The Metabolic Potential of the Human Lung Microbiome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Analysis of the BGCs from the Human Lung Microbiome
2.2. Network Analysis
2.3. Gene Cluster Comparisons
2.4. Phylogenetic Analysis
3. Results
3.1. Compilation of a Unique Genome Database of Lung-Associated Microbes
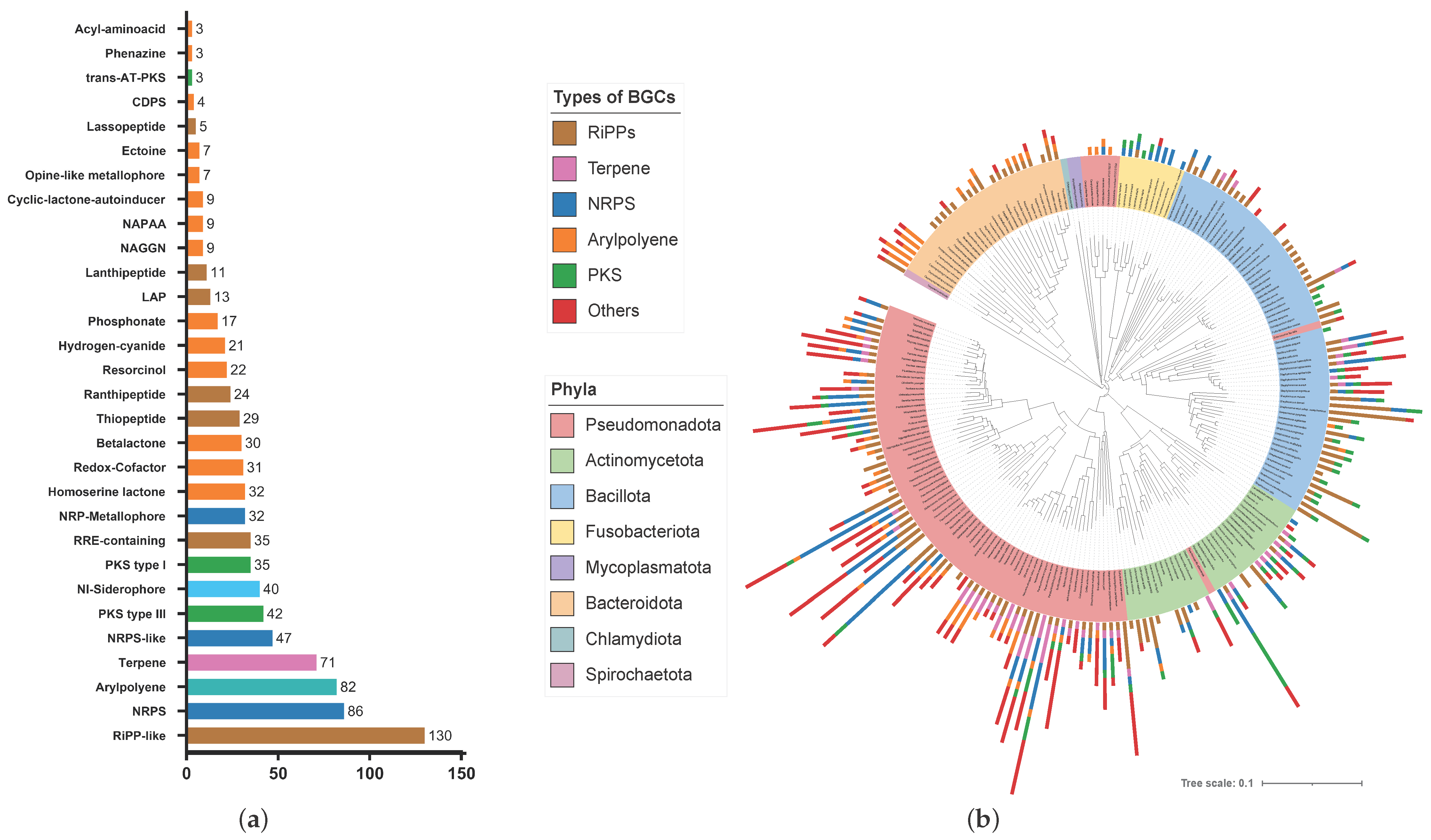
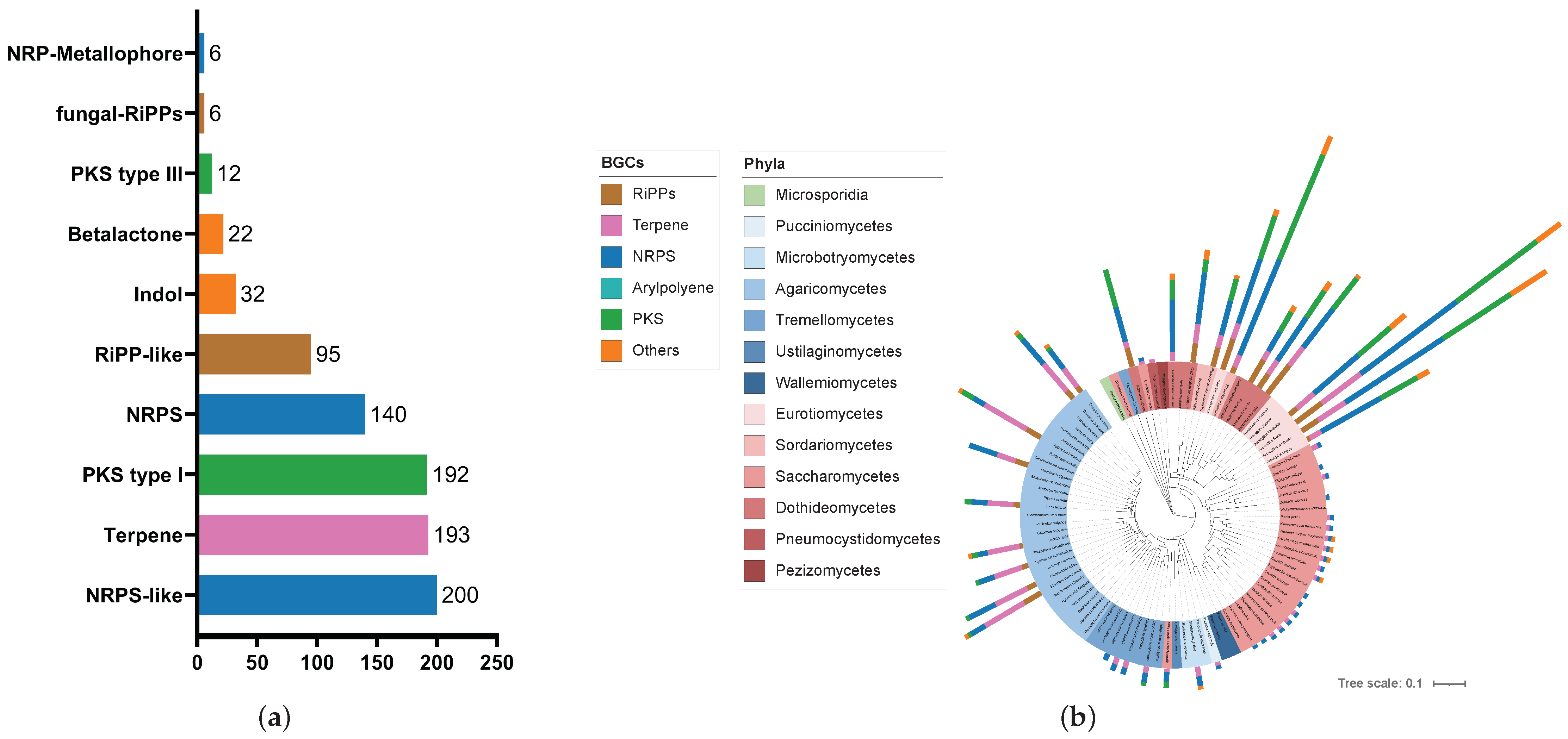
3.2. The Human Lung Microbiome Contains a Variety of Biosynthetic Gene Clusters across Phylogenetic Boundaries
3.3. Correlation between Genome Size and BGC Count
3.4. A Similarity Network of Gene Cluster Families Reveals a Multitude of New Putative Secondary Metabolites
4. Discussion
4.1. BGC Distribution and Metabolic Diversity
4.2. Assignment of Putative Pathways for Osmoadaptation
4.3. Assignment of Putative Pathways for Antioxidants
4.4. Assignment of Putative Pathways for Antimicrobials
4.5. Assignment of Putative Pathways for Communication
4.6. Assignment of Putative Pathways for Surfactants
4.7. Assignment of Putative Pathways for Metal Chelators
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yagi, K.; Huffnagle, G.B.; Lukacs, N.W.; Asai, N. The Lung Microbiome during Health and Disease. Int. J. Mol. Sci. 2021, 22, 10872. [Google Scholar] [CrossRef] [PubMed]
- Charlson, E.S.; Bittinger, K.; Haas, A.R.; Fitzgerald, A.S.; Frank, I.; Yadav, A.; Bushman, F.D.; Collman, R.G. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am. J. Respir. Crit. Care Med. 2011, 184, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Dickson, R.P.; Lukacs, N.W. The respiratory tract microbiome and lung inflammation: A two-way street. Mucosal Immunol. 2017, 10, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef]
- Chunxi, L.; Haiyue, L.; Yanxia, L.; Jianbing, P.; Jin, S. The Gut Microbiota and Respiratory Diseases: New Evidence. J. Immunol. Res. 2020, 2020, 2340670. [Google Scholar] [CrossRef]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The lung microbiome: Progress and promise. J. Clin. Investig. 2021, 131, e150473. [Google Scholar] [CrossRef]
- Maddi, A.; Sabharwal, A.; Violante, T.; Manuballa, S.; Genco, R.; Patnaik, S.; Yendamuri, S. The microbiome and lung cancer. J. Thorac. Dis. 2019, 11, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Pragman, A.A.; Lyu, T.; Baller, J.A.; Gould, T.J.; Kelly, R.F.; Reilly, C.S.; Isaacson, R.E.; Wendt, C.H. The lung tissue microbiota of mild and moderate chronic obstructive pulmonary disease. Microbiome 2018, 6, 7. [Google Scholar] [CrossRef]
- Bassis, C.M.; Erb-Downward, J.R.; Dickson, R.P.; Freeman, C.M.; Schmidt, T.M.; Young, V.B.; Beck, J.M.; Curtis, J.L.; Huffnagle, G.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015, 6, e00037. [Google Scholar] [CrossRef]
- Ibironke, O.; McGuinness, L.R.; Lu, S.-E.; Wang, Y.; Hussain, S.; Weisel, C.P.; Kerkhof, L.J. Species-level evaluation of the human respiratory microbiome. Gigascience 2020, 9, giaa038. [Google Scholar] [CrossRef]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Falkowski, N.R.; Huffnagle, G.B.; Curtis, J.L. Bacterial Topography of the Healthy Human Lower Respiratory Tract. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, M.F.; Cookson, W.O. The lung microbiome in health and disease. Clin. Med. 2017, 17, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Charlson, E.S.; Diamond, J.M.; Bittinger, K.; Fitzgerald, A.S.; Yadav, A.; Haas, A.R.; Bushman, F.D.; Collman, R.G. Lung-enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am. J. Respir. Crit. Care Med. 2012, 186, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Delhaes, L.; Monchy, S.; Fréalle, E.; Hubans, C.; Salleron, J.; Leroy, S.; Prevotat, A.; Wallet, F.; Wallaert, B.; Dei-Cas, E.; et al. The airway microbiota in cystic fibrosis: A complex fungal and bacterial community--implications for therapeutic management. PLoS ONE 2012, 7, e36313. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rebuffat, S. The manifold roles of microbial ribosomal peptide-based natural products in physiology and ecology. J. Biol. Chem. 2020, 295, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Meinwald, J. Natural products as molecular messengers. J. Nat. Prod. 2011, 74, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Fischbach, M.A. HUMAN MICROBIOTA. Small molecules from the human microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef] [PubMed]
- Milshteyn, A.; Colosimo, D.A.; Brady, S.F. Accessing Bioactive Natural Products from the Human Microbiome. Cell Host Microbe 2018, 23, 725–736. [Google Scholar] [CrossRef]
- Barber, C.C.; Zhang, W. Small molecule natural products in human nasal/oral microbiota. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab010. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, W. Signaling Natural Products from Human Pathogenic Bacteria. ACS Infect. Dis. 2020, 6, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Cimermancic, P.; Schulze, C.J.; Wieland Brown, L.C.; Martin, J.; Mitreva, M.; Clardy, J.; Linington, R.G.; Fischbach, M.A. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 2014, 158, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Aleti, G.; Baker, J.L.; Tang, X.; Alvarez, R.; Dinis, M.; Tran, N.C.; Melnik, A.V.; Zhong, C.; Ernst, M.; Dorrestein, P.C.; et al. Identification of the Bacterial Biosynthetic Gene Clusters of the Oral Microbiome Illuminates the Unexplored Social Language of Bacteria during Health and Disease. mBio 2019, 10, e00321-19. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Sun, T.; Jin, B.; Wang, S. sBGC-hm: An atlas of secondary metabolite biosynthetic gene clusters from the human gut microbiome. Bioinformatics 2023, 39, btad131. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, P.; Tagirdzhanov, A.; Kushnareva, A.; Olkhovskii, I.; Graf, S.; Schmartz, G.P.; Hegemann, J.D.; Bozhüyük, K.A.J.; Müller, R.; Keller, A.; et al. ABC-HuMi: The Atlas of Biosynthetic Gene Clusters in the Human Microbiome. Nucleic Acids Res. 2024, 52, D579–D585. [Google Scholar] [CrossRef] [PubMed]
