Indolizidines from Actinomycetes: An Overview of Producers, Biosynthesis and Bioactivities
Abstract
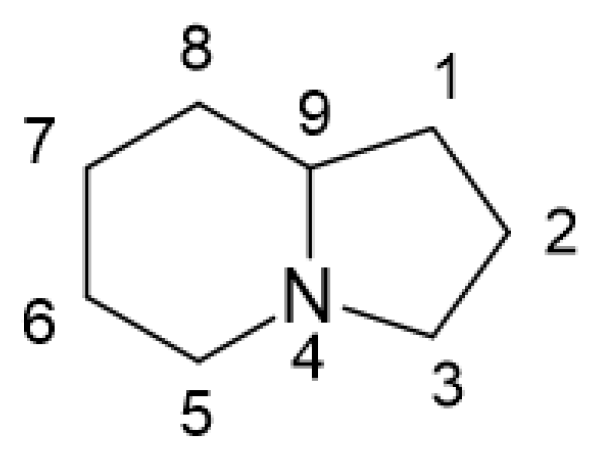
:1. Indolizidines: Structure, Sources, and Bioactivities
2. Bioactive Metabolites from Microorganisms
3. Known Actinomycetal Indolizidine Producers
3.1. Cyclizidine M146791 from Streptomyces sp. NCIB 11649
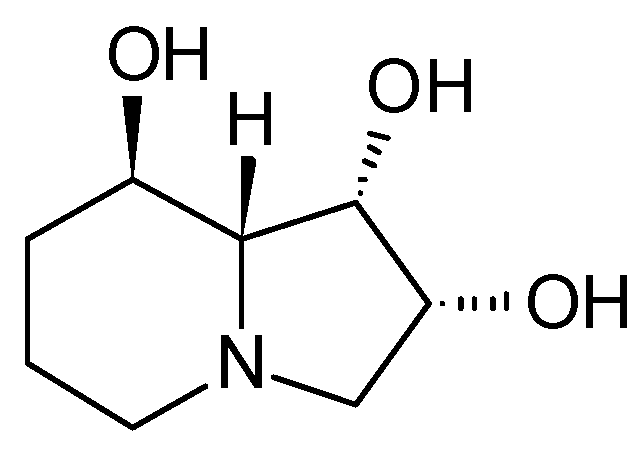
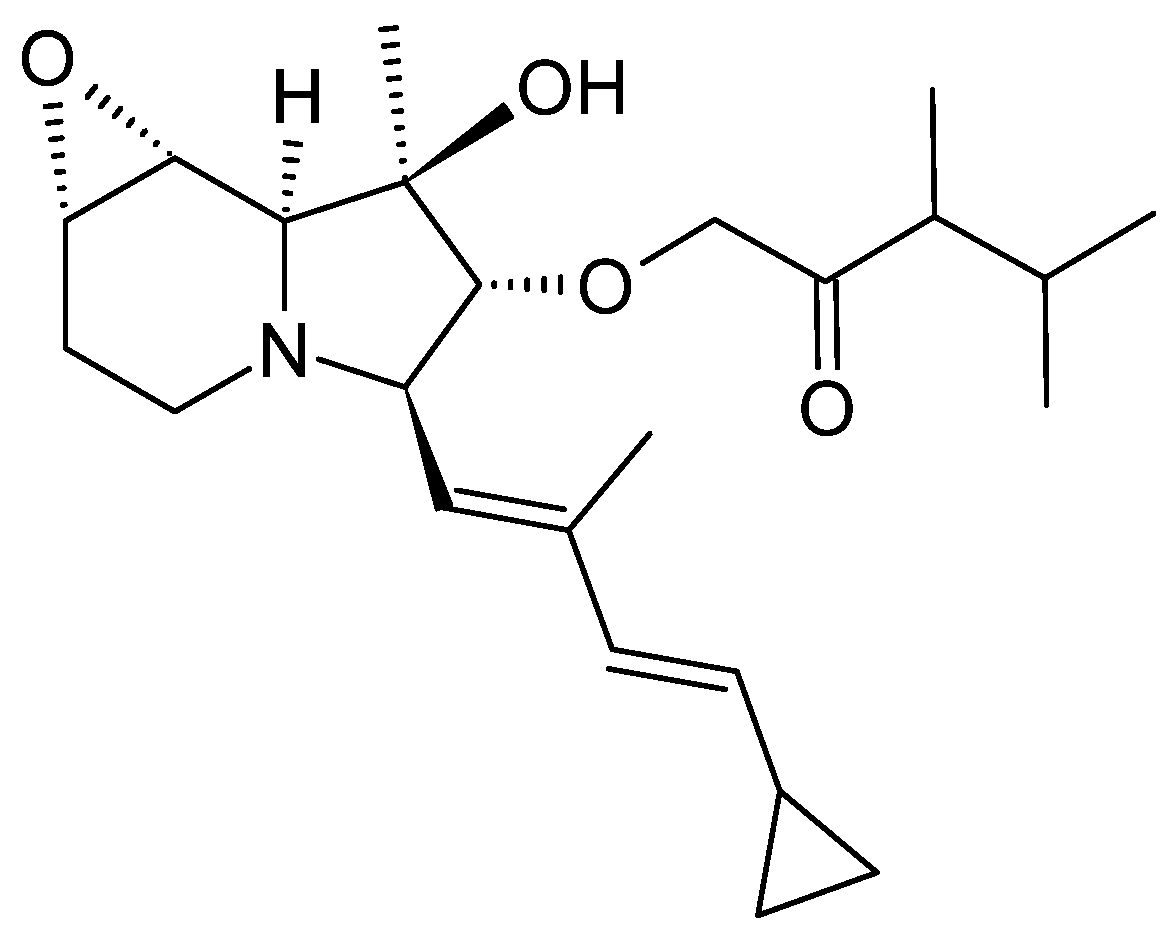
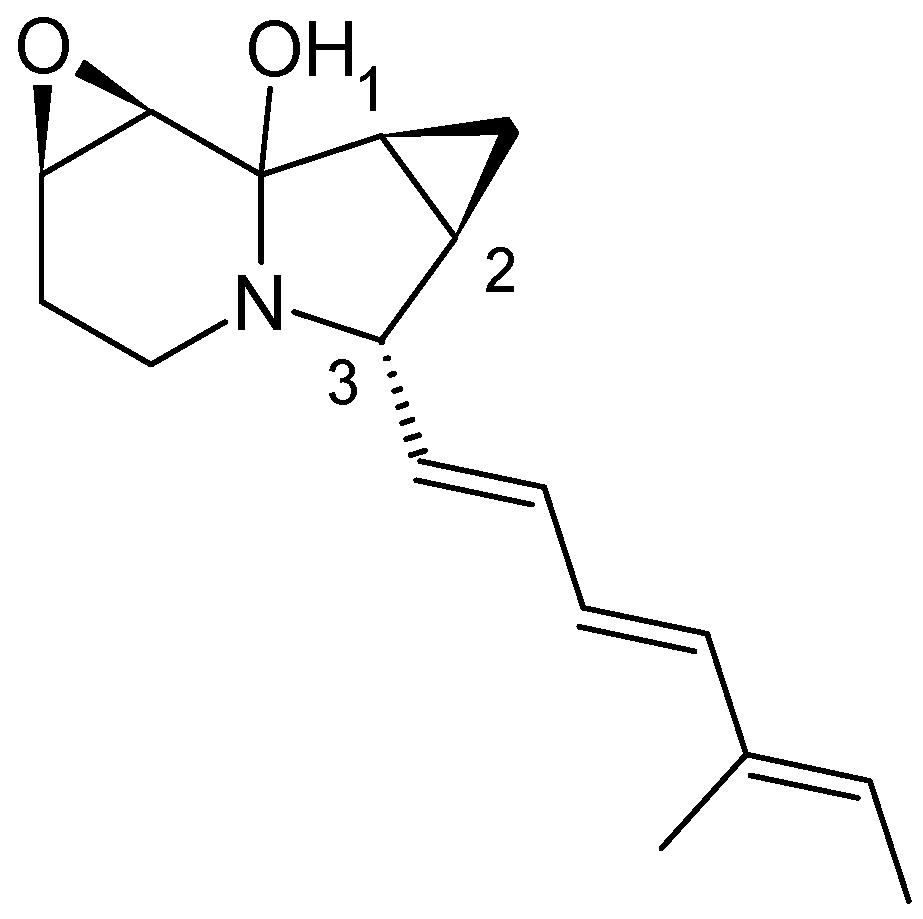
3.1.1. Structure and Isolation
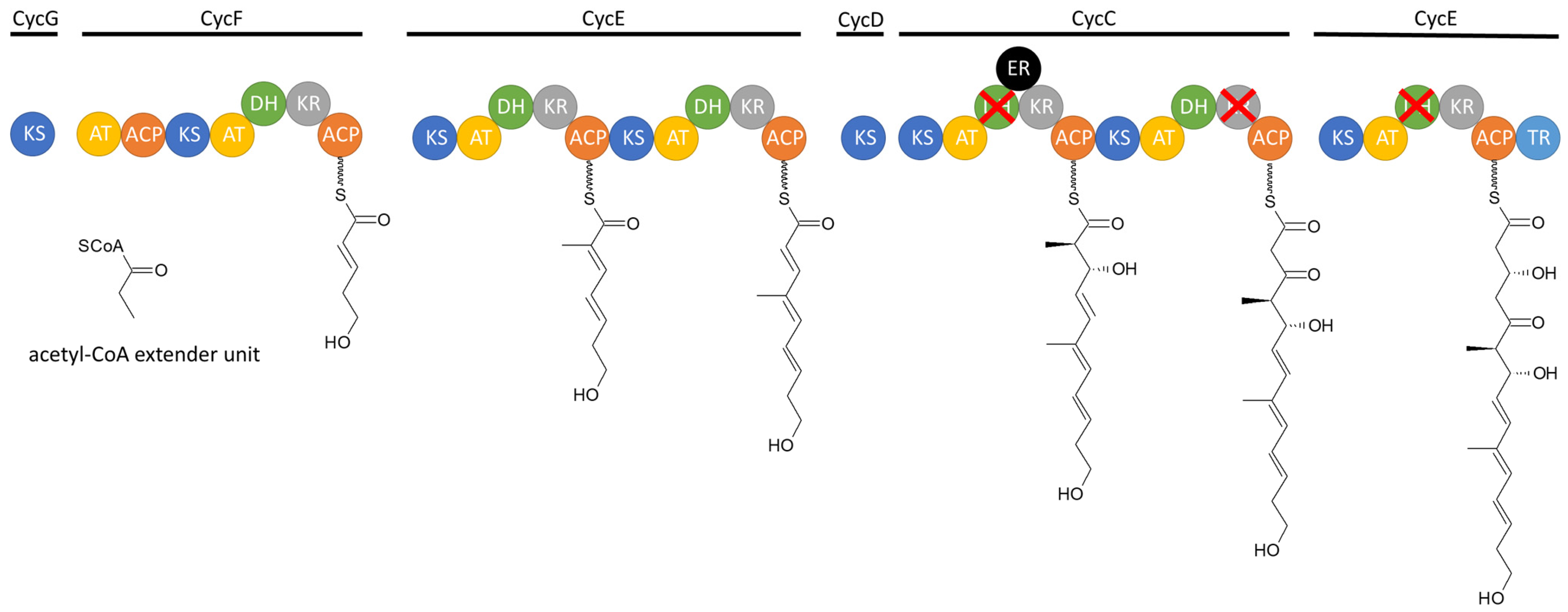
3.1.2. Biosynthesis
3.1.3. Bioactivity
3.2. Cyclizidines from Streptomyces sp. HNA39
3.2.1. Structure and Isolation
3.2.2. Bioactivity
3.3. Cyclizidines from Saccharopolyspora sp. RL78
3.3.1. Structure and Isolation
3.3.2. Bioactivity
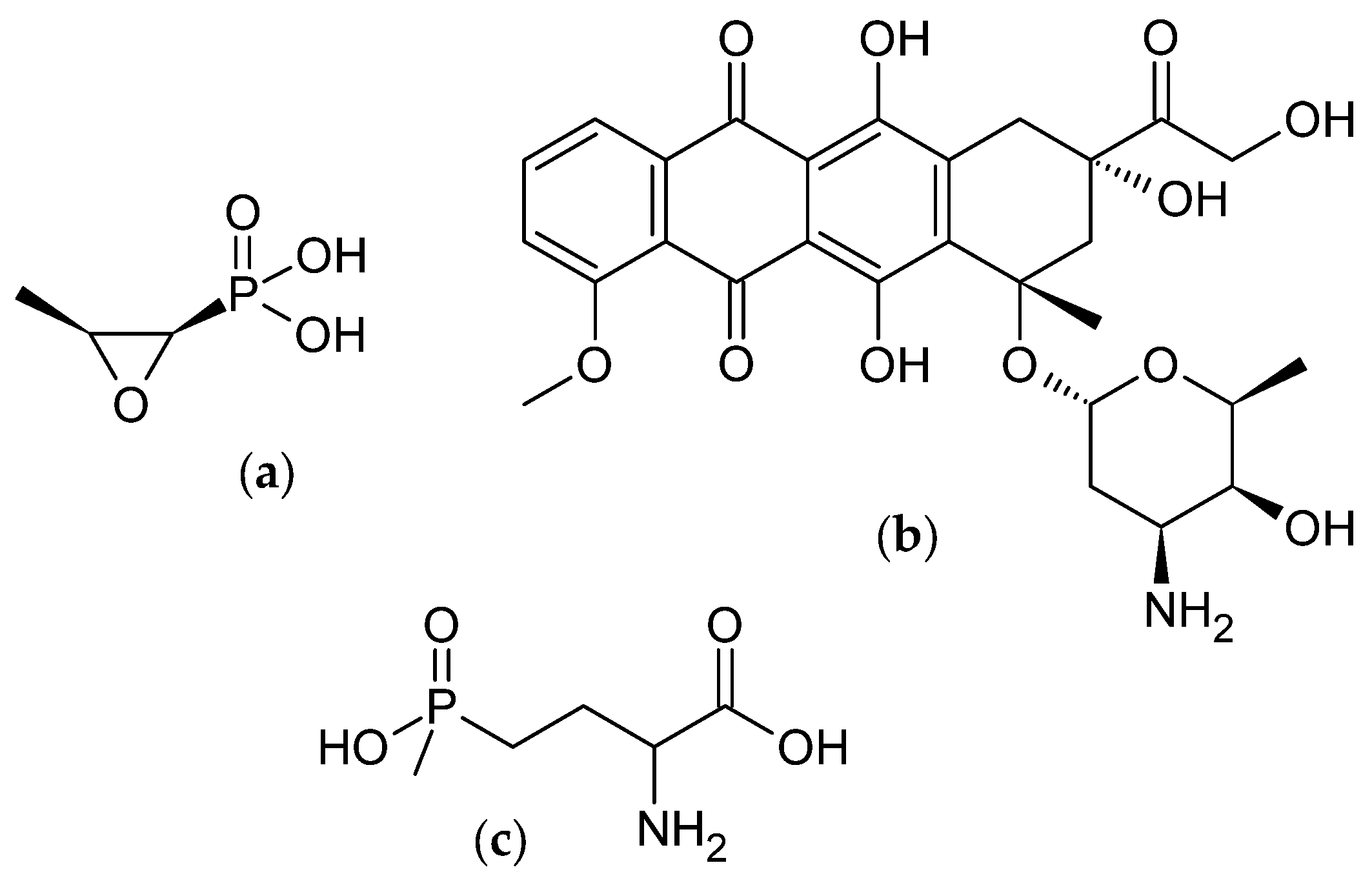
3.4. Iminimycins from Streptomyces griseus
3.4.1. Structure and Isolation
3.4.2. Biosynthesis
3.4.3. Bioactivity
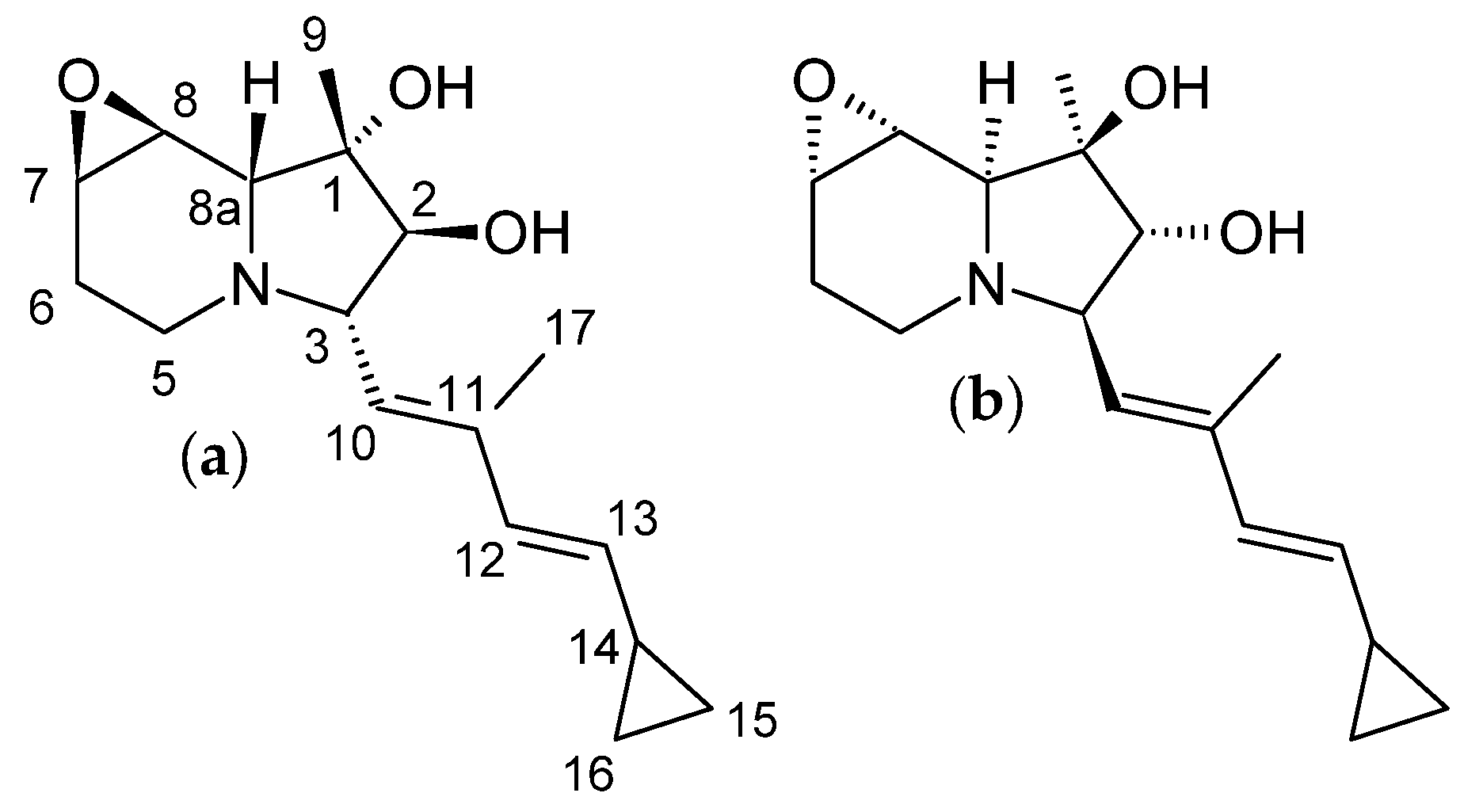
3.5. Indolizomycin from Streptomyces sp. SK2-52
3.5.1. Structure and Isolation
3.5.2. Bioactivity
4. Actinomycetes with a Silent Cyclizidine Cluster
