A Comprehensive Approach Combining Short-Chain Polyphosphate and Bacterial Biostimulants for Effective Nutrient Solubilization and Enhanced Wheat Growth
Abstract
:1. Introduction
2. Material and Methods
2.1. Soil Sampling and Preparation
2.2. Applied PolyP Fertilizer
2.3. Isolation of Rhizosphere Bacterial Strains
2.4. Species Determination of the Isolated Strains
2.5. In vitro Bacterial Screening for Plant Growth-Promoting Activities
2.5.1. Bacterial Phosphate Solubilization in Liquid Medium
2.5.2. Bacterial Potassium Solubilization
2.5.3. Bacterial Zinc Solubilization
2.5.4. Bacterial Indole-3-Acetic Acid (IAA) Production
2.5.5. Bacterial Ammonia Production
2.6. In vitro Screening for Biocontrol Activities
2.6.1. Bacterial Siderophores Production
2.6.2. Bacterial Hydrogen Cyanide Production
2.6.3. Bacterial Antifungal Activity
2.7. Assessment of Strains’ Activities on Seeds
2.7.1. Germination Assay
2.7.2. Assessment of Bacterial Activities on Plants
2.7.3. Biomass and Chlorophyll Index Measurements
2.8. Statistical Analysis
3. Results
3.1. Bacterial Isolates P1 to P8 belong to the Genera of Pseudomonas or Bacillus
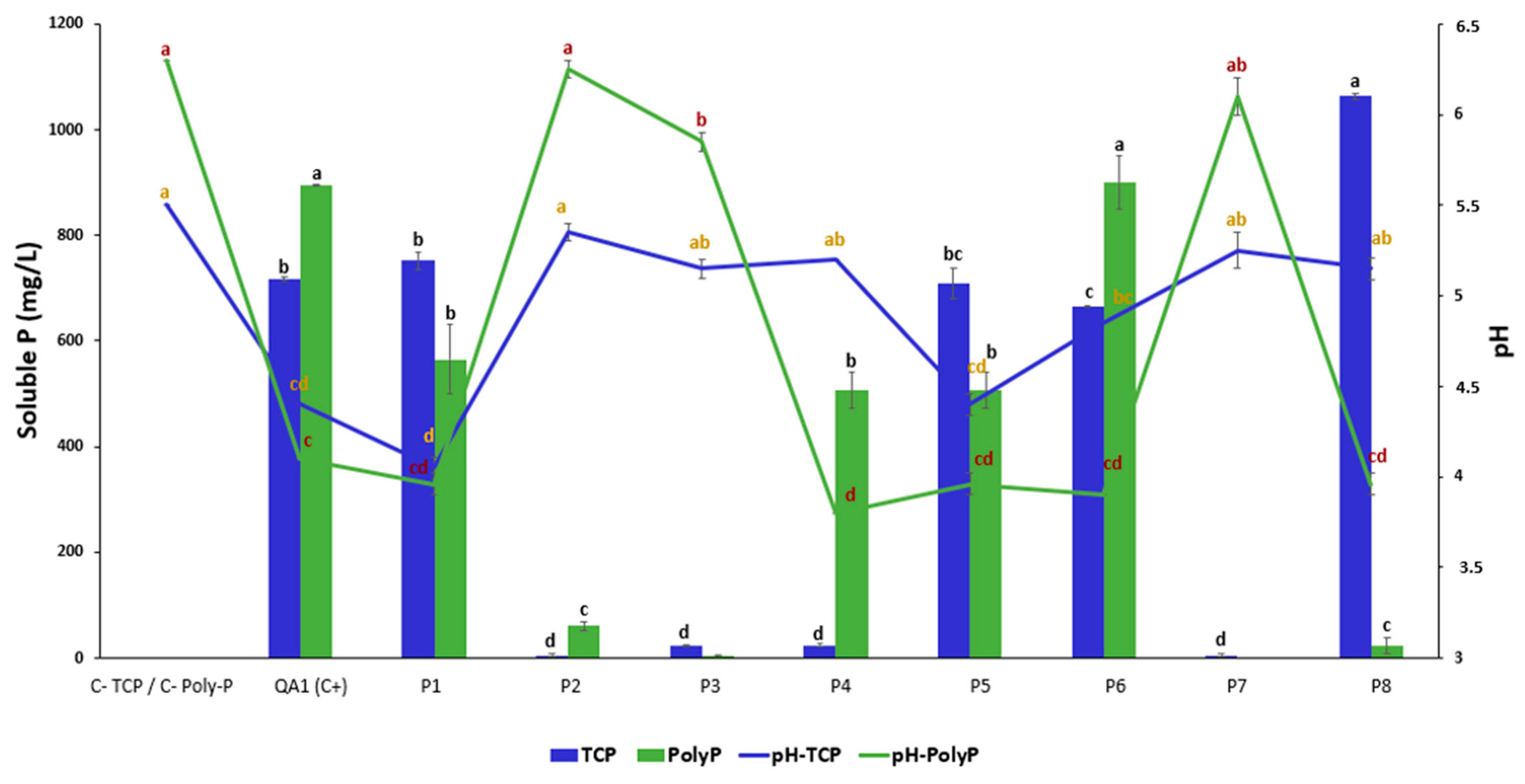
3.2. Bacterial Isolates P1, P5, and P6 Solubilize Both Insoluble Forms of P
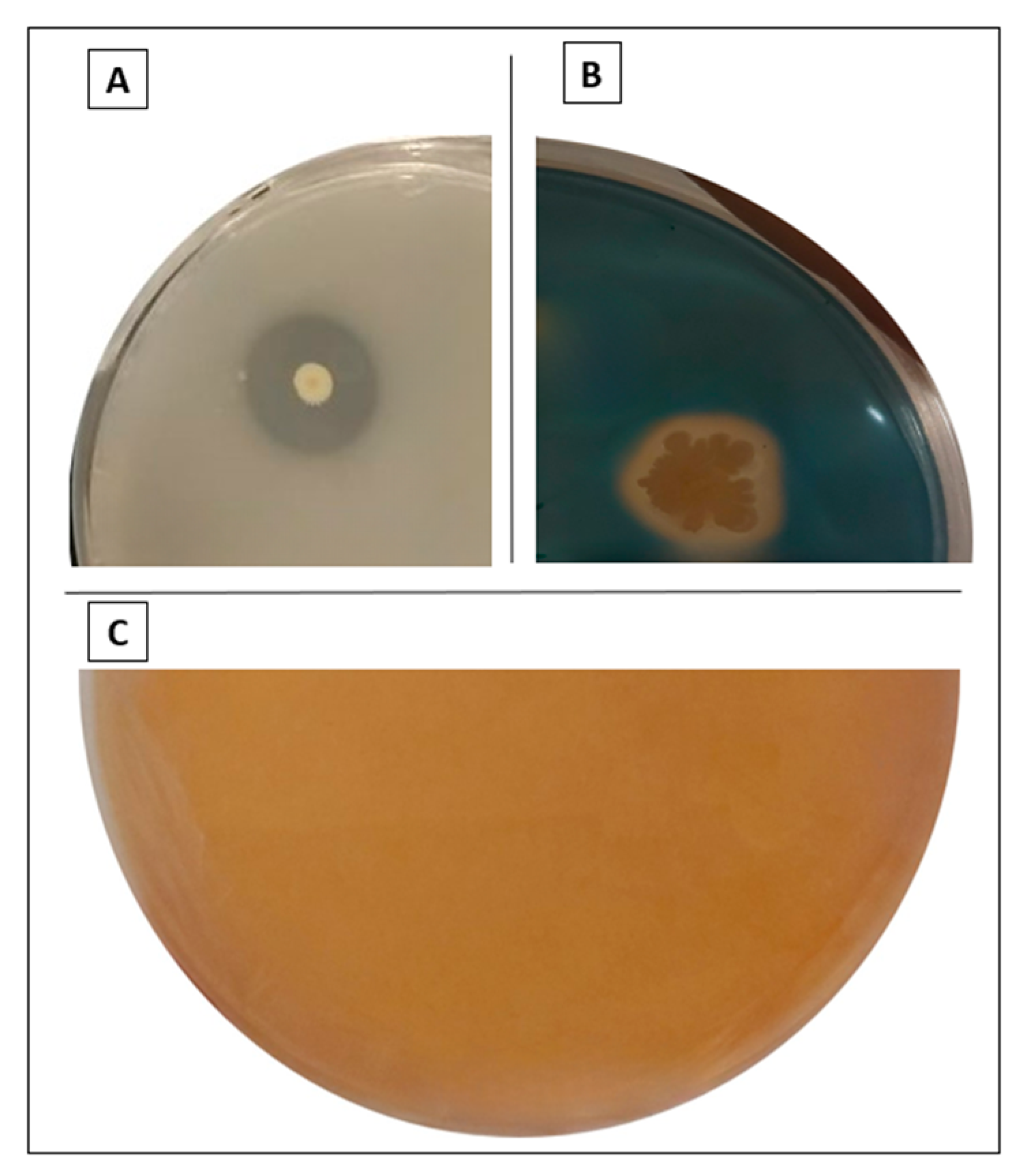
3.3. Bacterial Isolates P1, P2, and P4 Solubilized Potassium
3.4. P1, P2, P3, P7, and P8 Isolates Solubilized Both ZnO and ZnCO3, While P4 Solubilized Only ZnO
3.5. Bacillus Tropicus P5 Strain Is the Highest Ammonia Producer
3.6. Production of IAA Is Highly Pronounced in P. soyae P1 and B. tropicus P4 Strains
3.7. HCN Are Produced by All Strains
3.8. Siderophores Production Was Higher in the P. reinekei P2 Strain
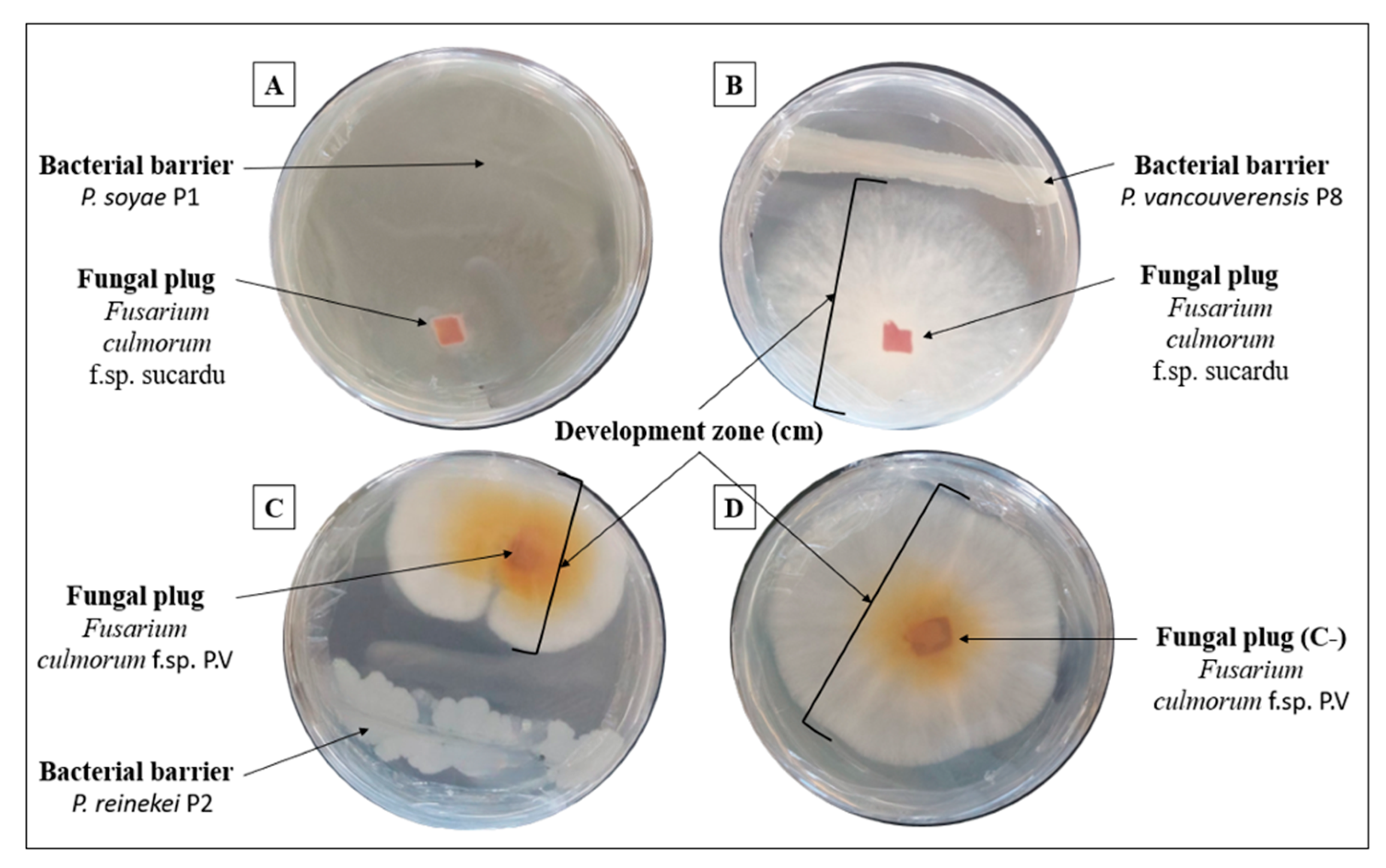
3.9. Pseudomonas reinekei P2 Strain Exhibited Stronger Anti-Fusarium Activity
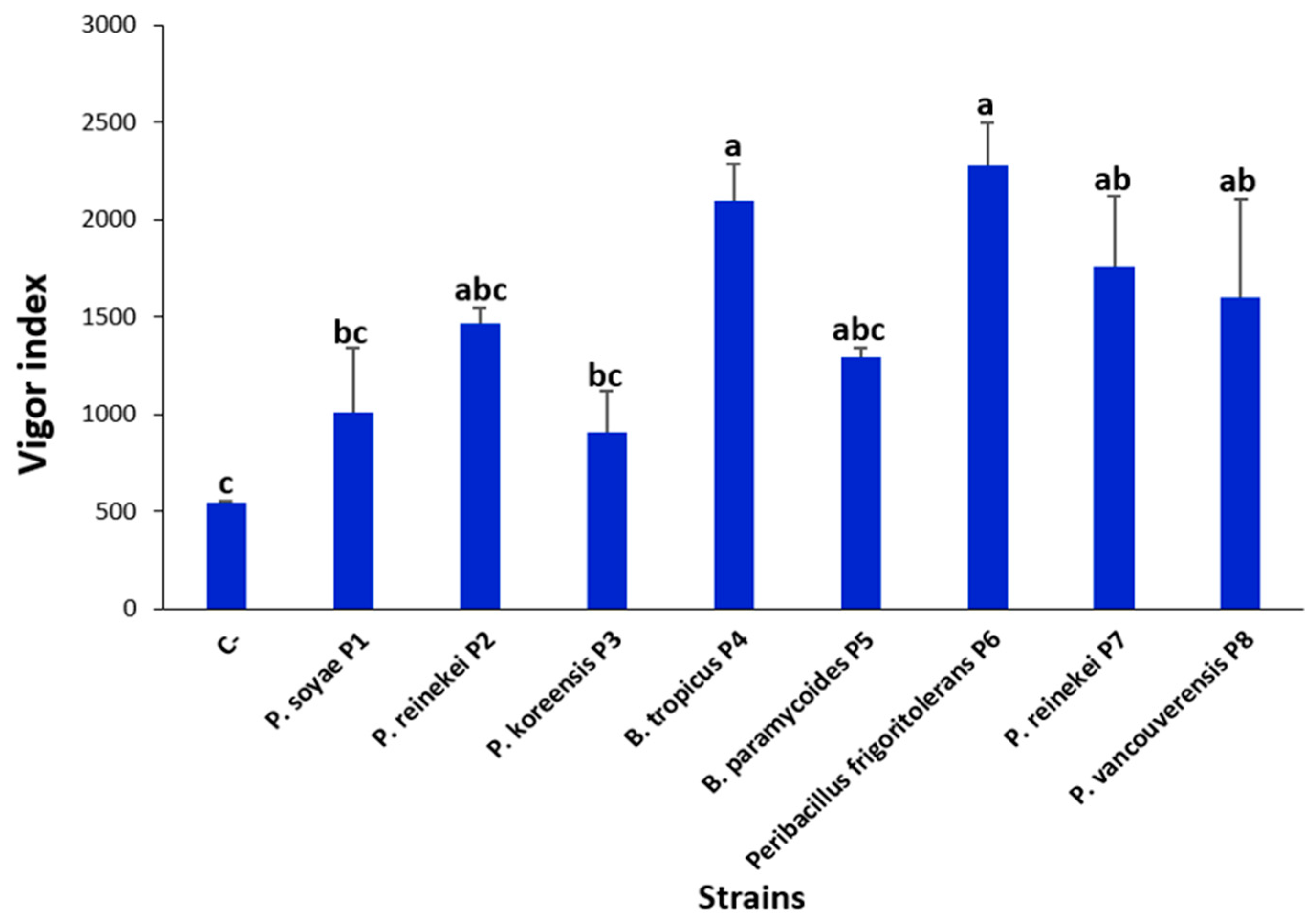
3.10. P. frigoritolerans P6 and B. tropicus P4 Strain Optimally Enhanced Wheat Seeds’ Germination
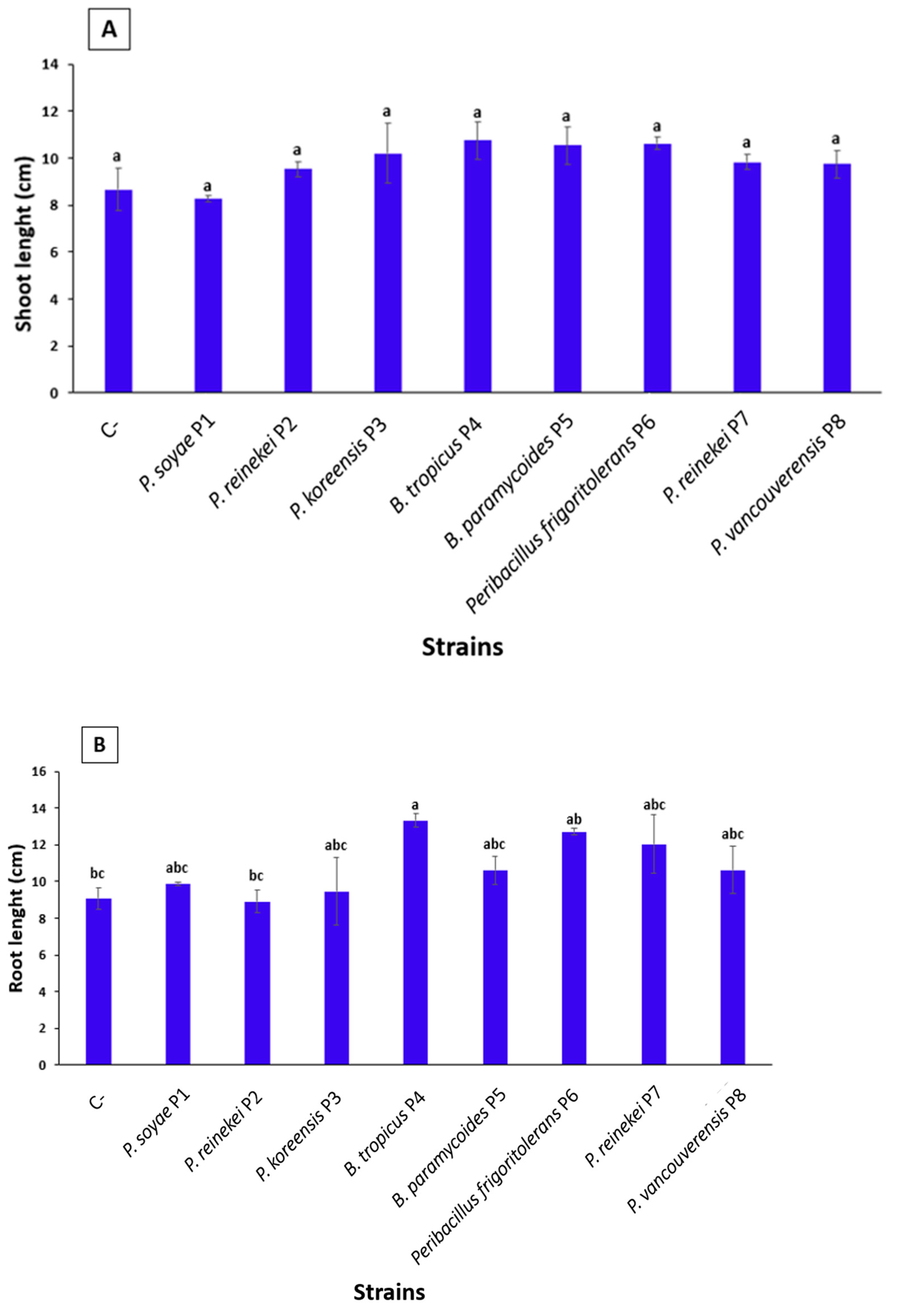
3.11. Co-Application of PolyP and P. soyae P1 Strain Boost Shoot Biomass While the Sole Application of PolyP Induced Root Development
4. Discussion
4.1. Solubilization of P, K, and Zn by P1-P8 Strains
4.2. Strains’ Biocontrol Activity against Fusarium Culmorum
4.3. Impact of PolyP-PSB Co-Application on Wheat Growth Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus Acquisition and Use: Critical Adaptations by Plants for Securing a Nonrenewable Resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- McBeath, T.M.; Lombi, E.; McLaughlin, M.J.; Bünemann, E.K. Polyphosphate-fertilizer Solution Stability with Time, Temperature, and pH. Z. Pflanzenernähr. Bodenk. 2007, 170, 387–391. [Google Scholar] [CrossRef]
