Recruitment of Vitronectin by Bacterial Pathogens: A Comprehensive Overview
Abstract
1. Introduction
1.1. Vitronectin Structure and Physio/Pathological Role in the Host
1.2. Vitronectin as a Regulator of the Complement System
1.3. Vitronectin as a Mediator of Cell Migration and Adhesion
2. Bacterial Engagement of Vitronectin as a Weapon to Escape the Immune System
3. Bacterial Targeting of Vitronectin for Host Colonization
3.1. Gram-Positive Bacteria
3.2. Gram-Negative Bacteria
4. Vitronectin Binding by Bacteria with a Yet-to-Be-Defined Activity
5. Vitronectin Adsorption on Biomaterial Surfaces: A Double-Edged Weapon
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gibson, A.D.; Peterson, C.B. Full-length and truncated forms of vitronectin provide insight into effects of proteolytic processing on function. Biochim. Biophys. Acta 2001, 1545, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Hayman, E.G.; Pierschbacher, M.D.; Ohgren, Y.; Ruoslahti, E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc. Natl. Acad. Sci. USA 1983, 80, 4003–4007. [Google Scholar] [CrossRef]
- Zhou, A. Functional structure of the somatomedin B domain of vitronectin. Protein Sci. 2007, 16, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Huntington, J.A.; Pannu, N.S.; Carrell, R.W.; Read, R.J. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat. Struct. Biol. 2003, 10, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Waltz, D.A.; Rao, N.; Drummond, R.J.; Rosenberg, S.; Chapman, H.A. Identification of the urokinase receptor as an adhesion receptor for vitronectin. J. Biol. Chem. 1994, 269, 32380–32388. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, D.; Smith, J.W. The cell adhesion domain in plasma vitronectin is cryptic. J. Biol. Chem. 1997, 272, 13705–13710. [Google Scholar] [CrossRef] [PubMed]
- Gebb, C.; Hayman, E.G.; Engvall, E.; Ruoslahti, E. Interaction of vitronectin with collagen. J. Biol. Chem. 1986, 261, 16698–16703. [Google Scholar] [CrossRef]
- Chillakuri, C.R.; Jones, C.; Mardon, H.J. Heparin binding domain in vitronectin is required for oligomerization and thus enhances integrin mediated cell adhesion and spreading. FEBS Lett. 2010, 584, 3287–3291. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.T.; Barker, W.C.; Chen, H.R. A domain structure common to hemopexin, vitronectin, interstitial collagenase, and a collagenase homolog. Protein Seq. Data Anal. 1987, 1, 21–26. [Google Scholar]
- Tschopp, J.; Masson, D.; Schäfer, S.; Peitsch, M.; Preissner, K.T. The heparin binding domain of S-protein/vitronectin binds to complement components C7, C8, and C9 and perforin from cytolytic T-cells and inhibits their lytic activities. Biochemistry 1988, 27, 4103–4109. [Google Scholar] [CrossRef]
- Preissner, K.T. Specific binding of plasminogen to vitronectin. Evidence for a modulatory role of vitronectin on fibrin(ogen)-induced plasmin formation by tissue plasminogen activator. Biochem. Biophys. Res. Commun. 1990, 168, 966–971. [Google Scholar] [CrossRef]
- de Boer, H.C.; Preissner, K.T.; Bouma, B.N.; de Groot, P.G. Binding of vitronectin-thrombin-antithrombin III complex to human endothelial cells is mediated by the heparin binding site of vitronectin. J. Biol. Chem. 1992, 267, 2264–2268. [Google Scholar] [CrossRef]
- Gechtman, Z.; Belleli, A.; Lechpammer, S.; Shaltiel, S. The cluster of basic amino acids in vitronectin contributes to its binding of plasminogen activator inhibitor-1, evidence from thrombin-, elastase- and plasmin-cleaved vitronectins and anti-peptide antibodies. Biochem, J. 1997, 325, 339–349. [Google Scholar] [CrossRef]