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Morris-Natschke, S.L.; Ma, D.; Shang, X.-F.; Yang, C.-J.; Liu, Y.-Q.; Lee, K.-H. Biologically active indolizidine alkaloids. Med. Res. Rev. 2021, 41, 928–960. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, M.; Parab, R.R.; Manju, S.L.; Desai, D.C.; Mahajan, G.B. Antifungal Activity of Hydrochloride Salts of Tylophorinidine and Tylophorinine. Nat. Prod. Commun. 2012, 7, 1171–1172. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Yatsuzuka, W.; Matsushima, S.; Harada, K.; Inoue, Y.; Miyamoto, H.; Matsumoto, M.; Fukuyama, Y. Antimalarial Phenanthroindolizine Alkaloids from Ficus septica. Chem. Pharm. Bull. 2016, 64, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-W.; Chen, W.-L.; Wu, P.-L.; Tseng, H.-Y.; Lee, S.-J. Anti-Inflammatory Mechanisms of Phenanthroindolizidine Alkaloids. Mol. Pharmacol. 2006, 69, 749–758. [Google Scholar] [CrossRef]
- Min, S.; Li, Y.; Nzabanita, C.; Liu, H.; Ting-Yan, M.; Li, Y.-Z. Locoweed Endophytes: A Review. J. Plant Physiol. Pathol. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Yang, H.; Miao, S.; Li, J.-P.; Wang, S.