- Kulakovskaya, T.V.; Vagabov, V.M.; Kulaev, I.S. Inorganic Polyphosphate in Industry, Agriculture and Medicine: Modern State and Outlook. Process. Biochem. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Frazier, A.W.; Smith, J.P.; Lehr, J.R. Fertilizer Materials, Characterization of Some Ammonium Polyphosphates. J. Agric. Food Chem. 1965, 13, 316–322. [Google Scholar] [CrossRef]
- Mukherjee, C.; Chowdhury, R.; Ray, K. Phosphorus Recycling from an Unexplored Source by Polyphosphate Accumulating Microalgae and Cyanobacteria—A Step to Phosphorus Security in Agriculture. Front. Microbiol. 2015, 6, 1421. [Google Scholar] [CrossRef]
- Kontárová, S.; Přikryl, R.; Škarpa, P.; Kriška, T.; Antošovský, J.; Gregušková, Z.; Figalla, S.; Jašek, V.; Sedlmajer, M.; Menčík, P.; et al. Slow-Release Nitrogen Fertilizers with Biodegradable Poly(3-Hydroxybutyrate) Coating: Their Effect on the Growth of Maize and the Dynamics of N Release in Soil. Polymers 2022, 14, 4323. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Dick, R.P.; Tabatabai, M.A. Hydrolysis of polyphosphates in soils. Soil. Sci. 1986, 142, 132–140. [Google Scholar] [CrossRef]
- Dick, W.A.; Tabatabai, M.A. Kinetics and activities of phosphatase-clay complexes1. Soil. Sci. 1987, 143, 5–15. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahmad, E. Mechanism of Phosphate Solubilization and Physiological Functions of Phosphate-Solubilizing Microorganisms. In Phosphate Solubilizing Microorganisms; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 31–62. ISBN 978-3-319-08215-8. [Google Scholar]
- Bhat, R.A.; Dervash, M.A.; Mehmood, M.A.; Skinder, B.M.; Rashid, A.; Bhat, J.I.A.; Singh, D.V.; Lone, R. Mycorrhizae: A Sustainable Industry for Plant and Soil Environment. In Mycorrhiza-Nutrient Uptake, Biocontrol, Ecorestoration; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 473–502. ISBN 978-3-319-68866-4. [Google Scholar]
- Hinsinger, P. Bioavailability of Soil Inorganic P in the Rhizosphere as Affected by Root-Induced Chemical Changes: A Review. Plant Soil. 2001, 237, 173–195. [Google Scholar] [CrossRef]
- White, P.J.; Hammond, J.P. Phosphorus Nutrition of Terrestrial Plants. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Plant Ecophysiology; Springer Netherlands: Dordrecht, The Netherlands, 2008; Volume 7, pp. 51–81. ISBN 978-1-4020-8434-8. [Google Scholar]
