Presence and Role of the Type 3 Fimbria in the Adherence Capacity of Enterobacter hormaechei subsp. hoffmannii
Abstract
:1. Introduction
2. Materials and Methods
3. Results
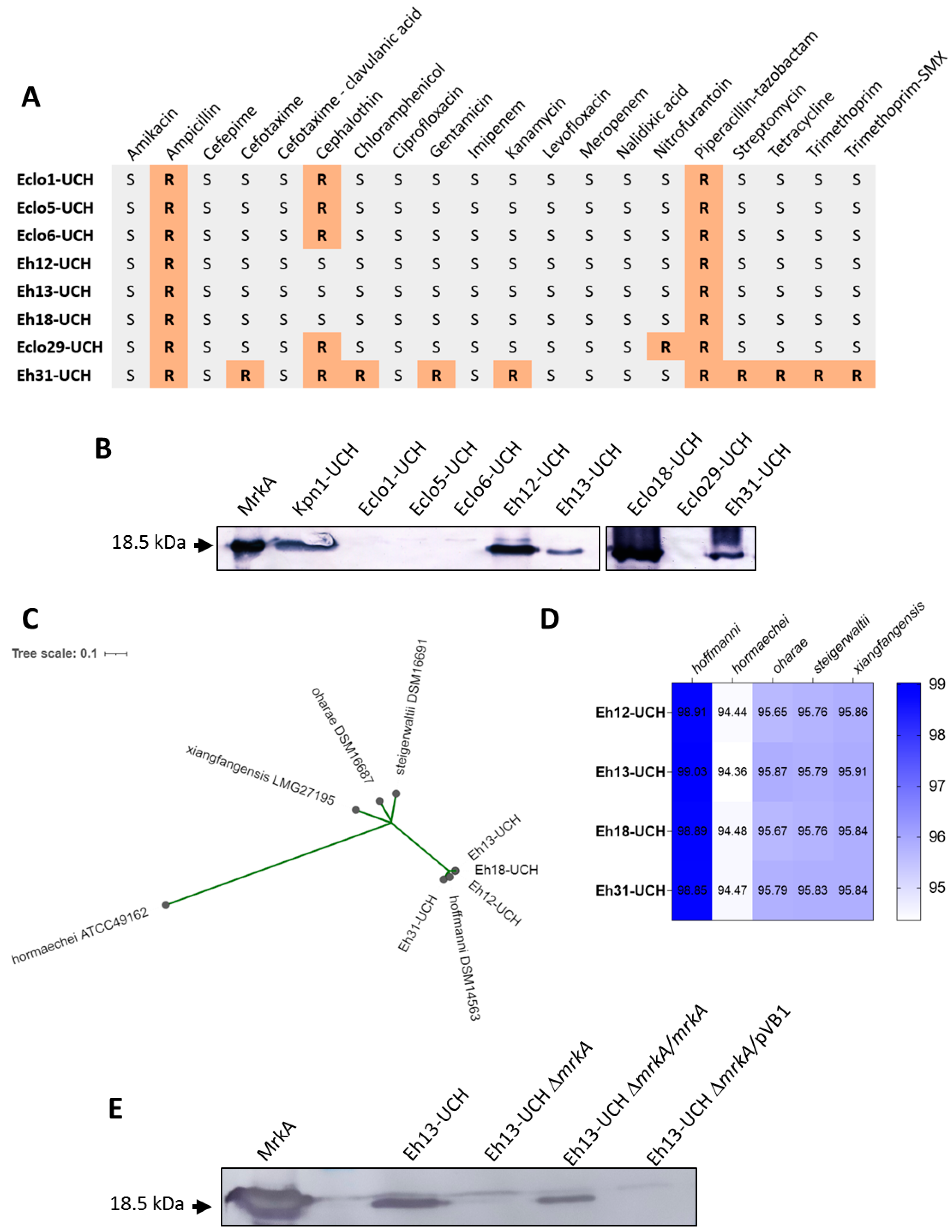
