Reverse Transcription PCR (RT-qPCR) Analysis
2.3.8. Statistical Analysis
3. Results and Discussion
3.1. Inactivation of E. coli by Pulsed Electric Field
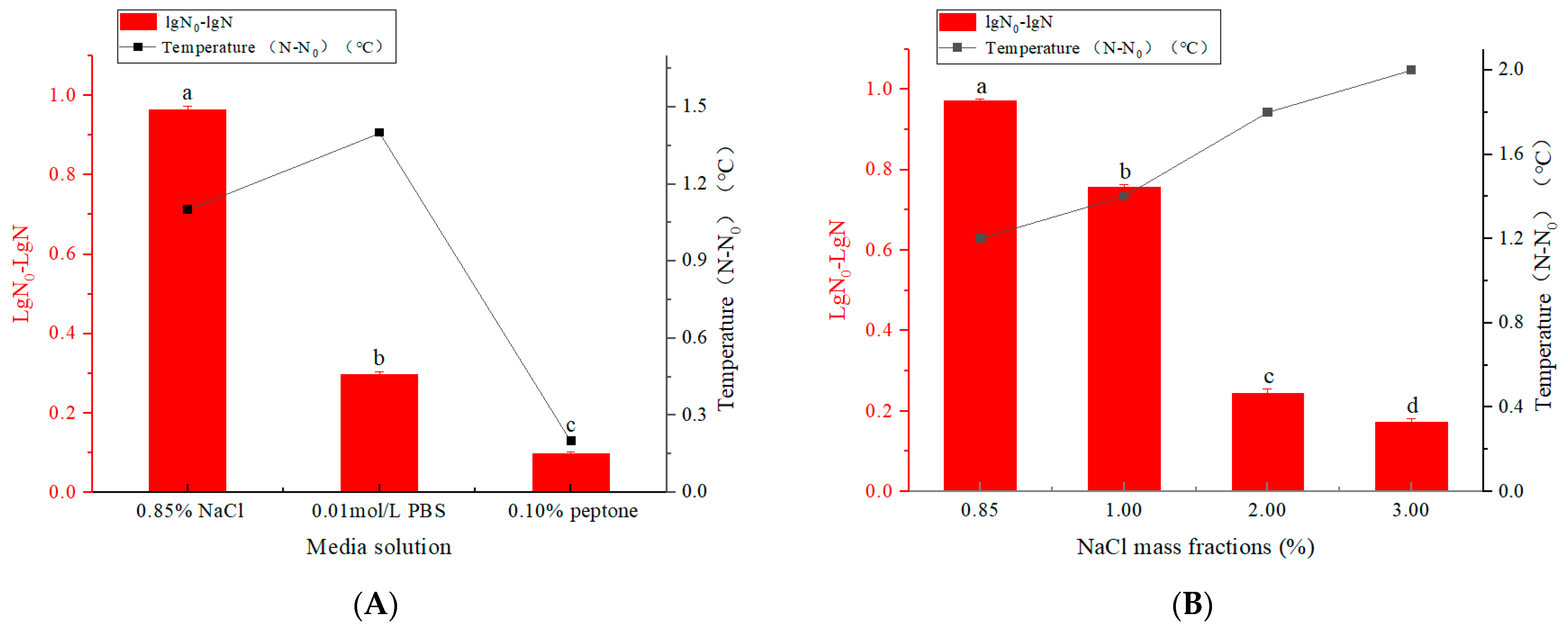
3.1.1. Inactivation of E. coli in Different Media Solutions by Pulsed Electric Field
3.1.2. Inactivation of E. coli in Different NaCl Mass Fractions by Pulsed Electric Field
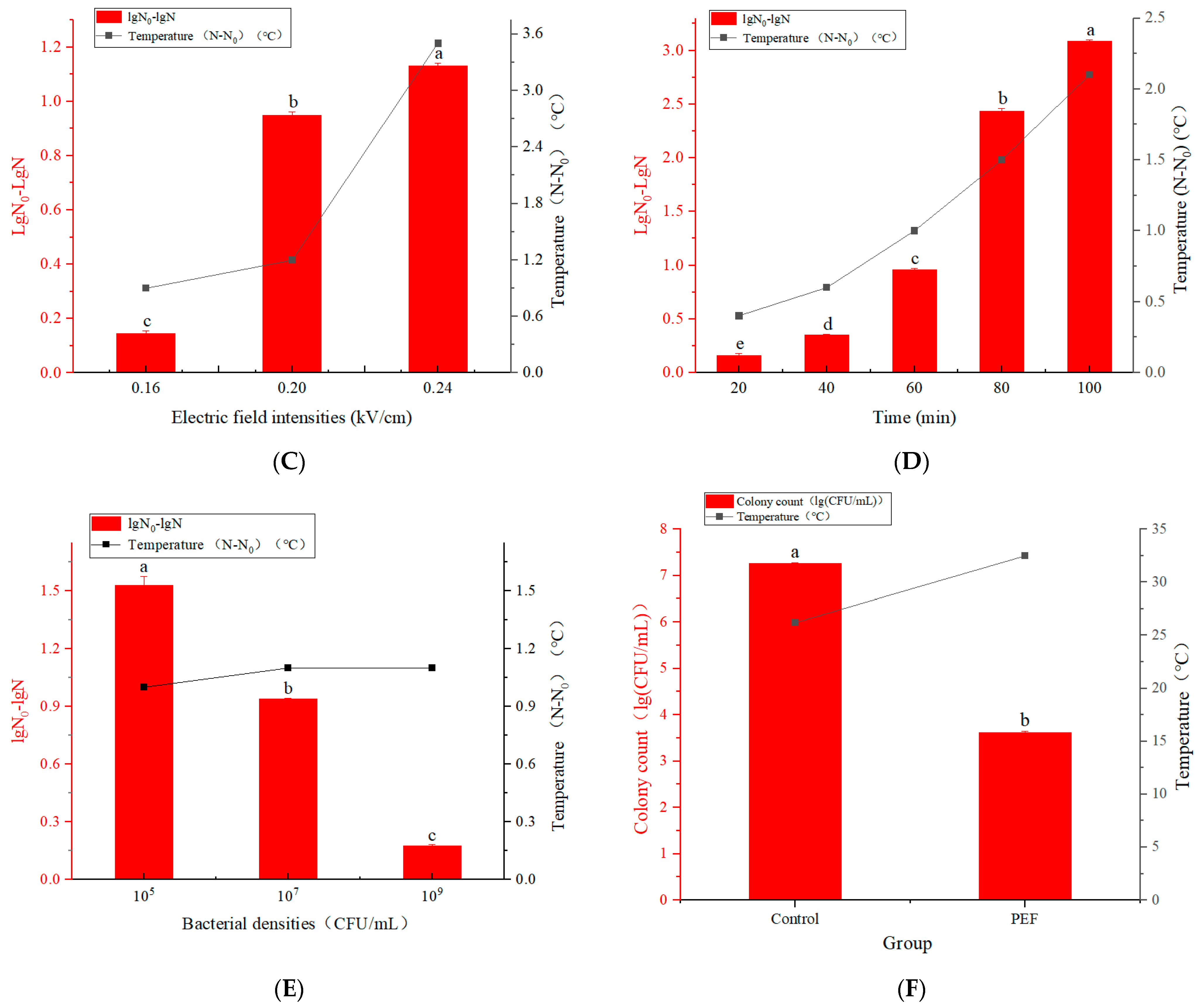
3.1.3. Inactivation of E. coli in Different Electric Field Intensities by Pulsed Electric Field
3.1.4. Inactivation of E. coli in Different Treatment Times by Pulsed Electric Field
3.1.5. Inactivation of E. coli in Different Bacterial Density by Pulsed Electric Field
3.1.6. Inactivation of E. coli by Pulsed Electric Field Treatment
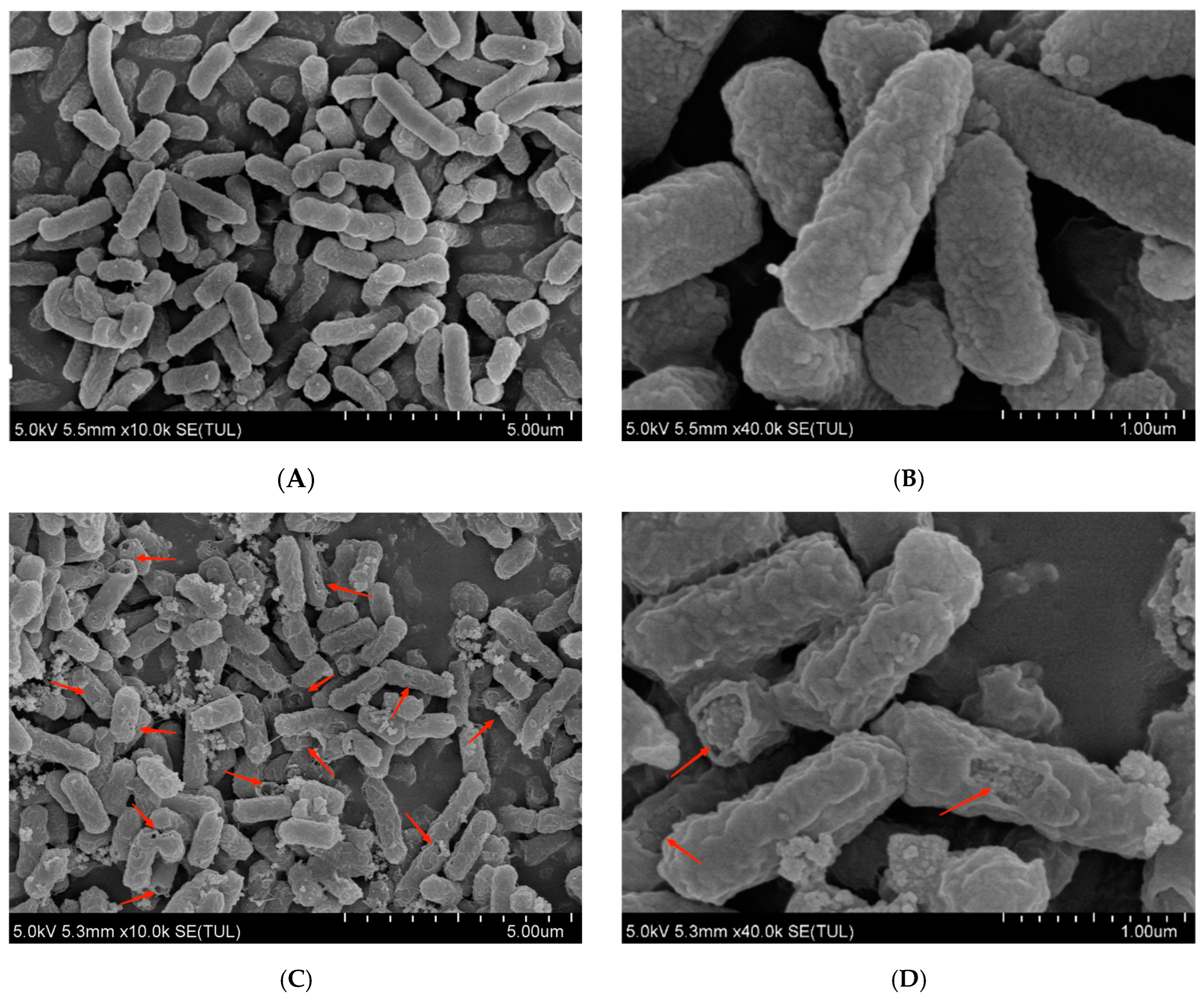
3.2. Morphological Changes of E. coli
3.3. Effect of Pulsed Electric Field on Bacterial Cell Membrane Permeability
3.4. Effect of Pulsed Electric Field on Pyruvate Kinase, Succinate Dehydrogenase, and Adenosine Triphosphatase
3.5. Transcriptomic Analysis
3.5.1. Sequence Analysis
3.5.2. Identification of Differentially Expressed Genes
3.5.3. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Enrichment Analysis
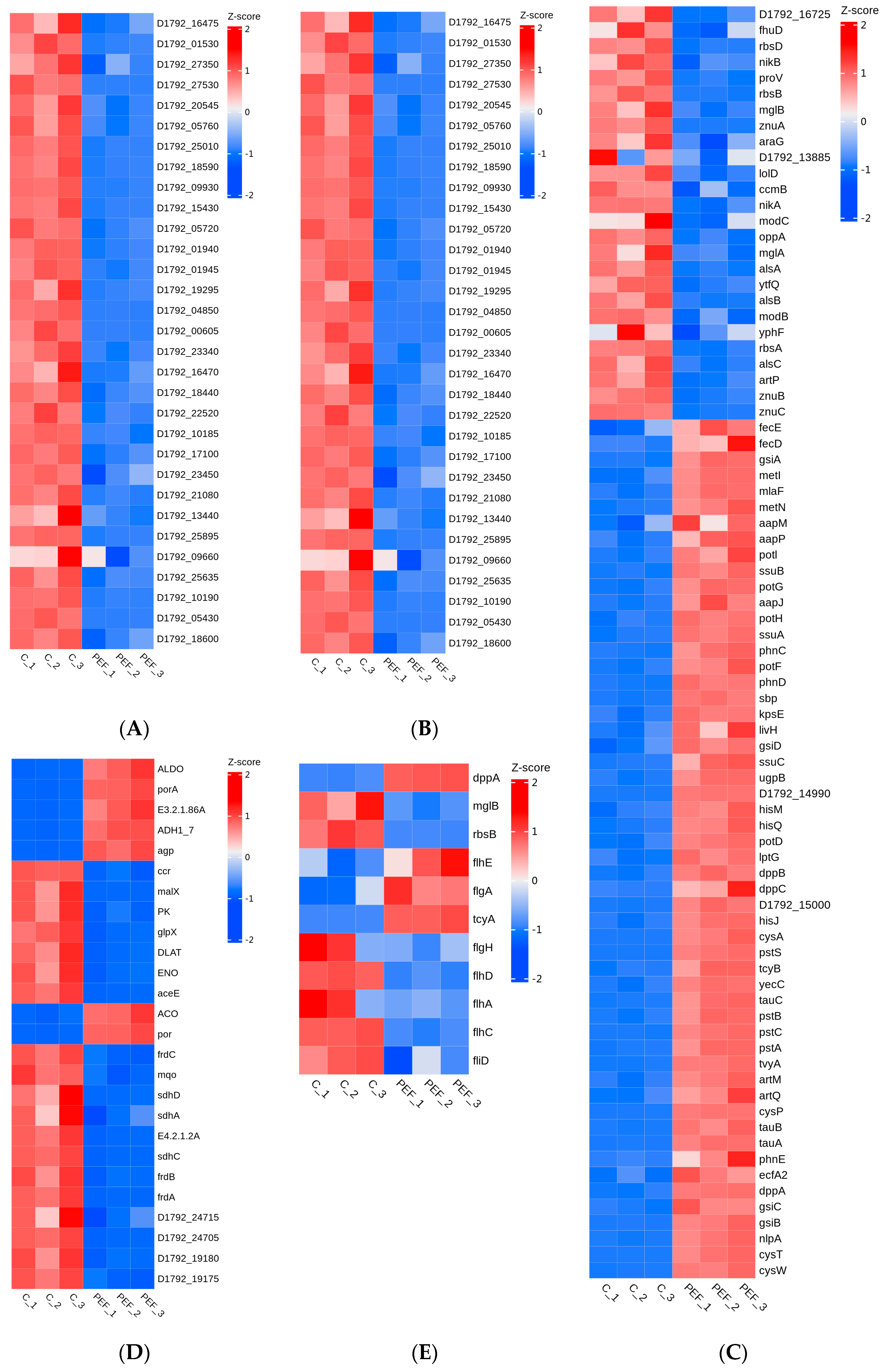
3.5.4. DEGs Related to Cell Structures
3.5.5. DEGs Related to DNA Replication and Repair
3.5.6. DEGs Related to Energy Metabolism
3.5.7. DEGs Related to Mobility
3.5.8. Quantitative Reverse Transcription PCR Verification of The Transcriptomics Sequence Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Huang, Z.; He, Z.; Yue, Y.; Zhou, Y.; Ross, R.P.; Stanton, C.; Zhang, H.; Zhao, J.; Chen, W. Protective effect of Bifidobacterium bifidum FSDJN7O5 and Bifidobacterium breve FHNFQ23M3 on diarrhea caused by enterotoxigenic Escherichia coli. Food Funct. 2021, 12, 7271–7282. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Brehmer, C.; Butt, S.; Vishram, B.; Harrison, M.; Marchant, E.; Ferris, S.; Jorgensen, F.; Smith, R.; Godbole, G.; et al. Outbreak of Shiga toxin-producing Escherichia coli O157 linked with consumption of a fast-food product containing imported cucumbers, United Kingdom, August 2020. Int. J. Infect. Dis. 2021, 110, S62–S68. [Google Scholar] [CrossRef] [PubMed]