- White, P.J.; Brown, P.H. Plant Nutrition for Sustainable Development and Global Health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- McGill, W.B.; Cole, C.V. Comparative Aspects of Cycling of Organic C, N, S and P through Soil Organic Matter. Geoderma 1981, 26, 267–286. [Google Scholar] [CrossRef]
- Singla, B.; Chugh, A.; Khurana, J.P.; Khurana, P. An Early Auxin-Responsive Aux/IAA Gene from Wheat (Triticum aestivum) Is Induced by Epibrassinolide and Differentially Regulated by Light and Calcium. J. Exp. Bot. 2006, 57, 4059–4070. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-Deaminase Producing Strain of Trichoderma Longibrachiatum T6 in Enhancing Wheat Seedling Tolerance to NaCl Stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate Solubilizing Bacteria with Glucose Dehydrogenase Gene for Phosphorus Uptake and Beneficial Effects on Wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Components of Rhizospheric Bacterial Communities of Barley and Their Potential for Plant Growth Promotion and Biocontrol of Fusarium Wilt of Watermelon. Braz. J. Microbiol. 2019, 50, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement Using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus Licheniformis QA1 and Enterobacter Asburiae QF11 Isolated from Chenopodium Quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Fahsi, N.; Mahdi, I.; Mesfioui, A.; Biskri, L.; Allaoui, A. Plant Growth-Promoting Rhizobacteria Isolated from the Jujube (Ziziphus Lotus) Plant Enhance Wheat Growth, Zn Uptake, and Heavy Metal Tolerance. Agriculture 2021, 11, 316. [Google Scholar] [CrossRef]
- Bourak, K.; Sare, A.R.; Allaoui, A.; Jijakli, M.H.; Massart, S. Impact of Two Phosphorus Fertilizer Formulations on Wheat Physiology, Rhizosphere, and Rhizoplane Microbiota. IJMS 2023, 24, 9879. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus Insoils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Sare, A.R.; Stouvenakers, G.; Eck, M.; Lampens, A.; Goormachtig, S.; Jijakli, M.H.; Massart, S. Standardization of Plant Microbiome Studies: Which Proportion of the Microbiota Is Really Harvested? Microorganisms 2020, 8, 342. [Google Scholar] [CrossRef]
- Fukatsu, T.; Nikoh, N.; Kawai, R.; Koga, R. The Secondary Endosymbiotic Bacterium of the Pea Aphid Acyrthosiphon Pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 2000, 66, 2748–2758. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The Molybdenum Blue Reaction for the Determination of Orthophosphate Revisited: Opening the Black Box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium Solubilizing Rhizobacteria (KSR): Isolation, Identification, and K-Release Dynamics from Waste Mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of Potassium- and Phosphate-Solubilizing Actinobacteria and the Assessment of Their Ability to Promote Wheat Growth Parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant Growth Promotion Induced by Phosphate Solubilizing Endophytic Pseudomonas Isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.J.; Lindow, S.E. Utilization of the Plant Hormone Indole-3-Acetic Acid for Growth by Pseudomonas Putida Strain 1290. Appl Env. Microbiol 2005, 71, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Halo, B.A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I.-J. Indole Acetic Acid and ACC Deaminase from Endophytic Bacteria Improves the Growth of Solanum Lycopersicum. Electron. J. Biotechnol. 2016, 21, 58–64. [Google Scholar] [CrossRef]
- Cappuccino, J.; Sherman, N. Microbiology: A Laboratory Manuala Laboratory Manual, 3rd ed.; Pearson: New York, NY, USA, 1992. [Google Scholar]
- Chrouqi, L.; Ouahmane, L.; Jadrane, I.; Koussa, T.; Alfeddy, M.N. Screening of Soil Rhizobacteria Isolated from Wheat Plants Grown in the Marrakech Region (Morocco, North Africa) for Plant Growth Promoting Activities. JMES 2017, 8, 3382–3390. [Google Scholar]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Benjelloun, S.; Allaoui, A.; Biskri, L. Rhizospheric Phosphate Solubilizing Bacillus Atrophaeus GQJK17 S8 Increases Quinoa Seedling, Withstands Heavy Metals, and Mitigates Salt Stress. Sustainability 2021, 13, 3307. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lorck, H. Production of Hydrocyanic Acid by Bacteria. Physiol. Plant. 1948, 1, 142–146. [Google Scholar] [CrossRef]
- Ji, S.H.; Gururani, M.A.; Chun, S.-C. Isolation and Characterization of Plant Growth Promoting Endophytic Diazotrophic Bacteria from Korean Rice Cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Lu, Y.; Liao, Y.; Nie, J.; Yuan, X.; Chen, F. Use of a Leaf Chlorophyll Content Index to Improve the Prediction of Above-Ground Biomass and Productivity. PeerJ 2019, 6, e6240. [Google Scholar] [CrossRef]
- Khourchi, S.; Delaplace, P.; Bargaz, A. Polyphosphate Fertilizer Use Efficiency Strongly Relies on Soil Physicochemical Properties and Root-Microbial Activities. Geoderma 2023, 429, 116281. [Google Scholar] [CrossRef]
- Khourchi, S.; Elhaissoufi, W.; Loum, M.; Ibnyasser, A.; Haddine, M.; Ghani, R.; Barakat, A.; Zeroual, Y.; Rchiad, Z.; Delaplace, P.; et al. Phosphate Solubilizing Bacteria Can Significantly Contribute to Enhance P Availability from Polyphosphates and Their Use Efficiency in Wheat. Microbiol. Res. 2022, 262, 127094. [Google Scholar] [CrossRef]
- Busman, L.M. Behavior of Polyphosphates in Soils; Iowa State University: Ames, IA, USA, 1984. [Google Scholar]