- Stoop, A.A.; Lupu, F.; Pannekoek, H. Colocalization of thrombin, PAI-1, and vitronectin in the atherosclerotic vessel wall: A potential regulatory mechanism of thrombin activity by PAI-1/vitronectin complexes. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1143–1149. [Google Scholar] [CrossRef]
- Yoneda, A.; Ogawa, H.; Kojima, K.; Matsumoto, I. Characterization of the ligand binding activities of vitronectin: Interaction of vitronectin with lipids and identification of the binding domains for various ligands using recombinant domains. Biochemistry 1998, 37, 6351–6360. [Google Scholar] [CrossRef]
- Jenne, D.; Hille, A.; Stanley, K.K.; Huttner, W.B. Sulfation of two tyrosine-residues in human complement S-protein (vitronectin). Eur J Biochem. 1989, 185, 391–395. [Google Scholar] [CrossRef]
- Schvartz, I.; Seger, D.; Shaltiel, S. Vitronectin. Int. J. Biochem. Cell Biol. 1999, 31, 539–544. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.; Miao, H.; Sun, T.; Lu, Y.; Zhang, Y. Circulating Vitronectin Predicts Liver Injury and Mortality in Children With Sepsis: A Prospective Observational Study. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620935201. [Google Scholar] [CrossRef]
- Semeraro, N.; Ammollo, C.T.; Semeraro, F.; Colucci, M. Sepsis, thrombosis and organ dysfunction. Thromb. Res. 2012, 129, 290–295. [Google Scholar] [CrossRef]
- Bera, A.; Subramanian, M.; Karaian, J.; Eklund, M.; Radhakrishnan, S.; Gana, N.; Rothwell, S.; Pollard, H.; Hu, H.; Shriver, C.D.; et al. Functional role of vitronectin in breast cancer. PLoS ONE 2020, 15, e0242141. [Google Scholar] [CrossRef]
- Burgos-Panadero, R.; Noguera, I.; Cañete, A.; Navarro, S.; Noguera, R. Vitronectin as a molecular player of the tumor microenvironment in neuroblastoma. BMC Cancer 2019, 19, 479. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Westra, D.; Volokhina, E.B.; van der Molen, R.G.; van der Velden, T.J.A.M.; Jeronimus-Klaasen, A.; Goertz, J.; Gracchi, V.; Dorresteijn, E.M.; Bouts, A.H.M.; Keijzer-Veen, M.G.; et al. Serological and genetic complement alterations in infection-induced and complement-mediated hemolytic uremic syndrome. Pediatr. Nephrol. 2017, 32, 297–309. [Google Scholar] [CrossRef]
- Laarman, A.; Milder, F.; van Strijp, J.; Rooijakkers, S. Complement inhibition by gram-positive pathogens: Molecular mechanisms and therapeutic implications. J. Mol. Med. 2010, 88, 115–120. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Podack, E.R.; Preissner, K.T.; Müller-Eberhard, H.J. Inhibition of C9 polymerization within the SC5b-9 complex of complement by S-protein. Acta Pathol Microbiol Immunol Scand Suppl. 1984, 284, 89–96. [Google Scholar]
- Meredith, J.E., Jr.; Winitz, S.; Lewis, J.M.; Hess, S.; Ren, X.-D.; Renshaw, M.W.; Schwartz, M.A. The regulation of growth and intracellular signaling by integrins. Endocr. Rev. 1996, 17, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Mayasundari, A.; Whittemore, N.A.; Serpersu, E.H.; Peterson, C.B. The solution structure of the N-terminal domain of human vitronectin: Proximal sites that regulate fibrinolysis and cell migration. J. Biol. Chem. 2004, 279, 29359–29366. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Su, Y.C.; Riesbeck, K. Vitronectin in bacterial pathogenesis: A host protein used in complement escape and cellular invasion. Mol. Microbiol. 2010, 78, 545–560. [Google Scholar] [CrossRef]