- Morton, V. Notes from the field: An outbreak of Shiga toxin–producing Escherichia coli O121 infections associated with flour-Canada, 2016–2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.; Trmcic, A.; Taylor, M.; Shyng, S.; Hasselback, P.; Man, S.; Tchao, C.; Stone, J.; Janz, L.; Hoang, L.; et al. Foodborne and Animal Contact Disease Outbreaks: Escherichia coli O121 outbreak associated with raw milk Gouda-like cheese in British Columbia, Canada, 2018. Can. Commun. Dis. Rep. 2021, 47, 11. [Google Scholar] [CrossRef] [PubMed]
- Mylius, M.; Dreesman, J.; Pulz, M.; Pallasch, G.; Beyrer, K.; Claußen, K.; Allerberger, F.; Fruth, A.; Lang, C.; Prager, R.; et al. Shiga toxin-producing Escherichia coli O103:H2 outbreak in Germany after school trip to Austria due to raw cow milk, 2017—The important role of international collaboration for outbreak investigations. Int. J. Med. Microbiol. 2018, 308, 539–544. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Ma, S.; Zhang, S.; Liu, D.; Wang, Y.; Wu, C. Surveillance of antimicrobial resistance in Escherichia coli and enterococci from food products at retail in Beijing, China. Food Control 2021, 119, 107483. [Google Scholar] [CrossRef]
- Yoo, J.H.; Baek, K.H.; Heo, Y.S.; Yong, H.I.; Jo, C. Synergistic bactericidal effect of clove oil and encapsulated atmospheric pressure plasma against Escherichia coli O157:H7 and Staphylococcus aureus and its mechanism of action. Food Microbiol. 2021, 93, 103611. [Google Scholar] [CrossRef] [PubMed]
- Bouras, M.; Grimi, N.; Bals, O.; Vorobiev, E. Impact of pulsed electric fields on polyphenols extraction from Norway spruce bark. Ind. Crops Prod. 2016, 80, 50–58. [Google Scholar] [CrossRef]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus spores in the food industry: A review on resistance and response to novel inactivation technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Balthazar, C.F.; Margalho, L.P.; Freitas, M.Q.; Sant’Ana, A.S.; Cruz, A.G. Pulsed electric field-based technology for microbial inactivation in milk and dairy products. Curr. Opin. Food Sci. 2023, 54, 101087. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Microbial decontamination of red wine by pulsed electric fields (PEF) after alcoholic and malolactic fermentation: Effect on Saccharomyces cerevisiae, Oenococcus oeni, and oenological parameters during storage. Foods 2023, 12, 278. [Google Scholar] [CrossRef] [PubMed]
- Oliveira dos Santos Júnior, L.C.; Schulz, M.; Knorr, D.; Regina Amante, E. Pulsed electric field for Escherichia coli inactivation in pumpkin juice and nectar. Acta Sci. Technol. 2023, 44, e56091. [Google Scholar] [CrossRef]
- Yang, N.; Huang, K.; Lyu, C.; Wang, J. Pulsed electric field technology in the manufacturing processes of wine, beer, and rice wine: A review. Food Control 2016, 61, 28–38. [Google Scholar] [CrossRef]
- Ghoshal, G. Comprehensive review in pulsed electric field (PEF) in food preservation: Gaps in current studies for potential future research. Heliyon 2023, 9, e17532. [Google Scholar] [CrossRef]
- Boudjema, N.; Kherat, M.; Drouiche, N.; Mameri, N. Investigation of the mechanisms of Escherichia coli cells sterilization by the application of an electric field. Int. J. Environ. Sci. Technol. 2019, 16, 6259–6266. [Google Scholar] [CrossRef]
- Wang, L.-H.; Pyatkovskyy, T.; Yousef, A.; Zeng, X.-A.; Sastry, S.K. Mechanism of Bacillus subtilis spore inactivation induced by moderate electric fields. Innov. Food Sci. Emerg. Technol. 2020, 62, 102349. [Google Scholar] [CrossRef]
- Al-Hilphy, A.R.; Abdulstar, A.R.; Gavahian, M. Moderate electric field pasteurization of milk in a continuous flow unit: Effects of process parameters, energy consumption, and shelf-life determination. Innov. Food Sci. Emerg. Technol. 2021, 67, 102568. [Google Scholar] [CrossRef]
- Yadav, D.; Tanveer, A.; Malviya, N.; Yadav, S. Overview and principles of bioengineering: The drivers of omics technologies. In Omics Technologies and Bio-Engineering; Academic Press: Cambridge, MA, USA, 2018; pp. 3–23. [Google Scholar]