- Wang, X.; Gao, Y.; Hu, B.; Chu, G. Comparison of the Hydrolysis Characteristics of Three Polyphosphates and Their Effects on Soil Phosphorus and Micronutrient Availability. Soil. Use Manag. 2019, 35, 664–674. [Google Scholar] [CrossRef]
- Othman, R.; Panhwar, Q.A. Phosphate-Solubilizing Bacteria Improves Nutrient Uptake in Aerobic Rice. In Phosphate Solubilizing Microorganisms; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 207–224. ISBN 978-3-319-08215-8. [Google Scholar]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium Solubilizing Bacteria (KSB): Mechanisms, Promotion of Plant Growth, and Future Prospects A Review. J. Soil. Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Meena, S.K.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A. Potassium Solubilization: Strategies to Mitigate Potassium Deficiency in Agricultural Soils. GJBAHS 2018, 7, 3. [Google Scholar] [CrossRef]
- Parmar, N.; Singh, A. (Eds.) Geomicrobiology and Biogeochemistry; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 39, ISBN 978-3-642-41836-5. [Google Scholar]
- Mohamed, H.M.; El-Homosy, R.F.; Abd-Ellatef, A.-E.H.; Salh, F.M.; Hussein, M.Y. Identification of Yeast Strains Isolated from Agricultural Soils for Releasing Potassium-Bearing Minerals. Geomicrobiol. J. 2017, 34, 261–266. [Google Scholar] [CrossRef]
- Olaniyan, F.T.; Alori, E.T.; Adekiya, A.O.; Ayorinde, B.B.; Daramola, F.Y.; Osemwegie, O.O.; Babalola, O.O. The Use of Soil Microbial Potassium Solubilizers in Potassium Nutrient Availability in Soil and Its Dynamics. Ann. Microbiol. 2022, 72, 45. [Google Scholar] [CrossRef]
- Cakmak, I.; Hoffland, E. Zinc for the Improvement of Crop Production and Human Health. Plant Soil. 2012, 361, 1–2. [Google Scholar] [CrossRef]
- Khanghahi, M.Y.; Ricciuti, P.; Allegretta, I.; Terzano, R.; Crecchio, C. Solubilization of Insoluble Zinc Compounds by Zinc Solubilizing Bacteria (ZSB) and Optimization of Their Growth Conditions. Environ. Sci. Pollut. Res. 2018, 25, 25862–25868. [Google Scholar] [CrossRef]
- Gandhi, A.; Muralidharan, G. Assessment of Zinc Solubilizing Potentiality of Acinetobacter sp. Isolated from Rice Rhizosphere. Eur. J. Soil. Biol. 2016, 76, 1–8. [Google Scholar] [CrossRef]
- Rehman, H.F.; Ashraf, A.; Muzammil, S.; Siddique, M.H.; Ali, T. Assessment of Zinc Solubilization Potential of Zinc-Resistant Pseudomonas Oleovorans Strain ZSB13 Isolated from Contaminated Soil. Braz. J. Biol. 2023, 83, e240015. [Google Scholar] [CrossRef]