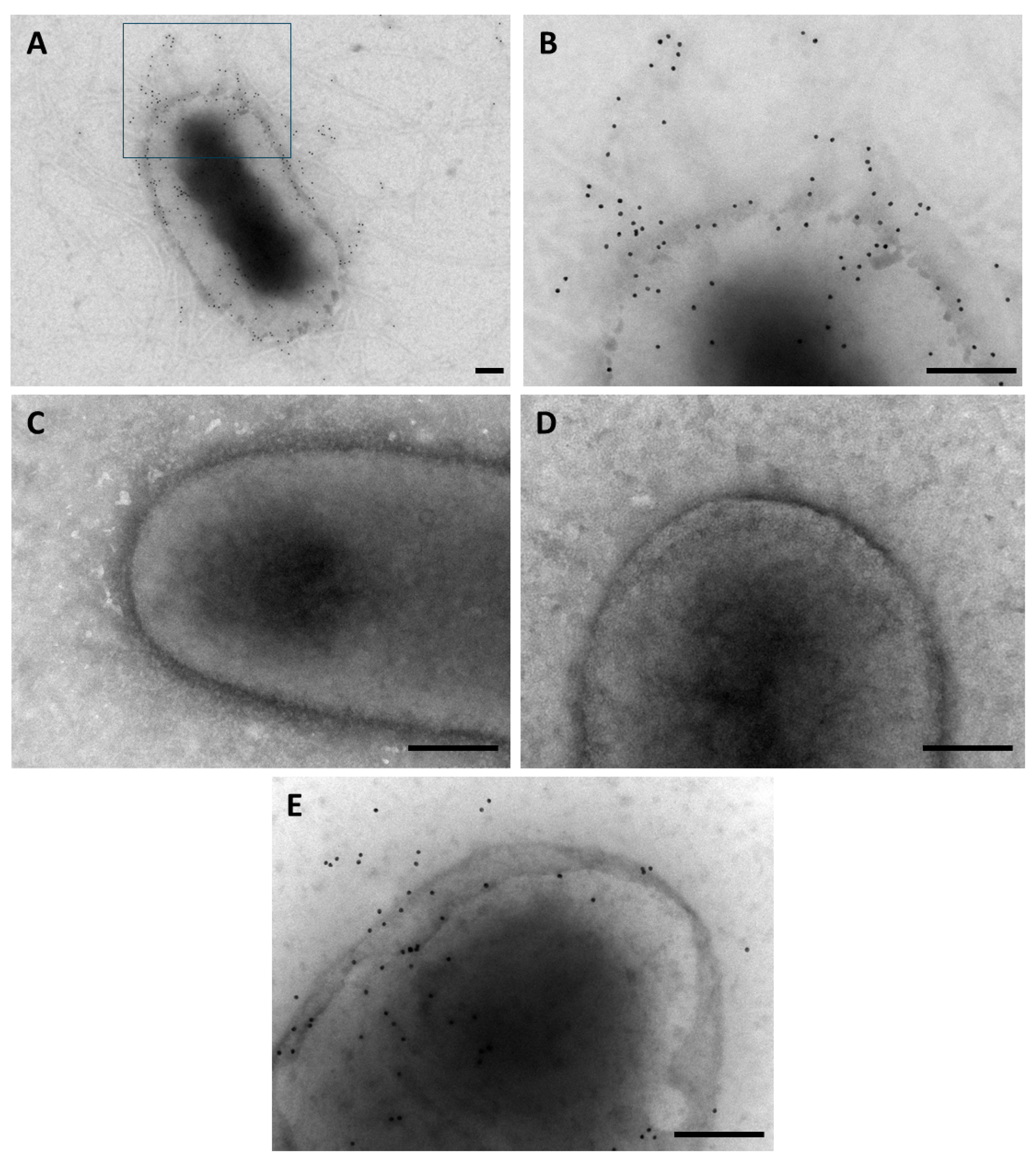
3.1. Production of Type 3 Fimbria by E. hormaechei subsp. hoffmannii
3.2. Role of the Type 3 Fimbria in Adherence Capacity of E. hormaechei subsp. hoffmannii Eh13-UCH Strain