- Hallström, T.; Singh, B.; Kraiczy, P.; Hammerschmidt, S.; Skerka, C.; Zipfel, P.F.; Riesbeck, K. Conserved Patterns of Microbial Immune Escape: Pathogenic Microbes of Diverse Origin Target the Human Terminal Complement Inhibitor Vitronectin via a Single Common Motif. PLoS ONE 2016, 11, e0147709. [Google Scholar] [CrossRef] [PubMed]
- Foxwell, A.R.; Kyd, J.M.; Cripps, A.W. Nontypeable Haemophilus influenzae: Pathogenesis and prevention. Microbiol. Mol. Biol. Rev. 1998, 62, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Hallström, T.; Blom, A.M.; Zipfel, P.F.; Riesbeck, K. Nontypeable Haemophilus influenzae protein E binds vitronectin and is important for serum resistance. J. Immunol. 2009, 183, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Jalalvand, F.; Mörgelin, M.; Zipfel, P.; Blom, A.M.; Riesbeck, K. Haemophilus influenzae protein E recognizes the C-terminal domain of vitronectin and modulates the membrane attack complex. Mol. Microbiol. 2011, 81, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Jalalvand, F.; Mörgelin, M.; Blom, A.M.; Singh, B.; Riesbeck, K. Haemophilus influenzae acquires vitronectin via the ubiquitous Protein F to subvert host innate immunity. Mol. Microbiol. 2013, 87, 1245–1266. [Google Scholar] [CrossRef]
- Singh, B.; Al-Jubair, T.; Mörgelin, M.; Thunnissen, M.M.; Riesbeck, K. The unique structure of Haemophilus influenzae protein E reveals multiple binding sites for host factors. Infect. Immun. 2013, 81, 801–814. [Google Scholar] [CrossRef][Green Version]
- Jalalvand, F.; Su, Y.-C.; Mörgelin, M.; Brant, M.; Hallgren, O.; Westergren-Thorsson, G.; Singh, B.; Riesbeck, K. Haemophilus influenzae protein F mediates binding to laminin and human pulmonary epithelial cells. J. Infect. Dis. 2013, 207, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Al-Jubair, T.; Mukherjee, O.; Oosterhuis, S.; Singh, B.; Su, Y.-C.; Fleury, C.; Blom, A.M.; Törnroth-Horsefield, S.; Riesbeck, K. Haemophilus influenzae Type f Hijacks Vitronectin Using Protein H To Resist Host Innate Immunity and Adhere to Pulmonary Epithelial Cells. J. Immunol. 2015, 195, 5688–5695. [Google Scholar] [CrossRef]
- Linke, D.; Riess, T.; Autenrieth, I.B.; Lupas, A.; Kempf, V.A. Trimeric autotransporter adhesins: Variable structure, common function. Trends Microbiol. 2006, 14, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Su, Y.-C.; Al-Jubair, T.; Mukherjee, O.; Hallström, T.; Mörgelin, M.; Blom, A.M.; Riesbeck, K. A fine-tuned interaction between trimeric autotransporter haemophilus surface fibrils and vitronectin leads to serum resistance and adherence to respiratory epithelial cells. Infect. Immun. 2014, 82, 2378–2389. [Google Scholar] [CrossRef][Green Version]
- Attia, A.S.; Ram, S.; Rice, P.A.; Hansen, E.J. Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade. Infect. Immun. 2006, 74, 1597–1611. [Google Scholar] [CrossRef]
- Singh, B.; Blom, A.M.; Unal, C.; Nilson, B.; Mörgelin, M.; Riesbeck, K. Vitronectin binds to the head region of Moraxella catarrhalis ubiquitous surface protein A2 and confers complement-inhibitory activity. Mol. Microbiol. 2010, 75, 1426–1444. [Google Scholar] [CrossRef]
- Su, Y.C.; Hallström, B.M.; Bernhard, S.; Singh, B.; Riesbeck, K. Impact of sequence diversity in the Moraxella catarrhalis UspA2/UspA2H head domain on vitronectin binding and antigenic variation. Microbes Infect. 2013, 15, 375–387. [Google Scholar] [CrossRef]