- Saravanan, V.S.; Kumar, M.R.; Sa, T.M. Microbial Zinc Solubilization and Their Role on Plants. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 47–63. ISBN 978-3-642-21060-0. [Google Scholar]
- Preston, G.M. Plant Perceptions of Plant Growth-Promoting Pseudomonas. Phil. Trans. R. Soc. Lond. B 2004, 359, 907–918. [Google Scholar] [CrossRef]
- Lally, R.D.; Galbally, P.; Moreira, A.S.; Spink, J.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Application of Endophytic Pseudomonas Fluorescens and a Bacterial Consortium to Brassica Napus Can Increase Plant Height and Biomass under Greenhouse and Field Conditions. Front. Plant Sci. 2017, 8, 2193. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Rhizobacteria (PGPR): Their Potential as Antagonists and Biocontrol Agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Pandey, S.S. The Role of Iron in Phytopathogenic Microbe–Plant Interactions: Insights into Virulence and Host Immune Response. Plants 2023, 12, 3173. [Google Scholar] [CrossRef]
- Efe, D. Potential Plant Growth-Promoting Bacteria with Heavy Metal Resistance. Curr. Microbiol. 2020, 77, 3861–3868. [Google Scholar] [CrossRef]
- Mohd Azim Khan, N.A.; Jaya Jothi, S.; Mohd Hata, E.; Parimannan, S.; Vadamalai, G.; Rajandas, H. Draft Genome Sequence of Bacillus Tropicus Strain UPM-CREST01, Isolated from the Bulk Paddy Soil at Kampung Gajah, Perak. Microbiol. Resour. Announc. 2022, 11, e01156-21. [Google Scholar] [CrossRef]
- Negi, R.; Kaur, T.; Devi, R.; Kour, D.; Sheikh, I.; Tyagi, V.; Yadav, A.N. First Report on Rahnella Sp. Strain EU-A3SNfb, a Plant Growth Promoting Endophytic Bacterium from Wild Wheat Relative Aegilops Kotschyi. Natl. Acad. Sci. Lett. 2022, 45, 393–396. [Google Scholar] [CrossRef]
- Dame, Z.T.; Rahman, M.; Islam, T. Bacilli as Sources of Agrobiotechnology: Recent Advances and Future Directions. Green. Chem. Lett. Rev. 2021, 14, 246–271. [Google Scholar] [CrossRef]
- Goudjal, Y.; Zamoum, M.; Sabaou, N.; Mathieu, F.; Zitouni, A. Potential of Endophytic Streptomyces Spp. for Biocontrol of Fusarium Root Rot Disease and Growth Promotion of Tomato Seedlings. Biocontrol Sci. Technol. 2016, 26, 1691–1705. [Google Scholar] [CrossRef]
- Alblooshi, A.A.; Purayil, G.P.; Saeed, E.E.; Ramadan, G.A.; Tariq, S.; Altaee, A.S.; El-Tarabily, K.A.; AbuQamar, S.F. Biocontrol Potential of Endophytic Actinobacteria against Fusarium Solani, the Causal Agent of Sudden Decline Syndrome on Date Palm in the UAE. JoF 2021, 8, 8. [Google Scholar] [CrossRef]
- Frydenvang, J.; Van Maarschalkerweerd, M.; Carstensen, A.; Mundus, S.; Schmidt, S.B.; Pedas, P.R.; Laursen, K.H.; Schjoerring, J.K.; Husted, S. Sensitive Detection of Phosphorus Deficiency in Plants Using Chlorophyll a Fluorescence. Plant Physiol. 2015, 169, 353–361. [Google Scholar] [CrossRef]