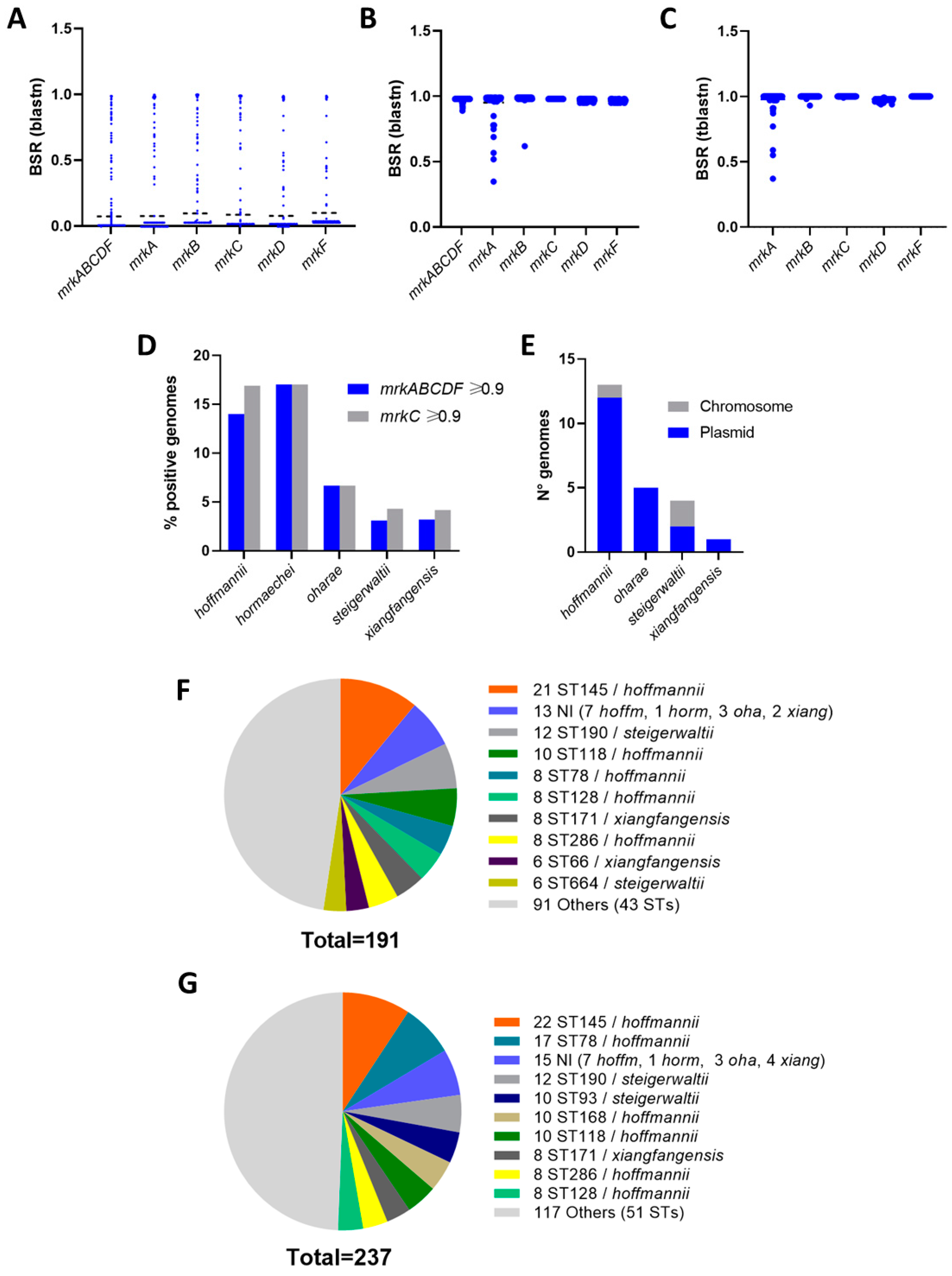
3.3. Distribution of the mrkABCDF Locus among E. hormaechei
4. Discussion
5. Conclusions
- The type 3 fimbria is an adherence determinant of Enterobacter hormaechei subsp. hoffmannii.