-W.; Zhu, M.-Z.; Xie, Y.-H.; Wang, J.-B.; Liu, Z.; Yang, Q. Suppressive effects of swainsonine on C6 glioma cell in vitro and in vivo. Phytomedicine 2009, 16, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jin, X.; Xie, L.; Xu, G.; Cui, Y.; Chen, Z. RETRACTED ARTICLE: Swainsonine represses glioma cell proliferation, migration and invasion by reduction of miR-92a expression. BMC Cancer 2019, 19, 247. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhu, M.-Z.; Wang, S.-W.; Miao, S.; Xie, Y.-H.; Wang, J.-B. Inhibition of the growth of human gastric carcinoma in vivo and in vitro by swainsonine. Phytomedicine 2007, 14, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, J.; Nakagawa, H.; Takahashi, M.; Kudo, T.; Kamiyama, N.; Sun, B.; Oshima, T.; Sato, Y.; Deguchi, K.; Todo, S.; et al. Swainsonine reduces 5-fluorouracil tolerance in the multistage resistance of colorectal cancer cell lines. Mol. Cancer 2007, 6, 58. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.-Y.; Peng, C.; Shu, H.-Z.; Liu, Z.-H.; Zhou, Q.-M.; Xiong, L. New indolizidine- and pyrrolidine-type alkaloids with anti-angiogenic activities from Anisodus tanguticus. Biomed. Pharmacother. 2023, 167, 115481. [Google Scholar] [CrossRef]