- Carstensen, A.; Szameitat, A.E.; Frydenvang, J.; Husted, S. Chlorophyll a Fluorescence Analysis Can Detect Phosphorus Deficiency under Field Conditions and Is an Effective Tool to Prevent Grain Yield Reductions in Spring Barley (Hordeum vulgare L.). Plant Soil. 2019, 434, 79–91. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant Growth-Promoting Rhizobacteria Act as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Złoch, M.; Thiem, D.; Gadzała-Kopciuch, R.; Hrynkiewicz, K. Synthesis of Siderophores by Plant-Associated Metallotolerant Bacteria under Exposure to Cd2+. Chemosphere 2016, 156, 312–325. [Google Scholar] [CrossRef]
- Hoyerová, K.; Hošek, P. New Insights Into the Metabolism and Role of Cytokinin N-Glucosides in Plants. Front. Plant Sci. 2020, 11, 741. [Google Scholar] [CrossRef]
- Brito, L.F.; López, M.G.; Straube, L.; Passaglia, L.M.P.; Wendisch, V.F. Inorganic Phosphate Solubilization by Rhizosphere Bacterium Paenibacillus Sonchi: Gene Expression and Physiological Functions. Front. Microbiol. 2020, 11, 588605. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic Insights of Plant Growth Promoting Bacteria Mediated Drought and Salt Stress Tolerance in Plants for Sustainable Agriculture. IJMS 2022, 23, 3741. [Google Scholar] [CrossRef]
- Ikhajiagbe, B.; Anoliefo, G.O.; Olise, O.F.; Rackelmann, F.; Sommer, M.; Adekunle, I.J. Major Phosphorus in Soils Is Unavailable, yet Critical for Plant Development. Not. Sci. Biol. 2020, 12, 500–535. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving Phosphorus Use Efficiency: A Complex Trait with Emerging Opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Upadhayay, V.K.; Singh, A.V.; Khan, A.; Sharma, A. Contemplating the Role of Zinc-Solubilizing Bacteria in Crop Biofortification: An Approach for Sustainable Bioeconomy. Front. Agron. 2022, 4, 903321. [Google Scholar] [CrossRef]
- Yin, X.; Struik, P.C.; Romero, P.; Harbinson, J.; Evers, J.B.; Van Der Putten, P.E.L.; Vos, J. Using Combined Measurements of Gas Exchange and Chlorophyll Fluorescence to Estimate Parameters of a Biochemical C3 Photosynthesis Model: A Critical Appraisal and a New Integrated Approach Applied to Leaves in a Wheat (Triticum aestivum) Canopy. Plant Cell Environ. 2009, 32, 448–464. [Google Scholar] [CrossRef] [PubMed]


| Strains | Ammonia Production (µmoL/mL) | Zn Solubilization | IAA Production (µg/mL) | |
|---|---|---|---|---|
| ZnO | ZnCO3 | |||
| Pseudomonas soyae P1 | 3.3 ± 0.1 c | ++ | +++ | 44.1 ± 2 a |