- Riley, S.P.; Patterson, J.L.; Nava, S.; Martinez, J.J. Pathogenic Rickettsia species acquire vitronectin from human serum to promote resistance to complement-mediated killing. Cell Microbiol. 2014, 16, 849–861. [Google Scholar] [CrossRef][Green Version]
- Fish, A.I.; Riley, S.P.; Singh, B.; Riesbeck, K.; Martinez, J.J. The Rickettsia conorii Adr1 Interacts with the C-Terminus of Human Vitronectin in a Salt-Sensitive Manner. Front. Cell Infect. Microbiol. 2017, 7, 61. [Google Scholar] [CrossRef]
- Sa, E.; Cunha, C.; Griffiths, N.J.; Virji, M. Neisseria meningitidis Opc invasin binds to the sulphated tyrosines of activated vitronectin to attach to and invade human brain endothelial cells. PLoS Pathog. 2010, 6, e1000911. [Google Scholar] [CrossRef]
- Andreae, C.A.; Sessions, R.B.; Virji, M.; Hill, D.J. Bioinformatic analysis of meningococcal Msf and Opc to inform vaccine antigen design. PLoS ONE 2018, 13, e0193940. [Google Scholar] [CrossRef]
- Hill, D.J.; Griffiths, N.J.; Borodina, E.; Andreae, C.A.; Sessions, R.B.; Virji, M. Identification and therapeutic potential of a vitronectin binding region of meningococcal msf. PLoS ONE 2015, 10, e0124133. [Google Scholar] [CrossRef]
- Krukonis, E.S.; Thomson, J.J. Complement evasion mechanisms of the systemic pathogens Yersiniae and Salmonellae. FEBS Lett. 2020, 594, 2598–2620. [Google Scholar] [CrossRef]
- Mühlenkamp, M.C.; Hallström, T.; Autenrieth, I.B.; Bohn, E.; Linke, D.; Rinker, J.; Riesbeck, K.; Singh, B.; Leo, J.C.; Hammerschmidt, S.; et al. Vitronectin Binds to a Specific Stretch within the Head Region of Yersinia Adhesin A and Thereby Modulates Yersinia enterocolitica Host Interaction. J. Innate Immun. 2017, 9, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Meuskens, I.; Leva-Bueno, J.; Millner, P.; Schütz, M.; Peyman, S.A.; Linke, D. The Trimeric Autotransporter Adhesin YadA of Yersinia enterocolitica Serotype O:9 Binds Glycan Moieties. Front. Microbiol. 2022, 12, 738818. [Google Scholar] [CrossRef] [PubMed]
- Bartra, S.S.; Ding, Y.; Fujimoto, L.M.; Ring, J.G.; Jain, V.; Ram, S.; Marassi, F.M.; Plano, G.V. Yersinia pestis uses the Ail outer membrane protein to recruit vitronectin. Microbiology 2015, 161, 2174–2183. [Google Scholar] [CrossRef]
- Shin, K.; Lechtenberg, B.C.; Fujimoto, L.M.; Yao, Y.; Bartra, S.S.; Plano, G.V.; Marassi, F.M. Structure of human Vitronectin C-terminal domain and interaction with Yersinia pestis outer membrane protein Ail. Sci. Adv. 2019, 5, eaax5068. [Google Scholar] [CrossRef]
- Richter, C.; Mukherjee, O.; Ermert, D.; Singh, B.; Su, Y.-C.; Agarwal, V.; Blom, A.M.; Riesbeck, K. Moonlighting of Helicobacter pylori catalase protects against complement-mediated killing by utilising the host molecule vitronectin. Sci. Rep. 2016, 6, 24391. [Google Scholar] [CrossRef]
- Hallström, T.; Uhde, M.; Singh, B.; Skerka, C.; Riesbeck, K.; Zipfel, P.F. Pseudomonas aeruginosa Uses Dihydrolipoamide Dehydrogenase (Lpd) to Bind to the Human Terminal Pathway Regulators Vitronectin and Clusterin to Inhibit Terminal Pathway Complement Attack. PLoS ONE 2015, 10, e0137630. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Yang, R.; Zhu, X.; Bai, H.; Deng, X.; Bai, J.; Zhang, Y.; Xiao, Y.; Li, Z.; et al. Outer membrane protein OMP76 of Riemerella anatipestifer contributes to complement evasion and virulence by binding to duck complement factor vitronectin. Virulence 2023, 14, 2223060. [Google Scholar] [CrossRef]