- Gokulan, K.; Joshi, M.; Khare, S.; Bartter, T. Lung microbiome, gut-lung axis and chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2022, 28, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Bajinka, O.; Simbilyabo, L.; Tan, Y.; Jabang, J.; Saleem, S.A. Lung-brain axis. Crit. Rev. Microbiol. 2022, 48, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, S.; Aldriwesh, M.; Alruways, M.; Freestone, P. Microbial endocrinology: Host-bacteria communication within the gut microbiome. J. Endocrinol. 2015, 225, R21–R34. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M. Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-20214-3. [Google Scholar]
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, e00187-18. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Navarro-Muñoz, J.C.; Selem-Mojica, N.; Mullowney, M.W.; Kautsar, S.A.; Tryon, J.H.; Parkinson, E.I.; De Los Santos, E.L.C.; Yeong, M.; Cruz-Morales, P.; Abubucker, S.; et al. A computational framework to explore large-scale biosynthetic diversity. Nat. Chem. Biol. 2020, 16, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, B.R.; Blin, K.; Navarro-Muñoz, J.C.; Avalon, N.E.; Chevrette, M.G.; Egbert, S.; Lee, S.; Meijer, D.; Recchia, M.J.J.; Reitz, Z.L.; et al. MIBiG 3.0: A community-driven effort to annotate experimentally validated biosynthetic gene clusters. Nucleic Acids Res. 2023, 51, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.M.; Chooi, Y.-H. clinker & clustermap.js: Automatic generation of gene cluster comparison figures. Bioinformatics 2021, 37, 2473–2475. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Saraiva, L.M.; Carvalho, S.M. Staphylococcus epidermidis biofilms undergo metabolic and matrix remodeling under nitrosative stress. Front. Cell. Infect. Microbiol. 2023, 13, 1200923. [Google Scholar] [CrossRef]
- Richardson, A.R. Virulence and Metabolism. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Mashabela, G.T.; De Wet, T.J.; Warner, D.F. Mycobacterium tuberculosis Metabolism. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Men, P.; Tang, S.; Lu, X. Aspergillus terreus as an industrial filamentous fungus for pharmaceutical biotechnology. Curr. Opin. Biotechnol. 2021, 69, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Jun, S.-C.; Han, K.-H.; Hong, S.-B.; Yu, J.-H. Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. Adv. Appl. Microbiol. 2017, 100, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Volke-Sepulveda, T.; Salgado-Bautista, D.; Bergmann, C.; Wells, L.; Gutierrez-Sanchez, G.; Favela-Torres, E. Secretomic Insight into Glucose Metabolism of Aspergillus brasiliensis in Solid-State Fermentation. J. Proteome Res. 2016, 15, 3856–3871. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Tian, J.; Keller, N.P. Post-translational modifications drive secondary metabolite biosynthesis in Aspergillus: A review. Environ. Microbiol. 2022, 24, 2857–2881. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, W.; Kjer, J.; El Amrani, M.; Wray, V.; Lin, W.; Ebel, R.; Lai, D.; Proksch, P. Pullularins E and F, two new peptides from the endophytic fungus Bionectria ochroleuca isolated from the mangrove plant Sonneratia caseolaris. Mar. Drugs 2012, 10, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, S.; Kurata, M.; Harimoto, Y.; Hatta, R.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. Complex regulation of secondary metabolism controlling pathogenicity in the phytopathogenic fungus Alternaria alternata. New Phytol. 2014, 202, 1297–1309. [Google Scholar] [CrossRef]
- Cimermancic, P.; Medema, M.H.; Claesen, J.; Kurita, K.; Wieland Brown, L.C.; Mavrommatis, K.; Pati, A.; Godfrey, P.A.; Koehrsen, M.; Clardy, J.; et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 2014, 158, 412–421. [Google Scholar] [CrossRef]
- Stubbendieck, R.M.; Zelasko, S.E.; Safdar, N.; Currie, C.R. Biogeography of Bacterial Communities and Specialized Metabolism in Human Aerodigestive Tract Microbiomes. Microbiol. Spectr. 2021, 9, e0166921. [Google Scholar] [CrossRef]
- Cummings, M.; Breitling, R.; Takano, E. Steps towards the synthetic biology of polyketide biosynthesis. FEMS Microbiol. Lett. 2014, 351, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Štiblariková, M.; Lásiková, A.; Gracza, T. Benzyl Alcohol/Salicylaldehyde-Type Polyketide Metabolites of Fungi: Sources, Biosynthesis, Biological Activities, and Synthesis. Mar. Drugs 2022, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef] [PubMed]
- Hallen, H.E.; Luo, H.; Scott-Craig, J.S.; Walton, J.D. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. USA 2007, 104, 19097–19101. [Google Scholar] [CrossRef] [PubMed]
- Ford, R.E.; Foster, G.D.; Bailey, A.M. Exploring fungal RiPPs from the perspective of chemical ecology. Fungal Biol. Biotechnol. 2022, 9, 12. [Google Scholar] [CrossRef]
- González, J.M.; Mayer, F.; Moran, M.A.; Hodson, R.E.; Whitman, W.B. Microbulbifer hydrolyticus gen. nov., sp. nov., and Marinobacterium georgiense gen. nov., sp. nov., two marine bacteria from a lignin-rich pulp mill waste enrichment community. Int. J. Syst. Bacteriol. 1997, 47, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Weinel, C.; Paulsen, I.T.; Dodson, R.J.; Hilbert, H.; Martins dos Santos, V.A.P.; Fouts, D.E.; Gill, S.R.; Pop, M.; Holmes, M.; et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 2002, 4, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Vanwijnsberghe, S.; Peeters, C.; De Ridder, E.; Dumolin, C.; Wieme, A.D.; Boon, N.; Vandamme, P. Genomic Aromatic Compound Degradation Potential of Novel Paraburkholderia Species: Paraburkholderia domus sp. nov., Paraburkholderia haematera sp. nov. and Paraburkholderia nemoris sp. nov. Int. J. Mol. Sci. 2021, 22, 7003. [Google Scholar] [CrossRef] [PubMed]