- Pyne, M.E.; Kevvai, K.; Grewal, P.S.; Narcross, L.; Choi, B.; Bourgeois, L.; Dueber, J.E.; Martin, V.J.J. A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids. Nat. Commun. 2020, 11, 3337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-J.; Li, J.-Q.; Zhang, H.-J.; Ding, W.-J.; Ma, Z.-J. Cyclizidine-Type Alkaloids from Streptomyces sp. HNA39. J. Nat. Prod. 2018, 81, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, M.; Hosoya, T.; Takagi, M.; Shin-Ya, K. A new cyclizidine analog—JBIR-102—From Saccharopolyspora sp. RL78 isolated from mangrove soil. J. Antibiot. 2012, 65, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Gomi, S.; Ikeda, D.; Nakamura, H.; Naganawa, H.; Yamashita, F.; Hotta, K.; Kondo, S.; Okami, Y.; Umezawa, H.; Iitaka, Y. Isolation and structure of a new antibiotic, indolizomycin, produced by a strain SK2-52 obtained by interspecies fusion treatment. J. Antibiot. 1984, 37, 1491–1494. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Miyano, R.; Iwatsuki, M.; Shirahata, T.; Kimura, T.; Asami, Y.; Kobayashi, Y.; Shiomi, K.; Petersson, G.A.; Takahashi, Y.; et al. Iminimycin A, the new iminium metabolite produced by Streptomyces griseus OS-3601. J. Antibiot. 2016, 69, 611–615. [Google Scholar] [CrossRef]
- Freer, A.A.; Gardner, D.; Greatbanks, D.; Poyser, J.P.; Sim, G.A. Structure of cyclizidine (antibiotic M146791): X-ray crystal structure of an indolizidinediol metabolite bearing a unique cyclopropyl side-chain. J. Chem. Soc. Chem. Commun. 1982, 20, 1160–1162. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nakashima, T. Actinomycetes, an inexhaustible source of naturally occurring antibiotics. Antibiotics 2018, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shanzeba; Gul, A.; Jehan, S.; Khan, Z.; Saeed, J.; Shirazi, R.R.; Raziq, A.; Khan, M.W.; Ullah, H. Biodiversity of Actinomycetes and Their Secondary Metabolites: A Comprehensive Review. J. Adv. Biomed. Pharm. Sci. 2023, 6, 36–48. [Google Scholar] [CrossRef]
- Hopwood, D.A. Soil to genomics: The Streptomyces chromosome. Annu. Rev. Genet. 2006, 40, 1–23. [Google Scholar] [CrossRef]