- The type 3 fimbria is uncommon among E. hormaechei, although it can be harbored by representatives of the five subspecies. E. hormaechei subsp. hoffmannii and E. hormaechei subsp. hormaechei displayed the highest positivity rates. Production of type 3 fimbria may confer fitness advantages regarding adherence and colonization capacities.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davin-Regli, A.; Lavigne, J.P.; Pagès, J.M. Enterobacter spp.: Update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin. Microbiol. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.R.; Bueno, M.F.C.; Francisco, G.R.; Casella, T.; de Oliveira Garcia, D.; Cerdeira, L.T.; Gerber, A.L.; de Almeida, L.G.P.; Lincopan, N.; de Vasconcelos, A.T.R.; et al. Genome and plasmid context of two rmtG-carrying Enterobacter hormaechei isolated from urinary tract infections in Brazil. J. Glob. Antimicrob. Resist. 2020, 20, 36–40. [Google Scholar] [CrossRef]
- da Silva, C.L.; Miranda, L.E.; Moreira, B.M.; Rebello, D.; Carson, L.A.; Kellum, M.E.; de Almeida, M.C.; Sampaio, J.L.; O’Hara, C.M. Enterobacter hormaechei bloodstream infection at three neonatal intensive care units in Brazil. Pediatr. Infect. Dis. J. 2002, 21, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Parte, A.C.; Sardà Carbasse, J.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, Y.; Zong, Z. Precise species identification for Enterobacter: A genome sequence-based study with reporting of two novel species, Enterobacter quasiroggenkampii sp. nov. and Enterobacter quasimori sp. nov. mSystems 2020, 5, e00527-20. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, F.G.; Corcione, S.; Pagani, N.; Di Perri, G. From ESKAPE to ESCAPE, from KPC to CCC. Clin. Infect. Dis. 2015, 60, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 17 May 2024).
- Campos, J.C.M.; Antunes, L.C.; Ferreira, R.B. Global priority pathogens: Virulence, antimicrobial resistance and prospective treatment options. Future Microbiol. 2020, 15, 649–677. [Google Scholar] [CrossRef]
- Gerlach, G.F.; Allen, B.L.; Clegg, S. Molecular characterization of the type 3 (MR/K) fimbriae of Klebsiella pneumoniae. J. Bacteriol. 1988, 170, 3547–3553. [Google Scholar] [CrossRef]
- Nuccio, S.P.; Bäumler, A.J. Evolution of the chaperone/usher assembly pathway: Fimbrial classification goes Greek. Microbiol. Mol. Biol. Rev. 2007, 71, 551–575. [Google Scholar] [CrossRef]
- Murphy, C.N.; Clegg, S. Klebsiella pneumoniae and type 3 fimbriae: Nosocomial infection, regulation and biofilm formation. Future Microbiol. 