- Bai, J.; Wu, Y.; Bu, Q.; Zhong, K.; Gao, H. Comparative study on antibacterial mechanism of shikimic acid and quinic acid against Staphylococcus aureus through transcriptomic and metabolomic approaches. LWT 2022, 153, 112441. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, M.; Cui, L.; Yuan, Y.; Yang, Y.; Wang, Z.; Yue, T. Antibacterial activity and mechanism of thymol against Alicyclobacillus acidoterrestris vegetative cells and spores. LWT 2019, 105, 377–384. [Google Scholar] [CrossRef]
- Zhao, J.; Peng, T.; Liang, S.; Ma, M.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Antibacterial activity and action mechanism of microencapsulated dodecyl gallate with methyl-β-cyclodextrin. Food Control 2020, 109, 106953. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Antimicrobial mechanism of pulsed light for the control of Escherichia coli O157:H7 and its application in carrot juice. Food Control 2019, 106, 106751. [Google Scholar] [CrossRef]
- Khan, I.; Khan, J.; Miskeen, S.; Tango, C.N.; Park, Y.-S.; Oh, D.-H. Prevalence and control of Listeria monocytogenes in the food industry—A review. Czech J. Food Sci. 2016, 34, 469–487. [Google Scholar] [CrossRef]
- Meneses, N.; Jaeger, H.; Moritz, J.; Knorr, D. Impact of insulator shape, flow rate and electrical parameters on inactivation of E. coli using a continuous co-linear PEF system. Innov. Food Sci. Emerg. Technol. 2011, 12, 6–12. [Google Scholar] [CrossRef]
- Siemer, C.; Toepfl, S.; Heinz, V. Inactivation of Bacillus subtilis spores by pulsed electric fields (PEF) in combination with thermal energy—I. Influence of process- and product parameters. Food Control 2014, 39, 163–171. [Google Scholar] [CrossRef]
- Zimmermann, U. Electrical breakdown, electropermeabilization and electrofusion. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 105, pp. 175–256. [Google Scholar]