- Komaki, H. Recent Progress of Reclassification of the Genus Streptomyces. Microorganisms 2023, 11, 831. [Google Scholar] [CrossRef]
- Chater, K.F. A Morphological and genetic mapping study of white colony mutants of Streptomyces coelicolor. J. Gen. Microbiol. 1972, 72, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, H.; Hopwood, D.A. Septation during sporulation in Streptomyces coelicolor. J. Gen. Microbiol. 1970, 60, 51–59. [Google Scholar] [CrossRef] [PubMed]
- van Wezel, G.P.; McDowall, K.J. The regulation of the secondary metabolism of Streptomyces: New links and experimental advances. Nat. Prod. Rep. 2011, 28, 1311–1333. [Google Scholar] [CrossRef] [PubMed]
- Schatz, A.; Bugle, E.; Waksman, S.A. Streptomycin, a Substance Exhibiting Antibiotic Activity Against Gram-Positive and Gram-Negative Bacteria. Exp. Biol. Med. 1944, 55, 66–69. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.O.; Birnbaum, J. Biosynthesis of fosfomycin by Streptomyces fradiae. Antimicrob. Agents Chemother. 1974, 5, 121–132. [Google Scholar] [CrossRef] [PubMed]
- WHO EML 22nd List. 2021. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 9 July 2024).