- da Silva, L.B.; Miragaia, L.d.S.; Breda, L.C.D.; Abe, C.M.; Schmidt, M.C.B.; Moro, A.M.; Monaris, D.; Conde, J.N.; Józsi, M.; Isaac, L.; et al. Pathogenic Leptospira species acquire factor H and vitronectin via the surface protein LcpA. Infect. Immun. 2015, 83, 888–897. [Google Scholar] [CrossRef]
- ElTahir, Y.; Al-Araimi, A.; Nair, R.R.; Autio, K.J.; Tu, H.; Leo, J.C.; Al-Marzooqi, W.; Johnson, E.H. Correction to: Binding of Brucella protein, Bp26, to select extracellular matrix molecules. BMC Mol. Cell Biol. 2020, 21, 16. [Google Scholar] [CrossRef]
- Sato, K.; Kumagai, Y.; Sekizuka, T.; Kuroda, M.; Hayashi, T.; Takano, A.; Gaowa; Taylor, K.R.; Ohnishi, M.; Kawabata, H. Vitronectin binding protein, BOM1093, confers serum resistance on Borrelia miyamotoi. Sci. Rep. 2021, 11, 5462. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, B.; Yu, Y.; Yuan, T.; Wei, Y.; Gan, Y.; Shao, J.; Shao, G.; Feng, Z.; et al. DnaK Functions as a Moonlighting Protein on the Surface of Mycoplasma hyorhinis Cells. Front. Microbiol. 2022, 13, 842058. [Google Scholar] [CrossRef]
- Leduc, I.; Olsen, B.; Elkins, C. Localization of the domains of the Haemophilus ducreyi trimeric autotransporter DsrA involved in serum resistance and binding to the extracellular matrix proteins fibronectin and vitronectin. Infect. Immun. 2009, 77, 657–666. [Google Scholar] [CrossRef]
- Kohler, S.; Hallström, T.; Singh, B.; Riesbeck, K.; Spartà, G.; Zipfel, P.F.; Hammerschmidt, S. Binding of vitronectin and Factor H to Hic contributes to immune evasion of Streptococcus pneumoniae serotype 3. Thromb. Haemost. 2015, 113, 125–142. [Google Scholar] [CrossRef]
- Voss, S.; Hallström, T.; Saleh, M.; Burchhardt, G.; Pribyl, T.; Singh, B.; Riesbeck, K.; Zipfel, P.F.; Hammerschmidt, S. The choline-binding protein PspC of Streptococcus pneumoniae interacts with the C-terminal heparin-binding domain of vitronectin. J. Biol. Chem. 2013, 288, 15614–15627. [Google Scholar] [CrossRef] [PubMed]
- Filippsen, L.F.; Valentin-Weigand, P.; Blobel, H.; Preissner, K.T.; Chhatwal, G.S. Role of complement S protein (vitronectin) in adherence of Streptococcus dysgalactiae to bovine epithelial cells. Am. J. Vet. Res. 1990, 51, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Liang, O.D.; Preissner, K.T.; Chhatwal, G.S. The hemopexin-type repeats of human vitronectin are recognized by Streptococcus pyogenes. Biochem. Biophys. Res. Commun. 1997, 234, 445–449. [Google Scholar] [CrossRef]
- Esgleas, M.; Lacouture, S.; Gottschalk, M. Streptococcus suis serotype 2 binding to extracellular matrix proteins. FEMS Microbiol. Lett. 2005, 244, 33–40. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.V.; Pietrocola, G.; Romeo, L.; Galbo, R.; Lentini, G.; Giardina, M.; Biondo, C.; Midiri, A.; Mancuso, G.; Venza, M.; et al. The Streptococcus agalactiae cell wall-anchored protein PbsP mediates adhesion to and invasion of epithelial cells by exploiting the host vitronectin/αv integrin axis. Mol. Microbiol. 2018, 110, 82–94. [Google Scholar] [CrossRef]
- Heilmann, C.; Hussain, M.; Peters, G.; Götz, F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 1997, 24, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Kohler, T.P.; Gisch, N.; Binsker, U.; Schlag, M.; Darm, K.; Völker, U.; Zähringer, U.; Hammerschmidt, S. Repeating structures of the major staphylococcal autolysin are essential for the interaction with human thrombospondin 1 and vitronectin. J. Biol. Chem. 2014, 289, 4070–4082. [Google Scholar] [CrossRef]
- Heilmann, C.; Thumm, G.; Chhatwal, G.S.; Hartleib, J.; Uekötter, A.; Peters, G. Identification and characterization of a novel autolysin (Aae) with adhesive properties from Staphylococcus epidermidis. Microbiology 2003, 149, 2769–2778. [Google Scholar] [CrossRef]