2012, 7, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Tennant, S.M.; Simon, R.; Cross, A.S. Progress towards the development of Klebsiella vaccines. Expert. Rev. Vaccines 2019, 18, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chang, C.S.; Pennini, M.; Pelletier, M.; Rajan, S.; Zha, J.; Chen, Y.; Cvitkovic, R.; Sadowska, A.; Heidbrink Thompson, J.; et al. Target-agnostic identification of functional monoclonal antibodies against Klebsiella pneumoniae multimeric MrkA fimbrial subunit. J. Infect. Dis. 2016, 213, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.L.; Beatson, S.A.; Totsika, M.; Forestier, C.; McEwan, A.G.; Schembri, M.A. Molecular analysis of type 3 fimbrial genes from Escherichia coli, Klebsiella and Citrobacter species. BMC Microbiol. 2010, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Old, D.C.; Adegbola, R.A. Antigenic relationships among type-3 fimbriae of Enterobacteriaceae revealed by immunoelectronmicroscopy. J. Med. Microbiol. 1985, 20, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Livrelli, V.; De Champs, C.; Di Martino, P.; Darfeuille-Michaud, A.; Forestier, C.; Joly, B. Adhesive properties and antibiotic resistance of Klebsiella, Enterobacter, and Serratia clinical isolates involved in nosocomial infections. J. Clin. Microbiol. 1996, 34, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Brust, F.R.; Boff, L.; da Silva Trentin, D.; Pedrotti Rozales, F.; Barth, A.L.; Macedo, A.J. Macrocolony of NDM-1 producing Enterobacter hormaechei subsp. oharae generates subpopulations with different features regarding the response of antimicrobial agents and biofilm formation. Pathogens 2019, 8, 49. [Google Scholar] [CrossRef]
- Gales, A.C.; Castanheira, M.; Jones, R.N.; Sader, H.S. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: Results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn. Microbiol. Infect. Dis. 2012, 73, 354–360. [Google Scholar] [CrossRef]
- Santiviago, C.A.; Reynolds, M.M.; Porwollik, S.; Choi, S.H.; Long, F.; Andrews-Polymenis, H.L.; McClelland, M. Analysis of pools of targeted Salmonella deletion mutants identifies novel genes affecting fitness during competitive infection in mice. PLoS Pathog. 2009, 5, e1000477. [Google Scholar] [CrossRef]
- Sharan, S.K.; Thomason, L.C.; Kuznetsov, S.G.; Court, D.L. Recombineering: A homologous recombination-based method of genetic engineering. Nat. Protoc. 2009, 4, 206–223. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing, M100, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 1 November 2022).
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bliss, C.M.; Bennett, J.S.; Bratcher, H.B.; Brehony, C.; Colles, F.M.; Wimalarathna, H.; Harrison, O.B.; Sheppard, S.K.; Cody, A.J.; et al. Ribosomal multilocus sequence typing: Universal characterization of bacteria from domain to strain. Microbiology 2012, 158, 1005–1015. [Google Scholar] [CrossRef]
- Gardner, S.N.; Slezak, T.; Hall, B.G. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics 2015, 31, 2877–2878. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Seemann, T. mlst. Github. Available online: https://github.com/tseemann/mlst (accessed on 1 May 2024).