- Simonis, P.; Kersulis, S.; Stankevich, V.; Sinkevic, K.; Striguniene, K.; Ragoza, G.; Stirke, A. Pulsed electric field effects on inactivation of microorganisms in acid whey. Int. J. Food Microbiol. 2019, 291, 128–134. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Palgan, I.; Noci, F.; Muñoz, A.; Whyte, P.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. The effect of pulsed electric fields (PEF) in combination with high intensity light pulses (HILP) on Escherichia coli inactivation and quality attributes in apple juice. Innov. Food Sci. Emerg. Technol. 2011, 12, 118–123. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Bursać Kovačević, D. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Jin, T.Z.; Campanella, O.H. Modeling the inactivation of Escherichia coli O157:H7 and Salmonella Typhimurium in juices by pulsed electric fields: The role of the energy density. J. Food Eng. 2020, 282, 110001. [Google Scholar] [CrossRef]
- Kamgang-Youbi, G.; Herry, J.-M.; Brisset, J.-L.; Bellon-Fontaine, M.-N.; Doubla, A.; Naïtali, M. Impact on disinfection efficiency of cell load and of planktonic/adherent/detached state: Case of Hafnia alvei inactivation by plasma activated water. Appl. Microbiol. Biotechnol. 2008, 81, 449–457. [Google Scholar] [CrossRef]
- Fernández, A.; Shearer, N.; Wilson, D.R.; Thompson, A. Effect of microbial loading on the efficiency of cold atmospheric gas plasma inactivation of Salmonella enterica serovar Typhimurium. Int. J. Food Microbiol. 2012, 152, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-S.; Zeng, X.-A.; Sun, D.-W.; Han, Z. Quantitative analysis of sublethally injured Saccharomyces cerevisiae cells induced by pulsed electric fields. LWT-Food Sci. Technol. 2015, 60, 672–677. [Google Scholar] [CrossRef]
- Ohshima, T.; Okuyama, K.; Sato, M. Effect of culture temperature on high-voltage pulse sterilization of Escherichia coli. J. Electrost. 2002, 55, 227–235. [Google Scholar] [CrossRef]
- Ananda, A.P.; Manukumar, H.M.; Krishnamurthy, N.B.; Nagendra, B.S.; Savitha, K.R. Assessment of antibacterial efficacy of a biocompatible nanoparticle PC@AgNPs against Staphylococcus aureus. Microb. Pathog. 2019, 126, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Yu, N.; Zhu, Y.; Wei, Y.; Zhang, H.; Sun, A. Inactivation of Pichia rhodanensis in relation to membrane and intracellular compounds due to microchip pulsed electric field (MPEF) treatment. PLoS ONE 2018, 13, e0198467. [Google Scholar] [CrossRef]