- Buijs, Y.; Bech, P.K.; Vazquez-Albacete, D.; Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L.; Zhang, S.-D. Marine Proteobacteria as a source of natural products: Advances in molecular tools and strategies. Nat. Prod. Rep. 2019, 36, 1333–1350. [Google Scholar] [CrossRef]
- Puri, A.W. Specialized Metabolites from Methylotrophic Proteobacteria. Curr. Issues Mol. Biol. 2019, 33, 211–224. [Google Scholar] [CrossRef]
- Timmermans, M.L.; Paudel, Y.P.; Ross, A.C. Investigating the Biosynthesis of Natural Products from Marine Proteobacteria: A Survey of Molecules and Strategies. Mar. Drugs 2017, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- D’Souza-Ault, M.R.; Smith, L.T.; Smith, G.M. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl. Environ. Microbiol. 1993, 59, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Sagot, B.; Gaysinski, M.; Mehiri, M.; Guigonis, J.-M.; Le Rudulier, D.; Alloing, G. Osmotically induced synthesis of the dipeptide N-acetylglutaminylglutamine amide is mediated by a new pathway conserved among bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 12652–12657. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, H.; Shi, M.; Jiang, M.; Li, L.; Zheng, Y. Microbial production of ectoine and hydroxyectoine as high-value chemicals. Microb. Cell Fact. 2021, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Hermann, L.; Mais, C.-N.; Czech, L.; Smits, S.H.J.; Bange, G.; Bremer, E. The ups and downs of ectoine: Structural enzymology of a major microbial stress protectant and versatile nutrient. Biol. Chem. 2020, 401, 1443–1468. [Google Scholar] [CrossRef] [PubMed]
- Poolman, B.; Glaasker, E. Regulation of compatible solute accumulation in bacteria. Mol. Microbiol. 1998, 29, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Bermon, S. Airway inflammation and upper respiratory tract infection in athletes: Is there a link? Exerc. Immunol. Rev. 2007, 13, 6–14. [Google Scholar]
- Csonka, L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 1989, 53, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2002, 26, 49–71. [Google Scholar] [CrossRef]
- Kempf, B.; Bremer, E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch. Microbiol. 1998, 170, 319–330. [Google Scholar] [CrossRef]
- Zhu, W.; Klinman, J.P. Biogenesis of the peptide-derived redox cofactor pyrroloquinoline quinone. Curr. Opin. Chem. Biol. 2020, 59, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.E.; Jones, A.D.; Mercer, R.S.; Rucker, R.B. Characterization of pyrroloquinoline quinone amino acid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal. Biochem. 1999, 269, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, T.; Seno, H.; Urakami, T.; Matsumoto, T.; Suzuki, O. Trace levels of pyrroloquinoline quinone in human and rat samples detected by gas chromatography/mass spectrometry. Biochim. Biophys. Acta 1992, 1156, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.S.; Rajpurohit, Y.S.; Khairnar, N.P. Pyrroloquinoline-quinone and its versatile roles in biological processes. J. Biosci. 2012, 37, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Kato, T. Nutritional biochemistry: A new redox-cofactor vitamin for mammals. Nature 2003, 422, 832. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.G.M.; Bryant, D.A. Chlorophyll biosynthesis in bacteria: The origins of structural and functional diversity. Annu. Rev. Microbiol. 2007, 61, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.S.; Rowe, J.J. Antibiotic action of pyocyanin. Antimicrob. Agents Chemother. 1981, 20, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Romero-Martinez, R.; Wheeler, M.; Guerrero-Plata, A.; Rico, G.; Torres-Guerrero, H. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect. Immun. 2000, 68, 3696–3703. [Google Scholar] [CrossRef]
- Paolo, W.F.; Dadachova, E.; Mandal, P.; Casadevall, A.; Szaniszlo, P.J.; Nosanchuk, J.D. Effects of disrupting the polyketide synthase gene WdPKS1 in Wangiella Exophiala dermatitidis on melanin production and resistance to killing by antifungal compounds, enzymatic degradation, and extremes in temperature. BMC Microbiol. 2006, 6, 55. [Google Scholar] [CrossRef]
- Chatfield, C.H.; Cianciotto, N.P. The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect. Immun. 2007, 75, 4062–4070. [Google Scholar] [CrossRef]
- van der Donk, W.A.; Nair, S.K. Structure and mechanism of lanthipeptide biosynthetic enzymes. Curr. Opin. Struct. Biol. 2014, 29, 58–66. [Google Scholar] [CrossRef]
- Takuma, M.; Kuroha, M.; Nagano, Y.; Kaweewan, I.; Hemmi, H.; Oyoshi, T.; Kodani, S. Heterologous production of coryneazolicin in Escherichia coli. J. Antibiot. 2019, 72, 800–806. [Google Scholar] [CrossRef]
- Bailly, C. The bacterial thiopeptide thiostrepton. An update of its mode of action, pharmacological properties and applications. Eur. J. Pharmacol. 2022, 914, 174661. [Google Scholar] [CrossRef]
- Borissenko, L.; Groll, M. 20S proteasome and its inhibitors: Crystallographic knowledge for drug development. Chem. Rev. 2007, 107, 687–717. [Google Scholar] [CrossRef]
- Cociancich, S.; Pesic, A.; Petras, D.; Uhlmann, S.; Kretz, J.; Schubert, V.; Vieweg, L.; Duplan, S.; Marguerettaz, M.; Noëll, J.; et al. The gyrase inhibitor albicidin consists of p-aminobenzoic acids and cyanoalanine. Nat. Chem. Biol. 2015, 11, 195–197. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Karimi, A.; Fallah, F.; Akhavan, M.M. Overview of ribosomal and non-ribosomal antimicrobial peptides produced by Gram positive bacteria. Cell. Mol. Biol. 2017, 63, 20–32. [Google Scholar] [CrossRef]