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.J.; Seto, H. Genetics and Biochemistry of Antibiotic Production; Butterworth-Heinemann: Oxford, UK, 1995; Chapter 6; pp. 197–222. ISBN 9780750690959. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary metabolites and biodiversity of actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Ezeobiora, C.E.; Igbokwe, N.H.; Amin, D.H.; Enwuru, N.V.; Okpalanwa, C.F.; Mendie, U.E. Uncovering the biodiversity and biosynthetic potentials of rare actinomycetes. Future J. Pharm. Sci. 2022, 8, 23. [Google Scholar] [CrossRef]
- Ngamcharungchit, C.; Chaimusik, N.; Panbangred, W.; Euanorasetr, J.; Intra, B. Bioactive metabolites from terrestrial and marine actinomycetes. Molecules 2023, 28, 5915. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Miyano, R.; Matsuo, H.; Iwatsuki, M.; Shirahata, T.; Kobayashi, Y.; Shiomi, K.; Petersson, G.A.; Takahashi, Y.; Ōmura, S. Absolute configuration of iminimycin B, a new indolizidine alkaloid, from Streptomyces griseus OS-3601. Tetrahedron Lett. 2016, 57, 3284–3286. [Google Scholar] [CrossRef]
- Huang, W.; Kim, S.J.; Liu, J.; Zhang, W. Identification of the polyketide biosynthetic machinery for the indolizidine alkaloid cyclizidine. Org. Lett. 2015, 17, 5344–5347. [Google Scholar] [CrossRef] [PubMed]
- Hanessian, S.; Soma, U.; Dorich, S.; Deschênes-Simard, B. Total synthesis of (+)-ent-cyclizidine: Absolute configurational confirmation of antibiotic M146791. Org. Lett. 2011, 13, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Leeper, F.J.; Shaw, S.E.; Satish, P. Biosynthesis of the indolizidine alkaloid cyclizidine: Incorporation of singly and doubly labelled precursors. Can. J. Chem. 2011, 72, 131–141. [Google Scholar] [CrossRef]
- Leeper, F.J.; Padmanabhan, P.; Kirby, G.W.; Sheldrake, G.N. Biosynthesis of the indolizidine alkaloid, cyclizidine. J. Chem. Soc. Chem. Commun. 1987, 505–506. [Google Scholar] [CrossRef]
- Staunton, J. The extraordinary enzymes involved in erythromycin biosynthesis. Angew. Chem. Int. Ed. 1991, 30, 1302–1306. [Google Scholar] [CrossRef]
- Walsh, C.T.; O’Connor, S.E.; Schneider, T.L. Polyketide-nonribosomal peptide epothilone antitumor agents: The EpoA, B, C subunits. J. Ind. Microbiol. Biotechnol. 2003, 30, 448–455. [Google Scholar] [CrossRef]
- Nivina, A.; Yuet, K.P.; Hsu, J.; Khosla, C. Evolution and Diversity of Assembly-Line Polyketide Synthases. Chem. Rev. 2019, 119, 12524–12547. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.-J.; Ma, Z.; Wang, N. Complete genome sequence of Streptomyces sp. HNA39, a new cyclizidine producer isolated from a South China Sea sediment. Mar. Genom. 2023, 70, 101033. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Li, J.-Q.; Jiang, Y.-J.; Liu, H.-Z.; Huo, C. A new indolizinium alkaloid from marine-derived Streptomyces sp. HNA39. J. Asian Nat. Prod. Res. 2020, 23, 913–918. [Google Scholar] [CrossRef]
- Kim, G.; Chu-Moyer, M.Y.; Danishefsky, S.J. Total synthesis of dl indolizomycin. Am. Chem. Soc. 1990, 112, 2003–2005. [Google Scholar] [CrossRef]