- Coustets, M.; Joubert-Durigneux, V.; Hérault, J.; Schoefs, B.; Blanckaert, V.; Garnier, J.-P.; Teissié, J. Optimization of protein electroextraction from microalgae by a flow process. Bioelectrochemistry 2015, 103, 74–81. [Google Scholar] [CrossRef]
- Bensalem, S.; Pareau, D.; Cinquin, B.; Français, O.; Le Pioufle, B.; Lopes, F. Impact of pulsed electric fields and mechanical compressions on the permeability and structure of Chlamydomonas reinhardtii cells. Sci. Rep. 2020, 10, 2668. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Cecchini, G.; Schröder, I.; Gunsalus, R.P.; Maklashina, E. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim. Biophys. Acta (BBA)-Bioenerg. 2002, 1553, 140–157. [Google Scholar] [CrossRef]
- Pei, Q.; Li, Y.; Ge, X.; Tian, P. Multipath effects of berberine on peach Brown rot fungus Monilinia fructicola. Crop Protection 2019, 116, 92–100. [Google Scholar] [CrossRef]
- Piasecka, M.; Wenda-Różewicka, L.; Ogoński, T. Computerized analysis of cytochemical reactions for dehydrogenases and oxygraphic studies as methods to evaluate the function of the mitochondrial sheath in rat spermatozoa. Andrologia 2001, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bénit, P.; Goncalves, J.; El Khoury, R.; Rak, M.; Favier, J.; Gimenez-Roqueplo, A.P.; Rustin, P. Succinate dehydrogenase, succinate, and superoxides: A genetic, epigenetic, metabolic, environmental explosive crossroad. Biomedicines 2022, 10, 1788. [Google Scholar] [CrossRef] [PubMed]
- Pinna, S.; Kunz, C.; Halpern, A.; Harrison, S.A.; Jordan, S.F.; Ward, J.; Werner, F.; Lane, N. A prebiotic basis for ATP as the universal energy currency. PLoS Biol. 2022, 20, e3001437. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Bernardi, P. Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium 2016, 60, 102–107. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial activity and mechanism of Larch bark procyanidins against Staphylococcus aureus. Acta Biochim. Et Biophys. Sin. 2017, 49, 1058–1066. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.; Fu, Y.B.; Deng, Y.; Shi, X.J.; Bian, Z.Y.; Ruhan, A.; Wang, X. Deep sequencing-based analysis of gene expression in bovine mammary epithelial cells after Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae infection. Genet. Mol. Res. 2015, 14, 16948–16965. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36 (Suppl. S1), D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Y.; Shan, M.; Zhao, H.; Lu, Z.; Lu, Y. A mini-review: Mechanism of antimicrobial action and application of surfactin. World J. Microbiol. Biotechnol. 2022, 38, 143. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Li, C.; Hua, S.; Sun, C.; Huang, L. Antibacterial mechanism of vanillin against Escherichia coli O157: H7. Heliyon 2023, 9, e19280. [Google Scholar] [CrossRef]
- Walker, J.E. The ATP synthase: The understood, the uncertain and the unknown. Biochem. Soc. Trans. 2013, 1817, S1. [Google Scholar] [CrossRef] [PubMed]
- Azi, F.; Li, Z.; Xu, P.; Dong, M. Transcriptomic analysis reveals the antibacterial mechanism of phenolic compounds from kefir fermented soy whey against Escherichia coli 0157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 2022, 383, 109953. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D. Cues from the Membrane: Bacterial Glycerophospholipids. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, Y.; Sun, H.; Fu, X.; Kong, Q.; Zhu, C.; Mou, H. Regulation of virulence factors expression during the intestinal colonization of Vibrio parahaemolyticus. Foodborne Pathog. Dis. 2022, 19, 169–178. [Google Scholar] [CrossRef]




| Genes | Forward (F) | Reverse (R) |
|---|---|---|
| 16SrRNA | TGACGTTACCCGCAGAAGAA | ATCTCTACGCATTTCACCGCTAC |
| D1792_18820 | AGCCGCATCGTGGAGTGG | TCGCTTCAGATACCGCCAGAG |
| D1792_24705 | CGTAGGTATTCGCCACATGATGATG | GAAAGCACGACAGTAATAACAAAGGAG |
| D1792_19850 | ACTGCGATCAACTGGTGGTAATG | CCGCTTCCACGCTGAATACTG |