- Bhatt, K.; Machado, H.; Osório, N.S.; Sousa, J.; Cardoso, F.; Magalhães, C.; Chen, B.; Chen, M.; Kim, J.; Singh, A.; et al. A Nonribosomal Peptide Synthase Gene Driving Virulence in Mycobacterium tuberculosis. mSphere 2018, 3. [Google Scholar] [CrossRef]
- Claus, S.P. The kiss of death: Limosilactobacillus reuteri PKS drives intraspecies competition. Cell Host Microbe 2022, 30, 757–759. [Google Scholar] [CrossRef]
- Bushin, L.B.; Clark, K.A.; Pelczer, I.; Seyedsayamdost, M.R. Charting an Unexplored Streptococcal Biosynthetic Landscape Reveals a Unique Peptide Cyclization Motif. J. Am. Chem. Soc. 2018, 140, 17674–17684. [Google Scholar] [CrossRef]
- Schramma, K.R.; Bushin, L.B.; Seyedsayamdost, M.R. Structure and biosynthesis of a macrocyclic peptide containing an unprecedented lysine-to-tryptophan crosslink. Nat. Chem. 2015, 7, 431–437. [Google Scholar] [CrossRef]
- Rued, B.E.; Covington, B.C.; Bushin, L.B.; Szewczyk, G.; Laczkovich, I.; Seyedsayamdost, M.R.; Federle, M.J. Quorum Sensing in Streptococcus mutans Regulates Production of Tryglysin, a Novel RaS-RiPP Antimicrobial Compound. mBio 2021, 12. [Google Scholar] [CrossRef]
- Mukherjee, S.; Biju, A.T. Recent Advances in the Organocatalytic Enantioselective Synthesis of Functionalized β-Lactones. Chem. Asian J. 2018, 13, 2333–2349. [Google Scholar] [CrossRef]
- Robinson, S.L.; Christenson, J.K.; Wackett, L.P. Biosynthesis and chemical diversity of β-lactone natural products. Nat. Prod. Rep. 2019, 36, 458–475. [Google Scholar] [CrossRef]
- Raushel, F.M. The Discovery of a β-Lactone Synthetase. Biochemistry 2017, 56, 1175–1176. [Google Scholar] [CrossRef]
- Tang, X.-X.; Yan, X.; Fu, W.-H.; Yi, L.-Q.; Tang, B.-W.; Yu, L.-B.; Fang, M.-J.; Wu, Z.; Qiu, Y.-K. New β-Lactone with Tea Pathogenic Fungus Inhibitory Effect from Marine-Derived Fungus MCCC3A00957. J. Agric. Food Chem. 2019, 67, 2877–2885. [Google Scholar] [CrossRef]
- Malhotra, S.; Hayes, D.; Wozniak, D.J. Cystic Fibrosis and Pseudomonas aeruginosa: The Host-Microbe Interface. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Létoffé, S.; Wu, Y.; Darch, S.E.; Beloin, C.; Whiteley, M.; Touqui, L.; Ghigo, J.-M. Pseudomonas aeruginosa Production of Hydrogen Cyanide Leads to Airborne Control of Staphylococcus aureus Growth in Biofilm and In Vivo Lung Environments. mBio 2022, 13, e0215422. [Google Scholar] [CrossRef]
- Jean-Pierre, V.; Boudet, A.; Sorlin, P.; Menetrey, Q.; Chiron, R.; Lavigne, J.-P.; Marchandin, H. Biofilm Formation by Staphylococcus aureus in the Specific Context of Cystic Fibrosis. Int. J. Mol. Sci. 2022, 24, 597. [Google Scholar] [CrossRef]
- Lenney, W.; Gilchrist, F.J. Pseudomonas aeruginosa and cyanide production. Eur. Respir. J. 2011, 37, 482–483. [Google Scholar] [CrossRef]
- Anand, A.; Falquet, L.; Abou-Mansour, E.; L’Haridon, F.; Keel, C.; Weisskopf, L. Biological hydrogen cyanide emission globally impacts the physiology of both HCN-emitting and HCN-perceiving Pseudomonas. mBio 2023, 14, e0085723. [Google Scholar] [CrossRef]
- Mangwani, N.; Dash, H.R.; Chauhan, A.; Das, S. Bacterial quorum sensing: Functional features and potential applications in biotechnology. J. Mol. Microbiol. Biotechnol. 2012, 22, 215–227. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Azimi, S.; Klementiev, A.D.; Whiteley, M.; Diggle, S.P. Bacterial Quorum Sensing During Infection. Annu. Rev. Microbiol. 2020, 74, 201–219. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: A signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 2000, 97, 8789–8793. [Google Scholar] [CrossRef]
- Qin, X.; Thota, G.K.; Singh, R.; Balamurugan, R.; Goycoolea, F.M. Synthetic homoserine lactone analogues as antagonists of bacterial quorum sensing. Bioorg. Chem. 2020, 98, 103698. [Google Scholar] [CrossRef]
- Fuqua, C.; Greenberg, E.P. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 685–695. [Google Scholar] [CrossRef]
- Ji, G.; Pei, W.; Zhang, L.; Qiu, R.; Lin, J.; Benito, Y.; Lina, G.; Novick, R.P. Staphylococcus intermedius produces a functional agr autoinducing peptide containing a cyclic lactone. J. Bacteriol. 2005, 187, 3139–3150. [Google Scholar] [CrossRef]
- Roongsawang, N.; Washio, K.; Morikawa, M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int. J. Mol. Sci. 2010, 12, 141–172. [Google Scholar] [CrossRef]
- Antonioli Júnior, R.; Poloni, J.d.F.; Pinto, É.S.M.; Dorn, M. Interdisciplinary Overview of Lipopeptide and Protein-Containing Biosurfactants. Genes 2022, 14, 76. [Google Scholar] [CrossRef]
- Morey, J.R.; Kehl-Fie, T.E. Bioinformatic Mapping of Opine-Like Zincophore Biosynthesis in Bacteria. mSystems 2020, 5, 10–1128. [Google Scholar] [CrossRef]
- Marchetti, M.; De Bei, O.; Bettati, S.; Campanini, B.; Kovachka, S.; Gianquinto, E.; Spyrakis, F.; Ronda, L. Iron Metabolism at the Interface between Host and Pathogen: From Nutritional Immunity to Antibacterial Development. Int. J. Mol. Sci. 2020, 21, 2145. [Google Scholar] [CrossRef]
- Nairz, M.; Weiss, G. Iron in infection and immunity. Mol. Aspects Med. 2020, 75, 100864. [Google Scholar] [CrossRef]
- Reitz, Z.L.; Medema, M.H. Genome mining strategies for metallophore discovery. Curr. Opin. Biotechnol. 2022, 77, 102757. [Google Scholar] [CrossRef]
- Kraemer, S.M.; Duckworth, O.W.; Harrington, J.M.; Schenkeveld, W.D.C. Metallophores and Trace Metal Biogeochemistry. Aquat Geochem 2015, 21, 159–195. [Google Scholar] [CrossRef]
- Kenney, G.E.; Rosenzweig, A.C. Chalkophores. Annu. Rev. Biochem. 2018, 87, 645–676. [Google Scholar] [CrossRef]