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Ramos, P.I.; Picão, R.C.; Almeida, L.G.; Lima, N.C.; Girardello, R.; Vivan, A.C.; Xavier, D.E.; Barcellos, F.G.; Pelisson, M.; Vespero, E.C.; et al. Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC Genom. 2014, 15, 54. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Yáñez, V.; Suazo, P.; Hormazábal, C.; Ibaceta, V.; Arenas-Salinas, M.; Vidal, R.M.; Silva-Ojeda, F.; Arellano, C.; Muñoz, I.; Del Canto, F. Distribution of papA and papG variants among Escherichia coli genotypes: Association with major extraintestinal pathogenic lineages. Int. J. Mol. Sci. 2024, 25, 6657. [Google Scholar] [CrossRef]
- Wang, L.; Wu, P.; Su, Y.; Wei, Y.; Guo, X.; Yang, L.; Wang, M.; Liu, B. Detection of genus and three important species of Cronobacter using novel genus- and species-specific genes identified by large-scale comparative genomic analysis. Front. Microbiol. 2022, 13, 885543. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.R.; Buller, N.; Rachlin, A.; Golledge, C.; Sarovich, D.S.; Price, E.P.; Mayo, M.; Currie, B.J. A persisting nontropical focus of Burkholderia pseudomallei with limited genome evolution over five decades. mSystems 2020, 5, e00726-20. [Google Scholar] [CrossRef] [PubMed]
- Del Canto, F.; Botkin, D.J.; Valenzuela, P.; Popov, V.; Ruiz-Perez, F.; Nataro, J.P.; Levine, M.M.; Stine, O.C.; Pop, M.; Torres, A.G.; et al. Identification of Coli Surface Antigen 23, a novel adhesin of enterotoxigenic Escherichia coli. Infect. Immun. 2012, 80, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, B.; Dolgilevich, S.; Kalachikov, S.; Morozova, I.; Ju, J.; Whittier, S.; Nowygrod, R.; Kozarov, E. Cultivation of Enterobacter hormaechei from human atherosclerotic tissue. J. Atheroscler. Thromb. 2011, 18, 72–81. [Google Scholar] [CrossRef]
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Yeh, T.K.; Lin, H.J.; Liu, P.Y.; Wang, J.H.; Hsueh, P.R. Antibiotic resistance in Enterobacter hormaechei. Int. J. Antimicrob. Agents 2022, 60, 106650. [Google Scholar] [CrossRef]
- Xu, T.; Xue, C.X.; Huang, J.; Wu, J.; Chen, R.; Zhou, K. Emergence of an epidemic hypervirulent clone of Enterobacter hormaechei coproducing mcr-9 and carbapenemases. Lancet Microbe 2022, 3, e474–e475. [Google Scholar] [CrossRef]
- Knecht, C.A.; García Allende, N.; Álvarez, V.E.; Prack Mc Cormick, B.; Massó, M.G.; Campos, J.; Fox, B.; Alonso, F.M.; Donis, N.; Canigia, L.F.; et al. New sequence type of an Enterobacter cloacae complex strain with the potential to become a high-risk clone. J. Glob. Antimicrob. Resist. 2022, 31, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Donà, V.; Nordmann, P.; Kittl, S.; Schuller, S.; Bouvier, M.; Poirel, L.; Endimiani, A.; Perreten, V. Emergence of OXA-48-producing Enterobacter hormaechei in a Swiss companion animal clinic and their genetic relationship to clinical human isolates. J. Antimicrob. Chemother. 2023, 78, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Krachler, A.M.; Orth, K. Targeting the bacteria-host interface: Strategies in anti-adhesion therapy. Virulence 2013, 4, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.Y.V.; Taylor, P.K.; Brinkman, F.S.L.; Lee, A.H.Y. Pathogen-associated gene discovery workflows for novel antivirulence therapeutic development. eBioMedicine 2023, 88, 104429. [Google Scholar] [CrossRef] [PubMed]