- Hamburger, M.; Wolfender, J.; Hostettmann, K. Search for chlorinated sesquiterpene lactones in the neurotoxic thistle Centaurea solstitialis by liquid chromatography-mass spectrometry, and model studies on their possible artifactual formation. Nat. Toxins 1993, 1, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Shin-Ya, K. New species of actinomycetes do not always produce new compounds with high frequency. J. Antibiot. 2011, 64, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.M.; Abdel-Wahab, N.M.; Hassan, H.M.; Abdelmohsen, U.R. Saccharopolyspora: An underexplored source for bioactive natural products. J. Appl. Microbiol. 2020, 128, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Waksman, S.A.; Reilly, H.C.; Harris, D.A. Streptomyces griseus (Krainsky) Waksman and Henrici. J. Bacteriol. 1948, 56, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, X.; Du, Y.; Li, Y.; Ren, W.; Lu, Y.; Shi, Y.; Sun, H.; Wang, L.; Li, Y.; et al. Genome-Directed Discovery of Bicyclic Cinnamoyl-Containing Nonribosomal Peptides with Anticoronaviral Activity from Streptomyces griseus. Org. Lett. 2023, 25, 4874–4879. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, H.; Katsuyama, Y.; Tezuka, T.; Miyano, R.; Inahashi, Y.; Takahashi, Y.; Nakashima, T.; Ohnishi, Y. Identification and Analysis of the Biosynthetic Gene Cluster for the Indolizidine Alkaloid Iminimycin in Streptomyces griseus. ChemBioChem 2021, 23, e202100517. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hotta, K.; Okami, Y.; Umezawa, H. Self-resistance of a Streptomyces which produces istamycins. J. Antibiot. 1981, 34, 824–829. [Google Scholar] [CrossRef]
- Kim, G.; Chu-Moyer, M.Y.; Danishefsky, S.J.; Schulte, G.K. The Total Synthesis of Indolizomycin. J. Am. Chem. Soc. 1993, 115, 30–39. [Google Scholar] [CrossRef]
- Hasegawa, T.; Lechevalier, M.P.; Lechevalier, H.A. New genus of the actinomycetales: Actinosynnema gen. nov. Int. J. Syst. Evol. Microbiol. 1978, 28, 304–310. [Google Scholar] [CrossRef]
- Watanabe, K.; Okuda, T.; Yokose, K.; Furumai, T.; Maruyama, H.B. Actinosynnema mirum, a new producer of nocardicin antibiotics. J. Antibiot. 1983, 36, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Land, M.; Lapidus, A.; Mayilraj, S.; Chen, F.; Copeland, A.; Del Rio, T.G.; Nolan, M.; Lucas, S.; Tice, H.; Cheng, J.-F.; et al. Complete genome sequence of Actinosynnema mirum type strain (101T). Stand. Genom. Sci. 2009, 1, 46–53. [Google Scholar] [CrossRef]
- Ehrlich, J.; Gottlieb, D.; Burkholder, P.R.; Anderson, L.E.; Pridham, T.G. Streptomyces venezuelae, n. sp., the Source of chloromycetin. J. Bacteriol. 1948, 56, 467–477. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krause, J. Indolizidines from Actinomycetes: An Overview of Producers, Biosynthesis and Bioactivities. Microorganisms 2024, 12, 1445. https://doi.org/10.3390/microorganisms12071445
Krause J. Indolizidines from Actinomycetes: An Overview of Producers, Biosynthesis and Bioactivities. Microorganisms. 2024; 12(7):1445. https://doi.org/10.3390/microorganisms12071445
Chicago/Turabian StyleKrause, Janina. 2024. "Indolizidines from Actinomycetes: An Overview of Producers, Biosynthesis and Bioactivities" Microorganisms 12, no. 7: 1445. https://doi.org/10.3390/microorganisms12071445