- Wang, W.; Qiu, Z.; Tan, H.; Cao, L. Siderophore production by actinobacteria. Biometals 2014, 27, 623–631. [Google Scholar] [CrossRef]
- Hossain, S.; Morey, J.R.; Neville, S.L.; Ganio, K.; Radin, J.N.; Norambuena, J.; Boyd, J.M.; McDevitt, C.A.; Kehl-Fie, T.E. Host subversion of bacterial metallophore usage drives copper intoxication. mBio 2023, 14, e0135023. [Google Scholar] [CrossRef]
- Uranga, C.C.; Arroyo, P.; Duggan, B.M.; Gerwick, W.H.; Edlund, A. Commensal Oral Rothia mucilaginosa Produces Enterobactin, a Metal-Chelating Siderophore. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Hwang, C.H.; Kim, W.J.; Jwa, H.Y.; Song, S.H. Community-acquired Achromobacter xylosoxidans infection presenting as a cavitary lung disease in an immunocompetent patient. Yeungnam Univ. J. Med. 2020, 37, 54–58. [Google Scholar] [CrossRef]
- Menetrey, Q.; Sorlin, P.; Jumas-Bilak, E.; Chiron, R.; Dupont, C.; Marchandin, H. Achromobacter xylosoxidans and Stenotrophomonas maltophilia: Emerging Pathogens Well-Armed for Life in the Cystic Fibrosis Patients’ Lung. Genes 2021, 12, 610. [Google Scholar] [CrossRef]
- Wienhold, S.-M.; Brack, M.C.; Nouailles, G.; Krishnamoorthy, G.; Korf, I.H.E.; Seitz, C.; Wienecke, S.; Dietert, K.; Gurtner, C.; Kershaw, O.; et al. Preclinical Assessment of Bacteriophage Therapy against Experimental Acinetobacter baumannii Lung Infection. Viruses 2021, 14, 33. [Google Scholar] [CrossRef]
- Cameron, S.J.S.; Lewis, K.E.; Huws, S.A.; Hegarty, M.J.; Lewis, P.D.; Pachebat, J.A.; Mur, L.A.J. A pilot study using metagenomic sequencing of the sputum microbiome suggests potential bacterial biomarkers for lung cancer. PLoS ONE 2017, 12, e0177062. [Google Scholar] [CrossRef]
- Toyoshima, M.; Chida, K.; Suda, T. Fulminant community-acquired pneumonia probably caused by Acinetobacter lwoffii. Respirology 2010, 15, 867–868. [Google Scholar] [CrossRef]
- Tabarsi, P.; Yousefi, S.; Jabbehdari, S.; Marjani, M.; Baghaei, P. Pulmonary Actinomycosis in a Patient with AIDS/HCV. J. Clin. Diagn. Res. 2017, 11, OD15–OD17. [Google Scholar] [CrossRef]
- Hussenet, C.; LeGoff, J.; Lorillon, G.; Simon, F.; Bergeron, A. Actinomyces naeslundii lung infection diagnosed by polymerase chain reaction coupled with electrospray-ionization mass spectrometry. Ann. Am. Thorac. Soc. 2014, 11, 1163–1165. [Google Scholar] [CrossRef]
- Supriya, B.G.; Harisree, S.; Savio, J.; Ramachandran, P. Actinomyces naeslundii causing pulmonary endobronchial Actinomycosis—A case report. Indian J. Pathol. Microbiol. 2019, 62, 326–328. [Google Scholar] [CrossRef]
- Suzuki, J.B.; Delisle, A.L. Pulmonary actinomycosis of periodontal origin. J. Periodontol. 1984, 55, 581–584. [Google Scholar] [CrossRef]
- Al-Nafeesah, A. Aggregatibacter actinomycetemcomitans pneumonia mimicking lung cancer in a previously healthy 12-year-old child from Saudi Arabia: A case report. Pan Afr. Med. J. 2020, 36, 89. [Google Scholar] [CrossRef]
- Matzumura-Kuan, M.; Jennings, J. Aggregatibacter actinomycetemcomitans infection mimicking lung cancer: A case report. Scand. J. Infect. Dis. 2014, 46, 669–672. [Google Scholar] [CrossRef]
- Sumer, J.; Haller, S.; Sawatzki, M.; Kellner, J.; Boggian, K. An unusual case of multiple hepatic and pulmonary abscesses caused by Aggregatibacter aphrophilus in a young man: A case report. J. Med. Case Rep. 2021, 15, 34. [Google Scholar] [CrossRef]
- Dworniczek, E.; Mordarska, H.; Bizuniak, I.; Smogór, W.; Szklarz, E. Beztlenowe pałeczki Propionibacterium w infekcjach oportunistycznych. Med. Dosw. Mikrobiol. 1993, 45, 219–222. [Google Scholar]
- Brock, D.W.; Georg, L.K.; Brown, J.M.; Hicklin, M.D. Actinomycosis caused by Arachnia propionica: Report of 11 cases. Am. J. Clin. Pathol. 1973, 59, 66–77. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Wu, W.; Booth, J.L.; Duggan, E.S.; Nagle, N.N.; Coggeshall, K.M.; Metcalf, J.P. Human lung innate immune response to Bacillus anthracis spore infection. Infect. Immun. 2007, 75, 3729–3738. [Google Scholar] [CrossRef]
- Young, G.A.; Zelle, M.R.; Lincoln, R.E. Respiratory pathogenicity of Bacillus anthracis spores; methods of study and observations on pathogenesis. J. Infect. Dis. 1946, 79, 233–246. [Google Scholar] [CrossRef]
- Lee, H.K.; Walls, G.; Anderson, G.; Sullivan, C.; Wong, C.A. Prolonged Bacteroides pyogenes infection in a patient with multiple lung abscesses. Respirol. Case Rep. 2024, 12, e01314. [Google Scholar] [CrossRef]
- Ganeshalingham, A.; Anderson, B.J.; Zuccollo, J.; Davies-Payne, D.; Beca, J. Porcelain lung: Calcification in severe Bordetella pertussis infection. Arch. Dis. Child. 2016, 101, 421. [Google Scholar] [CrossRef]
- Mihara, Y.; Yoshino, S.; Nakatani, K.; Nishimura, T.; Kan, H.; Yamamura, Y.; Tanaka, E.; Ishii, S.; Shimonodan, H.; Okada, K.; et al. Bordetella pertussis is a common pathogen in infants hospitalized for acute lower respiratory tract infection during the winter season. J. Infect. Chemother. 2021, 27, 497–502. [Google Scholar] [CrossRef]
- Ferreira, A.C.M.; Marson, F.A.L.; Cohen, M.A.; Bertuzzo, C.S.; Levy, C.E.; Ribeiro, A.F.; Ribeiro, J.D. Hypertonic Saline as a Useful Tool for Sputum Induction and Pathogen Detection in Cystic Fibrosis. Lung 2017, 195, 431–439. [Google Scholar] [CrossRef]
- Delsing, C.E.; Ruesen, C.; Boeree, M.J.; van Damme, P.A.; Kuipers, S.; van Crevel, R. An African woman with pulmonary cavities: TB or not TB? Neth. J. Med. 2014, 72, 426–428. [Google Scholar]
- Morgan, M.A.; Goldstein, E.J. Bulleidia extructa: An underappreciated anaerobic pathogen. Anaerobe 2021, 69, 102339. [Google Scholar] [CrossRef]
- Husain, S.; Singh, N. Burkholderia cepacia infection and lung transplantation. Semin. Respir. Infect. 2002, 17, 284–290. [Google Scholar] [CrossRef]