- Puente, J.L.; Bieber, D.; Ramer, S.W.; Murray, W.; Schoolnik, G.K. The bundle-forming pili of enteropathogenic Escherichia coli: Transcriptional regulation by environmental signals. Mol. Microbiol. 1996, 20, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Kenny, B.; Abe, A.; Stein, M.; Finlay, B.B. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect. Immun. 1997, 65, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Tarkkanen, A.M.; Virkola, R.; Clegg, S.; Korhonen, T.K. Binding of the type 3 fimbriae of Klebsiella pneumoniae to human endothelial and urinary bladder cells. Infect. Immun. 1997, 65, 1546–1549. [Google Scholar] [CrossRef]
- Murphy, C.N.; Mortensen, M.S.; Krogfelt, K.A.; Clegg, S. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. Infect. Immun. 2013, 81, 3009–3017. [Google Scholar] [CrossRef]
- Emeraud, C.; Petit, C.; Gauthier, L.; Bonnin, R.A.; Naas, T.; Dortet, L. Emergence of VIM-producing Enterobacter cloacae complex in France between 2015 and 2018. J. Antimicrob. Chemother. 2022, 77, 944–951. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Nian, H.; Wang, R.; Li, F.; Jiang, N.; Chu, Y. Carbapenem-resistant Enterobacter cloacae complex in a tertiary Hospital in Northeast China, 2010–2019. BMC Infect. Dis. 2021, 21, 611. [Google Scholar] [CrossRef] [PubMed]
- Izdebski, R.; Baraniak, A.; Zabicka, D.; Sekowska, A.; Gospodarek-Komkowska, E.; Hryniewicz, W.; Gniadkowski, M. VIM/IMP carbapenemase-producing Enterobacteriaceae in Poland: Epidemic Enterobacter hormaechei and Klebsiella oxytoca lineages. J. Antimicrob. Chemother. 2018, 73, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Lu, P.L.; Jian, S.H.; Fu, H.L.; Huang, P.H.; Chang, C.Y. Molecular epidemiology, risk factors and clinical outcomes of carbapenem-nonsusceptible Enterobacter cloacae complex infections in a Taiwan University Hospital. Pathogens 2022, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Morhart, P.; Gerlach, R.G.; Kunz, C.; Held, J.; Valenza, G.; Wölfle, J.; Reutter, H.; Hanslik, G.J.; Fahlbusch, F.B. Application of next-generation sequencing to Enterobacter hormaechei subspecies analysis during a neonatal intensive care unit outbreak. Children 2023, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Ganbold, M.; Seo, J.; Wi, Y.M.; Kwon, K.T.; Ko, K.S. Species identification, antibiotic resistance, and virulence in Enterobacter cloacae complex clinical isolates from South Korea. Front. Microbiol. 2023, 14, 1122691. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Ouzani, S.; Emeraud, C.; Gauthier, L.; Bonnin, R.A.; Le Sache, N.; Mokhtari, M.; Langlois, I.; Begasse, C.; Arangia, N.; et al. Uncovering the novel Enterobacter cloacae complex species responsible for septic shock deaths in newborns: A cohort study. Lancet Microbe 2021, 2, e536–e544. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Zong, Z. Reexamining the association of AmpC variants with Enterobacter species in the context of updated taxonomy. Antimicrob. Agents Chemother. 2021, 65, e0159621. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alonso, E.; Bourgeois-Nicolaos, N.; Lepainteur, M.; Derouin, V.; Barreault, S.; Waalkes, A.; Augusto, L.A.; Gera, S.; Gleizes, O.; Tissieres, P.; et al. Contaminated incubators: Source of a multispecies Enterobacter outbreak of neonatal sepsis. Microbiol. Spectr. 2022, 10, e0096422. [Google Scholar] [CrossRef]
- Lumbreras-Iglesias, P.; de Toro, M.; Vázquez, X.; García-Carús, E.; Rodicio, M.R.; Fernández, J. High-risk international clones ST66, ST171 and ST78 of Enterobacter cloacae complex causing blood stream infections in Spain and carrying blaOXA-48 with or without mcr-9. J. Infect. Public. Health 2023, 16, 272–279. [Google Scholar] [CrossRef]