- Biswas, R.; Voggu, L.; Simon, U.K.; Hentschel, P.; Thumm, G.; Götz, F. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 2006, 259, 260–268. [Google Scholar] [CrossRef]
- Pathak, H.; Sokkalingam, M.; Prasanth, L.; Devi, K.; Joshi, P. Staphylococcus aureus autolysins interact with caprine vitronectin without involving the heparin binding domain and the second arginine-glycine-aspartic acid motif of the host protein. Arch. Microbiol. 2019, 201, 639–647. [Google Scholar] [CrossRef]
- Pietrocola, G.; Pellegrini, A.; Alfeo, M.J.; Marchese, L.; Foster, T.J.; Speziale, P. The iron-regulated surface determinant B (IsdB) protein from Staphylococcus aureus acts as a receptor for the host protein vitronectin. J. Biol. Chem. 2020, 295, 10008–10022. [Google Scholar] [CrossRef]
- Mora-Uribe, P.; Miranda-Cárdenas, C.; Castro-Córdova, P.; Gil, F.; Calderón, I.; Fuentes, J.A.; Rodas, P.I.; Banawas, S.; Sarker, M.R.; Paredes-Sabja, D. Characterization of the Adherence of Clostridium difficile Spores: The Integrity of the Outermost Layer Affects Adherence Properties of Spores of the Epidemic Strain R20291 to Components of the Intestinal Mucosa. Front. Cell Infect. Microbiol. 2016, 6, 99. [Google Scholar] [CrossRef]
- Brooks, M.J.; Sedillo, J.L.; Wagner, N.; Laurence, C.A.; Wang, W.; Attia, A.S.; Hansen, E.J.; Gray-Owen, S.D. Modular arrangement of allelic variants explains the divergence in Moraxella catarrhalis UspA protein function. Infect. Immun. 2008, 76, 5330–5340. [Google Scholar] [CrossRef]
- Paulsson, M.; Che, K.F.; Ahl, J.; Tham, J.; Sandblad, L.; Smith, M.E.; Qvarfordt, I.; Su, Y.-C.; Lindén, A.; Riesbeck, K. Bacterial Outer Membrane Vesicles Induce Vitronectin Release Into the Bronchoalveolar Space Conferring Protection From Complement-Mediated Killing. Front. Microbiol. 2018, 9, 1559. [Google Scholar] [CrossRef]
- Brown, E.J. Interaction of gram-positive microorganisms with complement. Curr. Top. Microbiol. Immunol. 1985, 121, 159–187. [Google Scholar] [CrossRef]
- Janulczyk, R.; Iannelli, F.; Sjoholm, A.G.; Pozzi, G.; Bjorck, L. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 2000, 275, 37257–37263. [Google Scholar] [CrossRef]
- Adegbola, R.A. Bacterial adhesion and pathogenicity. Afr. J. Med. Med. Sci. 1988, 17, 63–69. [Google Scholar]
- Speziale, P.; Pietrocola, G.; Rindi, S.; Provenzano, M.; Provenza, G.; Di Poto, A.; Visai, L.; Arciola, C.R. Structural and functional role of Staphylococcus aureus surface components recognizing adhesive matrix molecules of the host. Future Microbiol. 2009, 4, 1337–1352. [Google Scholar] [CrossRef]
- Scibelli, A.; Roperto, S.; Manna, L.; Pavone, L.M.; Tafuri, S.; Della Morte, R.; Staiano, N. Engagement of integrins as a cellular route of invasion by bacterial pathogens. Vet. J. 2007, 173, 482–491. [Google Scholar] [CrossRef]
- Valentin-Weigand, P.; Grulich-Henn, J.; Chhatwal, G.S.; Müller-Berghaus, G.; Blobel, H.; Preissner, K.T. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin). Infect Immun. 1988, 56, 2851–2855. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Lang, A.; Rohde, M.; Agarwal, V.; Rennemeier, C.; Grashoff, C.; Preissner, K.T.; Hammerschmidt, S. Integrin-linked kinase is required for vitronectin-mediated internalization of Streptococcus pneumoniae by host cells. J. Cell Sci. 2009, 122, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Buscetta, M.; Firon, A.; Pietrocola, G.; Biondo, C.; Mancuso, G.; Midiri, A.; Romeo, L.; Galbo, R.; Venza, M.; Venza, I.; et al. PbsP, a cell wall-anchored protein that binds plasminogen to promote hematogenous dissemination of group B Streptococcus. Mol. Microbiol. 