- Mohapatra, P.R.; Shirgaonkar, R.B.; Behera, B.; Girija, A. Community-Acquired burkholderia cepacia complex (BCC) pneumonia in a lung cancer patient on erlotinib. Lung India 2023, 40, 364–365. [Google Scholar] [CrossRef]
- Miller, W.G.; Yee, E. Complete Genome Sequence of Campylobacter gracilis ATCC 33236T. Genome Announc. 2015, 3. [Google Scholar] [CrossRef]
- Ehrmann, E.; Jolivet-Gougeon, A.; Bonnaure-Mallet, M.; Fosse, T. Multidrug-resistant oral Capnocytophaga gingivalis responsible for an acute exacerbation of chronic obstructive pulmonary disease: Case report and literature review. Anaerobe 2016, 42, 50–54. [Google Scholar] [CrossRef]
- Fukuoka, K.; Mochizuki, Y.; Nakahara, Y.; Kawamura, T.; Watanabe, S.; Sasaki, S. A case report of lung abscess caused by Capnocytophaga gingivalis infection. Kansenshogaku Zasshi 2000, 74, 594–597. [Google Scholar] [CrossRef]
- van der Gast, C.J.; Walker, A.W.; Stressmann, F.A.; Rogers, G.B.; Scott, P.; Daniels, T.W.; Carroll, M.P.; Parkhill, J.; Bruce, K.D. Partitioning core and satellite taxa from within cystic fibrosis lung bacterial communities. ISME J. 2011, 5, 780–791. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Rogers, G.B.; Walker, A.W.; Oliver, A.; Hoffman, L.R.; Carroll, M.P.; Parkhill, J.; Bruce, K.D.; van der Gast, C.J. Implications of multiple freeze-thawing on respiratory samples for culture-independent analyses. J. Cyst. Fibros. 2015, 14, 464–467. [Google Scholar] [CrossRef]
- Bonatti, H.; Rossboth, D.W.; Nachbaur, D.; Fille, M.; Aspöck, C.; Hend, I.; Hourmont, K.; White, L.; Malnick, H.; Allerberger, F.J. A series of infections due to Capnocytophaga spp in immunosuppressed and immunocompetent patients. Clin. Microbiol. Infect. 2003, 9, 380–387. [Google Scholar] [CrossRef]
- Premachandra, N.M.; Jayaweera, J.A.A.S. Chlamydia pneumoniae infections and development of lung cancer: Systematic review. Infect. Agent. Cancer 2022, 17, 11. [Google Scholar] [CrossRef]
- Coenye, T.; Goris, J.; Spilker, T.; Vandamme, P.; LiPuma, J.J. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J. Clin. Microbiol. 2002, 40, 2062–2069. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, X.; Li, Z.; Guo, W.; Cheng, H.; Cao, Q. A Preliminary Study on Microbiota Characteristics of Bronchoalveolar Lavage Fluid in Patients with Pulmonary Nodules Based on Metagenomic Next-Generation Sequencing. Biomedicines 2023, 11, 631. [Google Scholar] [CrossRef]
- Minkin, R.; Shapiro, J.M. Corynebacterium afermentans lung abscess and empyema in a patient with human immunodeficiency virus infection. South. Med. J. 2004, 97, 395–397. [Google Scholar] [CrossRef]
- Kebbe, J.; Mador, M.J. Corynebacterium macginleyi: A cause of ventilator associated pneumonia in an immunocompromised patient. Respir. Med. Case Rep. 2015, 16, 154–156. [Google Scholar] [CrossRef]
- Michel, C.; Raimo, M.; Lazarevic, V.; Gaïa, N.; Leduc, N.; Knoop, C.; Hallin, M.; Vandenberg, O.; Schrenzel, J.; Grimaldi, D.; et al. Case Report: About a Case of Hyperammonemia Syndrome Following Lung Transplantation: Could Metagenomic Next-Generation Sequencing Improve the Clinical Management? Front. Med. 2021, 8, 684040. [Google Scholar] [CrossRef]
- van Roeden, S.E.; Thijsen, S.F.; Sankatsing, S.U.C.; Limonard, G.J.M. Clinical relevance of Corynebacterium pseudodiphtheriticum in lower respiratory tract specimens. Infect. Dis. 2015, 47, 862–868. [Google Scholar] [CrossRef]
- Severo, C.B.; Guazzelli, L.S.; Barra, M.B.; Hochhegger, B.; Severo, L.C. Multiple pulmonary nodules caused by Corynebacterium striatum in an immunocompetent patient. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 89–91. [Google Scholar] [CrossRef]
- Bowstead, T.T.; Santiago, S.M. Pleuropulmonary infection due to Corynebacterium striatum. Br. J. Dis. Chest 1980, 74, 198–200. [Google Scholar] [CrossRef]
- Ekanayake, A.; Madegedara, D.; Chandrasekharan, V.; Magana-Arachchi, D. Respiratory Bacterial Microbiota and Individual Bacterial Variability in Lung Cancer and Bronchiectasis Patients. Indian J. Microbiol. 2020, 60, 196–205. [Google Scholar] [CrossRef]
- Yildiz, H.; Sünnetçioğlu, A.; Ekin, S.; Baran, A.İ.; Özgökçe, M.; Aşker, S.; Üney, İ.; Turgut, E.; Akyüz, S. Delftia acidovorans pneumonia with lung cavities formation. Colomb. Med. 2019, 50, 215–221. [Google Scholar] [CrossRef]
- Bittar, F.; Richet, H.; Dubus, J.-C.; Reynaud-Gaubert, M.; Stremler, N.; Sarles, J.; Raoult, D.; Rolain, J.-M. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS ONE 2008, 3, e2908. [Google Scholar] [CrossRef] [PubMed]
- Cawcutt, K.A.; Bhatti, M.M.; Nelson, D.R. Pleural fluid infection caused by Dietzia cinnamea. Diagn. Microbiol. Infect. Dis. 2016, 85, 496–497. [Google Scholar] [CrossRef]
- Duport, P.; Miltgen, G.; Kebbabi, C.; Belmonte, O.; Coolen-Allou, N.; Allyn, J.; Allou, N. First case of pleural empyema and pulmonary abscess caused by Eggerthia catenaformis. Anaerobe 2018, 50, 9–11. [Google Scholar] [CrossRef]
- Kentos, A.; Vuyst, P.d.; Stuelens, M.J.; Jacobs, F.; Francquen, P.d.; Delaere, B.; Demaeyer, P.; Thys, J.P. Lung abscess due to Eikenella corrodens: Three cases and review. Eur. J. Clin. Microbiol. Infect. Dis. 1995, 14, 146–148. [Google Scholar] [CrossRef]
- Jiménez-Castillo, R.A.; Aguilar-Rivera, L.R.; Carrizales-Sepúlveda, E.F.; Gómez-Quiroz, R.A.; Llantada-López, A.R.; González-Aguirre, J.E.; Náñez-Terreros, H.; Rendón-Ramírez, E.J. A case of round pneumonia due to Enterobacter hormaechei: The need for a standardized diagnosis and treatment approach in adults. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e3. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, M.; Guo, H.; Wang, K.; Liu, J.; Wang, Y.; Lin, Y.; Li, J.; Li, P.; Yang, L.; et al. Altered Respiratory Microbiomes, Plasma Metabolites, and Immune Responses in Influenza A Virus and Methicillin-Resistant Staphylococcus aureus Coinfection. Microbiol. Spectr. 2023, 11, e0524722. [Google Scholar] [CrossRef]