| D1792_16885 | GGTGATGGCGTACTGGAGATATTG | AGGTTGAAACGGCGGATTTGG |
| D1792_02420 | CGGCATCAATACTTACGCTCAGG | ACATACTTTATTCTCACCCAGCAACAC |
| D1792_19100 | TGGCAGCAGAAGCAGGTCAG | AGCAAGCGTTGGTCAGAATGTG |
| D1792_09930 | TTTGATGAAGTGGATGTAGGGATTAGC | ACCTGAGTTGATTCGCCAAGTTG |
| E4.2.1.2A | CTCCTGTTCTGCTGACCGTAATATC | ACCGCTTCGCCTTCTCCTG |
| rbsB | CGTCAGTGCGAATGCGATGG | CCACCAGGTTATAGCCAAGTTTATCC |
| Different Solutions | Electrical Conductivity (mS/cm) |
|---|---|
| 0.01 mol/L PBS | 16.29 ± 0.00 |
| Mass fraction of 0.85% NaCl | 15.06 ± 0.01 |
| Mass fraction of 1.00% NaCl | 17.29 ± 0.00 |
| Mass fraction of 2.00% NaCl | 32.82 ± 0.02 |
| Mass fraction of 3.00% NaCl | 47.21 ± 0.02 |
| Mass fraction of 0.10% Peptone | 0.12 ± 0.00 |
| Sample Name | Raw Reads | Clean Reads | Clean Q20 (%) | Clean Q30 (%) |
|---|---|---|---|---|
| Control 1 | 29,156,142 | 28,864,672 | 98.53 | 96.07 |
| Control 2 | 26,599,110 | 26,278,048 | 98.51 | 96.03 |
| Control 3 | 26,553,670 | 26,228,896 | 98.46 | 95.94 |
| PEF 1 | 24,870,364 | 24,507,490 | 98.14 | 95.47 |
| PEF 2 | 27,193,094 | 26,839,560 | 98.30 | 95.76 |
| PEF 3 | 24,118,414 | 23,765,962 | 98.33 | 95.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, J.; Lin, Y.; Wang, L.; Yan, Z.; Wei, J.; Du, J.; Li, Z. Effects of PEF on Cell and Transcriptomic of Escherichia coli. Microorganisms 2024, 12, 1380. https://doi.org/10.3390/microorganisms12071380
Kuang J, Lin Y, Wang L, Yan Z, Wei J, Du J, Li Z. Effects of PEF on Cell and Transcriptomic of Escherichia coli. Microorganisms. 2024; 12(7):1380. https://doi.org/10.3390/microorganisms12071380
Chicago/Turabian StyleKuang, Jinyan, Ying Lin, Li Wang, Zikang Yan, Jinmei Wei, Jin Du, and Zongjun Li. 2024. "Effects of PEF on Cell and Transcriptomic of Escherichia coli" Microorganisms 12, no. 7: 1380. https://doi.org/10.3390/microorganisms12071380
APA StyleKuang, J., Lin, Y., Wang, L., Yan, Z., Wei, J., Du, J., & Li, Z. (2024). Effects of PEF on Cell and Transcriptomic of Escherichia coli. Microorganisms, 12(7), 1380. https://doi.org/10.3390/microorganisms12071380