2016, 101, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Coates, R.; Moran, J.; Horsburgh, M.J. Staphylococci: Colonizers and pathogens of human skin. Future Microbiol. 2014, 9, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Chhatwal, G.S.; Preissner, K.T.; Müller-Berghaus, G.; Blobel, H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect. Immun. 1987, 55, 1878–1883. [Google Scholar] [CrossRef]
- Paulsson, M.; Wadström, T. Vitronectin and type-I collagen binding by Staphylococcus aureus and coagulase-negative staphylococci. FEMS Microbiol. Immunol. 1990, 2, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Lundberg, F.; Ljungh, A. Characterization of vitronectin-binding proteins of Staphylococcus epidermidis. Curr. Microbiol. 2001, 42, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zoll, S.; Schlag, M.; Shkumatov, A.V.; Rautenberg, M.; Svergun, D.I.; Götz, F.; Stehle, T. Ligand-binding properties and conformational dynamics of autolysin repeat domains in staphylococcal cell wall recognition. J. Bacteriol. 2012, 194, 3789–3802. [Google Scholar] [CrossRef]
- Mathelié-Guinlet, M.; Viela, F.; Pietrocola, G.; Speziale, P.; Dufrêne, Y.F. Nanonewton forces between Staphylococcus aureus surface protein IsdB and vitronectin. Nanoscale Adv. 2020, 2, 5728–5736. [Google Scholar] [CrossRef]
- Ikeda, M.; Enomoto, N.; Hashimoto, D.; Fujisawa, T.; Inui, N.; Nakamura, Y.; Suda, T.; Nagata, T. Nontypeable Haemophilus influenzae exploits the interaction between protein-E and vitronectin for the adherence and invasion to bronchial epithelial cells. BMC Microbiol. 2015, 15, 263. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, X.; He, Y.; Jiang, L.; Zhang, S.; Bartra, S.S.; Plano, G.V.; Klena, J.D.; Skurnik, M.; Chen, H.; et al. Invasiveness of the Yersinia pestis ail protein contributes to host dissemination in pneumonic and oral plague. Microb. Pathog. 2020, 141, 103993. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.C. Pseudomonas aeruginosa in cystic fibrosis: Pathogenesis and persistence. Paediatr. Respir. Rev. 2002, 3, 128–134. [Google Scholar] [CrossRef]
- Leroy-Dudal, J.; Gagnière, H.; Cossard, E.; Carreiras, F.; Di Martino, P. Role of alphavbeta5 integrins and vitronectin in Pseudomonas aeruginosa PAK interaction with A549 respiratory cells. Microbes Infect. 2004, 6, 875–881. [Google Scholar] [CrossRef]
- Abdallah, M.-N.; Tran, S.D.; Abughanam, G.; Laurenti, M.; Zuanazzi, D.; Mezour, M.A.; Xiao, Y.; Cerruti, M.; Siqueira, W.L.; Tamimi, F. Biomaterial surface proteomic signature determines interaction with epithelial cells. Acta Biomater. 2017, 54, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hao, L.; Li, J.; Du, C.; Wang, Y. Insight into vitronectin structural evolution on material surface chemistries: The mediation for cell adhesion. Bioact. Mater. 2020, 5, 1044–1052. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Reis, A.C.D. Bacterial adhesion to biomaterials: What regulates this attachment? A review. Jpn. Dent. Sci. Rev. 2021, 57, 85–96. [Google Scholar] [CrossRef]
- Hessenauer, M.E.; Lauber, K.; Zuchtriegel, G.; Uhl, B.; Hussain, T.; Canis, M.; Strieth, S.; Berghaus, A.; Reichel, C.A. Vitronectin promotes the vascularization of porous polyethylene biomaterials. Acta Biomater. 2018, 82, 24–33. [Google Scholar] [CrossRef]