- Mendes, A.R.; Costa, A.; Ferreira, H.; Ferreira, C. Enterococcus faecalis-associated lung abscess in a male adolescent—A case report. BMC Pediatr. 2020, 20, 98. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, R.; Zhu, Y.; Li, Z.; She, X.; Jian, X.; Yu, F.; Deng, X.; Sai, B.; Wang, L.; et al. Lung microbiome alterations in NSCLC patients. Sci. Rep. 2021, 11, 11736. [Google Scholar] [CrossRef]
- George, W.L.; Kirby, B.D.; Sutter, V.L.; Citron, D.M.; Finegold, S.M. Gram-negative anaerobic bacilli: Their role in infection and patterns of susceptibility to antimicrobial agents. II. Little-known Fusobacterium species and miscellaneous genera. Rev. Infect. Dis. 1981, 3, 599–626. [Google Scholar] [CrossRef]
- Sonti, R.; Fleury, C. Fusobacterium necrophorum presenting as isolated lung nodules. Respir. Med. Case Rep. 2015, 15, 80–82. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, D.H.; Kim, S.-Y.; Kang, N.; Jhun, B.W. Comparison of the sputum microbiome between patients with stable nontuberculous mycobacterial pulmonary disease and patients requiring treatment. BMC Microbiol. 2024, 24, 172. [Google Scholar] [CrossRef]
- Da Costa, C.T.; Porter, C.; Parry, K.; Morris, A.; Quoraishi, A.H. Empyema thoracis and lung abscess due to Gemella morbillorum. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.K.; Groote, M.A.d.; Sagel, S.D.; Zemanick, E.T.; Kapsner, R.; Penvari, C.; Kaess, H.; Deterding, R.R.; Accurso, F.J.; Pace, N.R. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc. Natl. Acad. Sci. USA 2007, 104, 20529–20533. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.S.R.; Al-Alo, K.Z.K.; Al-Yasiri, M.H.; Lateef, Z.M.; Ghasemian, A. Microbiome Dysbiosis and Predominant Bacterial Species as Human Cancer Biomarkers. J. Gastrointest. Cancer 2020, 51, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, L.; Xu, L.; Huang, X.; Sun, X.; Yang, L.; Xu, L. Lung abscess secondary to lung cancer with a coinfection of Granulicatellaadiacens and other bacteria: A case report. BMC Infect. Dis. 2021, 21, 662. [Google Scholar] [CrossRef] [PubMed]
- Reissig, A.; Mempel, C.; Schumacher, U.; Copetti, R.; Gross, F.; Aliberti, S. Microbiological diagnosis and antibiotic therapy in patients with community-acquired pneumonia and acute COPD exacerbation in daily clinical practice: Comparison to current guidelines. Lung 2013, 191, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.J.; Ochoa, C.E.; Sethi, S.; Dickey, B.F. Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 113–123. [Google Scholar] [CrossRef] [PubMed]
- García-Fojeda, B.; González-Carnicero, Z.; Lorenzo, A.d.; Minutti, C.M.; Tapia, L.d.; Euba, B.; Iglesias-Ceacero, A.; Castillo-Lluva, S.; Garmendia, J.; Casals, C. Lung Surfactant Lipids Provide Immune Protection Against Haemophilus influenzae Respiratory Infection. Front. Immunol. 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Chatziparasidis, G.; Kantar, A.; Grimwood, K. Pathogenesis of nontypeable Haemophilus influenzae infections in chronic suppurative lung disease. Pediatr. Pulmonol. 2023, 58, 1849–1860. [Google Scholar] [CrossRef]
- Hong, B.-Y.; Paulson, J.N.; Stine, O.C.; Weinstock, G.M.; Cervantes, J.L. Meta-analysis of the lung microbiota in pulmonary tuberculosis. Tuberculosis 2018, 109, 102–108. [Google Scholar] [CrossRef]
- Boucher, M.B.; Bedotto, M.; Couderc, C.; Gomez, C.; Reynaud-Gaubert, M.; Drancourt, M. Haemophilus pittmaniae respiratory infection in a patient with siderosis: A case report. J. Med. Case Rep. 2012, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Bachman, M.A.; Breen, P.; Deornellas, V.; Mu, Q.; Zhao, L.; Wu, W.; Cavalcoli, J.D.; Mobley, H.L.T. Genome-Wide Identification of Klebsiella pneumoniae Fitness Genes during Lung Infection. mBio 2015, 6, e00775. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, T.; Ghimire, L.; Jin, L.; Le, J.; Periasamy, S.; Paudel, S.; Cai, S.; Jeyaseelan, S. Host Defense against Klebsiella pneumoniae Pneumonia Is Augmented by Lung-Derived Mesenchymal Stem Cells. J. Immunol. 2021, 207, 1112–1127. [Google Scholar] [CrossRef] [PubMed]
- Morgenstein, A.A.; Citron, D.M.; Orisek, B.; Finegold, S.M. Serious infection with Leptotrichia buccalis. Report of a case and review of the literature. Am. J. Med. 1980, 69, 782–785. [Google Scholar] [CrossRef]
- Eribe, E.R.K.; Olsen, I. Leptotrichia species in human infections II. J. Oral Microbiol. 2017, 9, 1368848. [Google Scholar] [CrossRef]
- Anezaki, H.; Terada, N.; Kawamura, T.; Kurai, H. Moraxella catarrhalis bacteremic pneumonia. IDCases 2020, 19, e00712. [Google Scholar] [CrossRef] [PubMed]
- Versi, A.; Ivan, F.X.; Abdel-Aziz, M.I.; Bates, S.; Riley, J.; Baribaud, F.; Kermani, N.Z.; Montuschi, P.; Dahlen, S.-E.; Djukanovic, R.; et al. Haemophilus influenzae and Moraxella catarrhalis in sputum of severe asthma with inflammasome and neutrophil activation. Allergy 2023, 78, 2906–2920. [Google Scholar] [CrossRef]
- Davis, J.M.; Whipp, M.J.; Ashhurst-Smith, C.; DeBoer, J.C.; Peel, M.M. Mucoid nitrate-negative Moraxella nonliquefaciens from three patients with chronic lung disease. J. Clin. Microbiol. 2004, 42, 3888–3890. [Google Scholar] [CrossRef]
- Rosett, W.; Heck, D.M.; Hodges, G.R. Pneumonitis and pulmonary abscess associated with Moraxella nonliquefaciens. Chest 1976, 70, 664–665. [Google Scholar] [CrossRef]