- Qiu, X.; Ye, K.; Ma, Y.; Zhao, Q.; Wang, L.; Yang, J. Genome sequence-based species classification of Enterobacter cloacae complex: A study among clinical isolates. Microbiol. Spectr. 2024, 12, e0431223. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]

| Strain | Specie/Complex | Origin/Illness | Utility | Reference |
|---|---|---|---|---|
| Eclo1-UCH | Enterobacter cloacae | Pneumonia | Detection of type 3 fimbria | This study |
| Eclo5-UCH | Enterobacter cloacae | Sepsis | Detection of type 3 fimbria | This study |
| Eclo6-UCH | Enterobacter cloacae | Sepsis | Detection of type 3 fimbria | This study |
| Eh12-UCH | Enterobacter hormaechei subsp. hoffmannii | Sepsis | Detection of type 3 fimbria | This study |
| Eh13-UCH | Enterobacter hormaechei subsp. hoffmannii | Sepsis | Detection of type 3 fimbria and functional analyses | This study |
| Eh18-UCH | Enterobacter hormaechei subsp. hoffmannii | Pneumonia | Detection of type 3 fimbria | This study |
| Eclo29-UCH | Enterobacter cloacae | Sepsis | Detection of type 3 fimbria | This study |
| Eh31-UCH | Enterobacter hormaechei subsp. hoffmannii | Sepsis | Detection of type 3 fimbria | This study |
| Kpn1-UCH | Klebsiella pneumoniae | Pneumonia | Detection of type 3 fimbria | This study |
| Eh13-UCHΔmrkA | Enterobacter hormaechei subsp. hoffmannii | Obtained after removal of the mrkA gene in Eh13-UCH | Detection of type 3 fimbria | This study |
| Eh13-UCHΔmrkA/pVB1 | Enterobacter hormaechei subsp. hoffmannii | Eh13-UCHΔmrkA transformed with the empty pVB1 plasmid | Detection of type 3 fimbria | This study |
| Eh13-UCHΔmrkA/mrkA | Enterobacter hormaechei subsp. hoffmannii | Eh13-UCHΔmrkA transformed with the pVB1 plasmid containing mrkA | Detection of type 3 fimbria | This study |
| Plasmids | ||||
| pCLF4 | - | - | Template to amplify the kanamycin resistance cassette used for allelic exchange | [20] |
| pSIM9 | - | - | Provision of the λ Red recombinase system | [21] |
| pVB1 | - | - | Expression plasmid, promoter inducible with m-tuolic acid | DualSystems Biotech, (Schlieren, Switzerland) |
| Strain | Sequence Type | Genome Length (bp) | N50 | Completeness (%) | Contamination (%) | NCBI Nucleotide Accession Code |
|---|---|---|---|---|---|---|
| Eh12-UCH | ST145 | 5,115,276 | 270,304 | 99.89 | 0.2 | JBCNUO000000000 |
| Eh13-UCH | ST145 | 4,744,088 | 309,640 | 99.89 | 0.2 | JBCNUP000000000 |
| Eh18-UCH | ST145 | 5,112,462 | 269,969 | 99.89 | 0.2 | JBCNUQ000000000 |
| Eh31-UCH | ST118 | 5,900,299 | 165,594 | 99.78 | 12.04 | JBCNUR000000000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Yáñez, V.; Ibaceta, V.; Torres, A.; Vidal, R.M.; Schneider, I.; Schilling, V.; Toro, C.; Arellano, C.; Scavone, P.; Muñoz, I.; et al. Presence and Role of the Type 3 Fimbria in the Adherence Capacity of Enterobacter hormaechei subsp. hoffmannii. Microorganisms 2024, 12, 1441. https://doi.org/10.3390/microorganisms12071441
Fernández-Yáñez V, Ibaceta V, Torres A, Vidal RM, Schneider I, Schilling V, Toro C, Arellano C, Scavone P, Muñoz I, et al. Presence and Role of the Type 3 Fimbria in the Adherence Capacity of Enterobacter hormaechei subsp. hoffmannii. Microorganisms. 2024; 12(7):1441. https://doi.org/10.3390/microorganisms12071441
Chicago/Turabian StyleFernández-Yáñez, Valentina, Valentina Ibaceta, Alexia Torres, Roberto M. Vidal, Isidora Schneider, Valeria Schilling, Cecilia Toro, Carolina Arellano, Paola Scavone, Ignacio Muñoz, and et al. 2024. "Presence and Role of the Type 3 Fimbria in the Adherence Capacity of Enterobacter hormaechei subsp. hoffmannii" Microorganisms 12, no. 7: 1441. https://doi.org/10.3390/microorganisms12071441