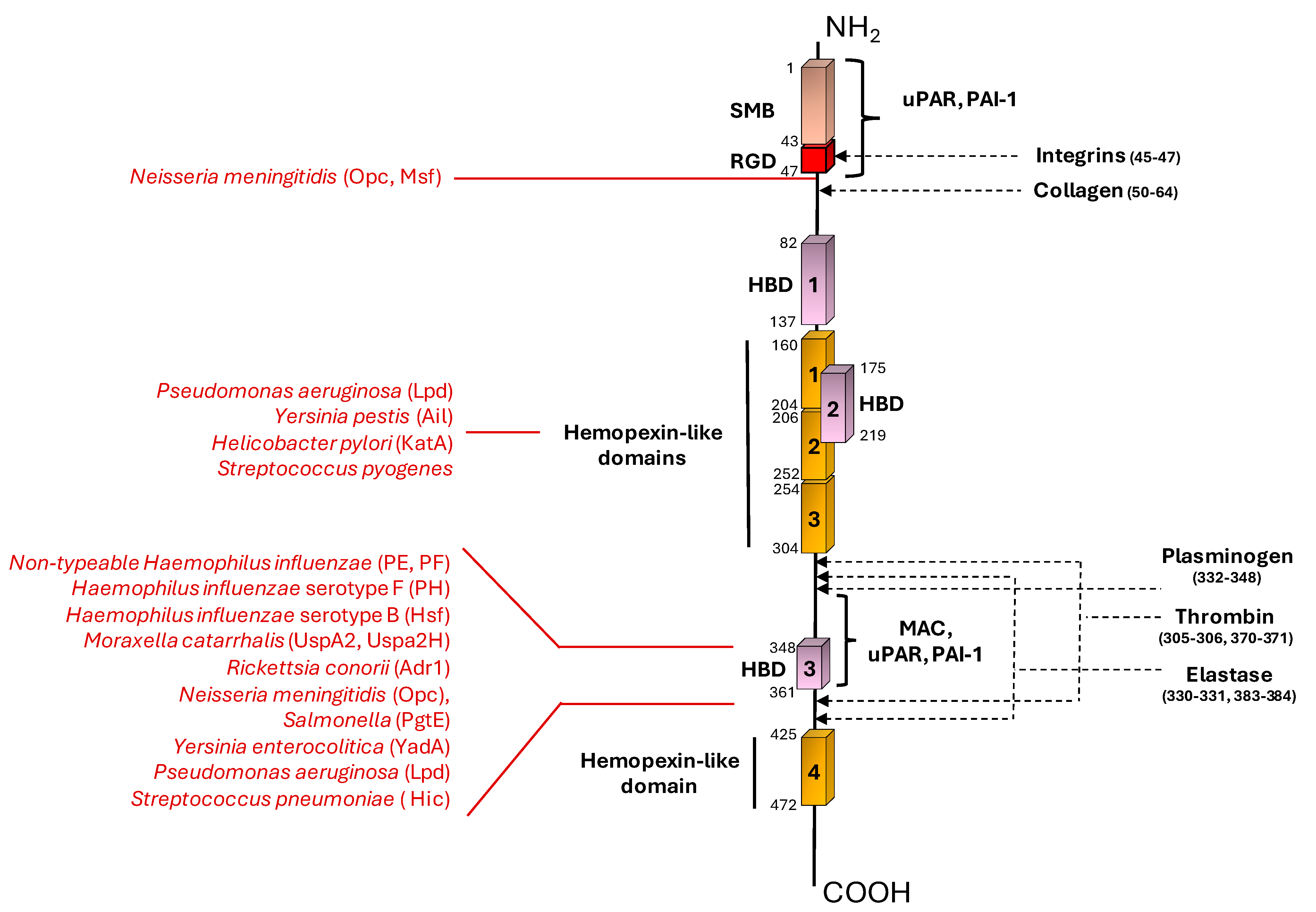
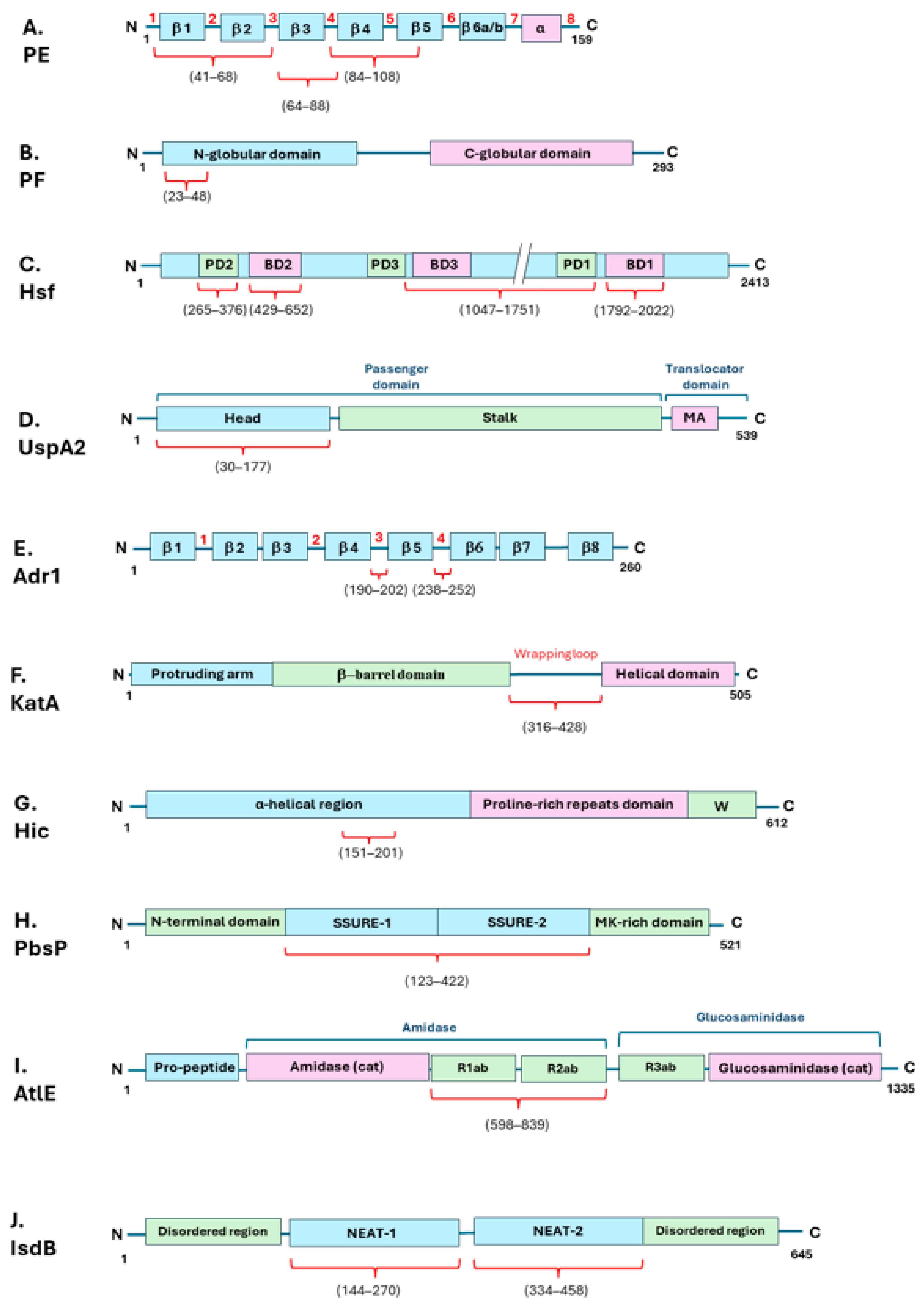
| Bacterial Species | Bacterial Protein Interacting with Vn | Bacterial Protein Region Involved in Vn Binding (Amino Acid Residues) | Vn Region Bound (Amino Acid Residues) | Role in Complement Evasion | Role in Cell Adhesion/Invasion | Ref. |
|---|---|---|---|---|---|---|
| Gram-negative | ||||||
| Nontypeable Haemophilus influenzae | PE | 84–108, 41–68, 64–88, | HBD-3 (353–363) | + | U | [33,35] |
| “ | PF | 23–48 | HBD-3 (348–361), PAI-1 binding site (348–370) | + | U | [34,36] |
| Haemophilus influenzae serotype F | PH | U | C-terminal (352–362) | + | U | [37] |
| Haemophilus influenzae serotype B | Hsf | 265–376, 429–652, 1047–1751, 1792–2022 | C-terminal (352–374) | + | + | [38,39] |
| Moraxella catarrhalis | UspA2 | 30–177 | HBD-3 (312–396) | + | U | [40,41,42] |
| “ | UspA2H | 30–177 | HBD-3 (312–396) | + | U | [42] |
| Rickettsia conorii | Adr1 | 190–202, 238–252 | C-terminal (363–373) | + | U | [43,44] |
| Neisseria meningititis | Opc | U | N-terminal (43–68), HBD-3 | + | + | [45] |
| “ | Msf | 39–82 | N-terminal (43–68) | + | U | [46,47] |
| Salmonella | Pgte | U | C-terminal | + | U | [48] |
| Yersinia enterocolitica | YadA | Head domain | HBD-3 | + | U | [49,50] |
| Yersinia pestis | Ail | U | Hemopexin domain | + | U | [51,52] |
| Helicobacter pylori | KatA | 316–428 | C-terminal (229–339) | + | U | [53] |
| Pseudomonas aeruginosa | LpD | U | C-terminal (354–363) Hemopexin-like repeats (161–287) | + | U | [54] |
| Riemerella anatipestifer | OMP76 | U | U | + | U | [55] |
| Leptospira interrogans | LcpA | U | HBD (s) | + | U | [56] |
| Brucella | Bp26 | 46–65, 96–115, 146–160, 176–190, 231–250 | U | U | U | [57] |
| Borrelia miyamotoi | BOM1093 | 209–308 | U | + | U | [58] |
| Mycoplasma hyorhinis | DnaK | U | U | U | + | [59] |
| Haemophilus ducreyi | DsrA | C-terminal passenger domain | U | U | U | [60] |
| Gram-positive bacteria | ||||||
| Streptococcus pneumoniae | Hic | 151–201 | C-terminal (HBD-3) | + | U | [61,62] |
| Streptococcus dysagalactiae | U | U | U | U | + | [63] |
| Streptococcus pyogenes | U | U | Hemopexin-type repeats | U | U | [64] |
| Streptococcus suis | U | U | U | U | U | [65] |
| Streptococcus agalactiae | PbsP | 123–422 | U | U | + | [66] |
| Staphylococcus epidermidis | AtlE | 598–839 | U | U | + | [67,68] |
| “ | Aae | U | U | U | U | [69] |
| Staphylococcus aureus | AtlA | U | U | U | U | [70,71] |
| “ | IsdB | 144–270, 334–458 | HBD (s) | U | + | [72] |
| Clostridioides difficile | BclA3 | U | U | U | + | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrini, A.; Pietrocola, G. Recruitment of Vitronectin by Bacterial Pathogens: A Comprehensive Overview. Microorganisms 2024, 12, 1385. https://doi.org/10.3390/microorganisms12071385
Pellegrini A, Pietrocola G. Recruitment of Vitronectin by Bacterial Pathogens: A Comprehensive Overview. Microorganisms. 2024; 12(7):1385. https://doi.org/10.3390/microorganisms12071385
Chicago/Turabian StylePellegrini, Angelica, and Giampiero Pietrocola. 2024. "Recruitment of Vitronectin by Bacterial Pathogens: A Comprehensive Overview" Microorganisms 12, no. 7: 1385. https://doi.org/10.3390/microorganisms12071385
APA StylePellegrini, A., & Pietrocola, G. (2024). Recruitment of Vitronectin by Bacterial Pathogens: A Comprehensive Overview. Microorganisms, 12(7), 1385. https://doi.org/10.3390/microorganisms12071385