| Pseudomonas reinekei P2 | 5.1 ± 0.2 b | ++ | ++ | 9.7 ± 0.1 cde |
| Pseudomonas koreensis P3 | 1.7 ± 0.1 d | +++ | +++ | 8.6 ± 0.06 de |
| Bacillus tropicus P4 | 3.01 ± 0.1 c | + | − | 7.5 ± 0.3 de |
| Bacillus paramycoides P5 | 9.1 ± 0.4 a | − | − | 9.7 ± 0.07 cde |
| Peribacillus frigolitolerans P6 | 4.8 ± 0.1 b | − | −v | 25.6 ± 10.3 b |
| Pseudomonas reinekei P7 | 5.6 ± 0.1 b | ++ | ++ | 17.2 ± 0.8 bcd |
| Pseudomonas vancouverenesis P8 | 5.6 ± 0.2 b | ++ | +++ | 24.8 ± 1.2 bc |
| Strains | Siderophores | HCN | Antifungal (Development Zone (cm)) | |
|---|---|---|---|---|
| F. culmorum Sucardu | F. culmorum P.V | |||
| Control | − | − | 8.5 ± 0.03 a | 6.5 ± 0.04 a |
| P. soyae P1 | + | +++ | 0 | 4.13 ± 0.11 d |
| P. reinekei P2 | +++ | +++ | 0 | 4.53 ± 0.04 c |
| P. koreensis P3 | + | +++ | 4.33 ± 0.11 c | 4.54 ± 0.04 c |
| B. tropicus P4 | + | +++ | 3.30 ± 0.13 d | 4.00 ± 0.06 d |
| B. paramycoide P5 | + | +++ | 1.66 ± 0.11 e | 5.53 ± 0.11 b |
| P. frigotolerans P6 | + | ++ | 1.76 ± 0.15 e | 4.63 ± 0.08 c |
| P. reinekei P7 | ++ | ++ | 4.33 ± 0.11 c | 4.13 ± 0.11 d |
| P. vancouverensi P8 | ++ | +++ | 5.06 ± 0.08 d | 4.3 ± 0.13 cd |
| Strains | Germination Rate (%) |
|---|---|
| Control | 30.76 d |
| P. soyae P1 | 53.84 ± 10.25 bcd |
| P. reinekei P2 | 79.48 ± 3.41 abc |
| P. koreensis P3 | 46.15 ± 10.25 cd |
| B. tropicus P4 | 87.17 ± 8.54 ab |
| B. paramycoides P5 | 61.53 ± 5.12 abcd |
| P. frigoritolerans P6 | 97.43 ± 3.41 a |
| P. reinekei P7 | 79.92 ± 11.96 abc |
| P. vancouverensis P8 | 76.92 ± 20.5 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourak, K.; Oulkhir, F.E.; Maghnia, F.Z.; Massart, S.; Biskri, L.; Jijakli, M.H.; Allaoui, A. A Comprehensive Approach Combining Short-Chain Polyphosphate and Bacterial Biostimulants for Effective Nutrient Solubilization and Enhanced Wheat Growth. Microorganisms 2024, 12, 1423. https://doi.org/10.3390/microorganisms12071423
Bourak K, Oulkhir FE, Maghnia FZ, Massart S, Biskri L, Jijakli MH, Allaoui A. A Comprehensive Approach Combining Short-Chain Polyphosphate and Bacterial Biostimulants for Effective Nutrient Solubilization and Enhanced Wheat Growth. Microorganisms. 2024; 12(7):1423. https://doi.org/10.3390/microorganisms12071423
Chicago/Turabian StyleBourak, Kaoutar, Fatima Ezzahra Oulkhir, Fatima Zahra Maghnia, Sebastien Massart, Latefa Biskri, M. Haissam Jijakli, and Abdelmounaaim Allaoui. 2024. "A Comprehensive Approach Combining Short-Chain Polyphosphate and Bacterial Biostimulants for Effective Nutrient Solubilization and Enhanced Wheat Growth" Microorganisms 12, no. 7: 1423. https://doi.org/10.3390/microorganisms12071423