- Vuori-Holopainen, E.; Salo, E.; Saxen, H.; Vaara, M.; Tarkka, E.; Peltola, H. Clinical “pneumococcal pneumonia” due to Moraxella osloensis: Case report and a review. Scand. J. Infect. Dis. 2001, 33, 625–627. [Google Scholar] [CrossRef]
- Silva, C.A.M.; Danelishvili, L.; McNamara, M.; Berredo-Pinho, M.; Bildfell, R.; Biet, F.; Rodrigues, L.S.; Oliveira, A.V.; Bermudez, L.E.; Pessolani, M.C.V. Interaction of Mycobacterium leprae with human airway epithelial cells: Adherence, entry, survival, and identification of potential adhesins by surface proteome analysis. Infect. Immun. 2013, 81, 2645–2659. [Google Scholar] [CrossRef] [PubMed]
- Rees, R.J.; McDougall, A.C. Airborne infection with Mycobacterium leprae in mice. J. Med. Microbiol. 1977, 10, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Cao, L.-J.; Xia, H.-L.; Ji, Z.-M.; Hu, N.-N.; Leng, Z.-J.; Xie, W.; Fang, Y.; Zhang, J.-Q.; Xia, D.-Q. The performance of detecting Mycobacterium tuberculosis complex in lung biopsy tissue by metagenomic next-generation sequencing. BMC Pulm. Med. 2022, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.M.; Alspaugh, J.A.; Meredith, F.T.; Harrell, L.J.; Tapson, V.; Davis, R.D.; Kanj, S.S. Mycoplasma hominis pneumonia complicating bilateral lung transplantation: Case report and review of the literature. Chest 1997, 112, 1428–1432. [Google Scholar] [CrossRef]
- Moneke, I.; Hornuss, D.; Serr, A.; Kern, W.V.; Passlick, B.; Senbaklavaci, O. Lung Abscess and Recurrent Empyema After Infection With Mycoplasma hominis: A Case Report and Review of the Literature. Open Forum Infect. Dis. 2022, 9, ofab406. [Google Scholar] [CrossRef]
- Chaudhry, R.; Ghosh, A.; Chandolia, A. Pathogenesis of Mycoplasma pneumoniae: An update. Indian J. Med. Microbiol. 2016, 34, 7–16. [Google Scholar] [CrossRef]
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef]
- Kovaleva, V.V.; Andreeva, Z.M.; Fedoseeva, V.N.; Chitaeva, V.G. Vydelenie i identifikatsiia neĭsseriĭ pri bronkhial’noĭ astme. Zh. Mikrobiol. Epidemiol. Immunobiol. 1975, 8, 59–62. [Google Scholar]
- Kamar, N.; Chabbert, V.; Ribes, D.; Chabanon, G.; Faguer, S.; Mari, A.; Guitard, J.; Durand, D.; Rostaing, L. Neisseria-cinerea-induced pulmonary cavitation in a renal transplant patient. Nephrol. Dial. Transplant 2007, 22, 2099–2100. [Google Scholar] [CrossRef]
- Moriya, T.; Ohsumi, A.; Tanizawa, K.; Matsumura, Y.; Date, H. Infected lung bulla caused by Neisseria elongata: A case report. Respir. Med. Case Rep. 2022, 40, 101758. [Google Scholar] [CrossRef]
- Zavascki, A.P.; Fritscher, L.; Superti, S.; Dias, C.; Kroth, L.; Traesel, M.A.; Antonello, I.C.F.; Saitovitch, D. First case report of Neisseria lactamica causing cavitary lung disease in an adult organ transplant recipient. J. Clin. Microbiol. 2006, 44, 2666–2668. [Google Scholar] [CrossRef] [PubMed]
- Changal, K.H.; Raina, A.; Altaf, S.S. Neisseria lactamica Causing a Lung Cavity and Skin Rash in a Renal Transplant Patient: First Report from India. Case Rep. Infect. Dis. 2016, 2016, 1932963. [Google Scholar] [CrossRef]
- Jang, H.J.; Choi, J.Y.; Kim, K.; Yong, S.H.; Kim, Y.W.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; et al. Relationship of the lung microbiome with PD-L1 expression and immunotherapy response in lung cancer. Respir. Res. 2021, 22, 322. [Google Scholar] [CrossRef]
- Ying, K.L.; Brasky, T.M.; Freudenheim, J.L.; McElroy, J.P.; Nickerson, Q.A.; Song, M.-A.; Weng, D.Y.; Wewers, M.D.; Whiteman, N.B.; Mathe, E.A.; et al. Saliva and Lung Microbiome Associations with Electronic Cigarette Use and Smoking. Cancer Prev. Res. 2022, 15, 435–446. [Google Scholar] [CrossRef]
- Diao, W.; Shen, N.; Du, Y.; Erb-Downward, J.R.; Sun, X.; Guo, C.; Ke, Q.; Huffnagle, G.B.; Gyetko, M.R.; He, B. Symptom-related sputum microbiota in stable chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2289–2299. [Google Scholar] [CrossRef]
- Carvalho Baptista, I.M.d.; Martinho, F.C.; Nascimento, G.G.; Da Rocha Santos, C.E.; Prado, R.F.d.; Valera, M.C. Colonization of oropharynx and lower respiratory tract in critical patients: Risk of ventilator-associated pneumonia. Arch. Oral Biol. 2018, 85, 64–69. [Google Scholar] [CrossRef]
- Rutebemberwa, A.; Stevens, M.J.; Perez, M.J.; Smith, L.P.; Sanders, L.; Cosgrove, G.; Robertson, C.E.; Tuder, R.M.; Harris, J.K. Novosphingobium and its potential role in chronic obstructive pulmonary diseases: Insights from microbiome studies. PLoS ONE 2014, 9, e111150. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.; Li, S.; Liu, S.; Jia, N.; Liu, Y.; Liu, Q.; Li, J.; Han, C. Olsenella uli-induced pneumonia: A case report. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 9. [Google Scholar] [CrossRef]
- van Charante, F.; Wieme, A.; Rigole, P.; Canck, E.d.; Ostyn, L.; Grassi, L.; Deforce, D.; Crabbé, A.; Vandamme, P.; Joossens, M.; et al. Microbial diversity and antimicrobial susceptibility in endotracheal tube biofilms recovered from mechanically ventilated COVID-19 patients. Biofilm 2022, 4, 100079. [Google Scholar] [CrossRef] [PubMed]
- Gawron-Gzella, A.; Michalak, A.; Kędzia, A. Antimicrobial activity of preparation Bioaron C. Acta Pol. Pharm. 2014, 71, 795–802. [Google Scholar]
- Miyatake, H.; Takagi, K.; Yamaki, K.; Gonda, H.; Watanabe, H.; Satake, T.; Suzuki, R.; Mizuno, K.; Aihara, H.; Hara, M. A clinical study on ceftazidime in the treatment of intractable respiratory infections. Jpn. J. Antibiot. 1990, 43, 1164–1173. [Google Scholar] [PubMed]
- Guilloux, C.-A.; Lamoureux, C.; Beauruelle, C.; Héry-Arnaud, G. Porphyromonas: A neglected potential key genus in human microbiomes. Anaerobe 2021, 68, 102230. [Google Scholar] [CrossRef]
- Brook, I. Role of encapsulated Bacteroides sp. in upper respiratory tract infection. Méd. Mal. Infect. 1990, 20, 37–44. [Google Scholar] [CrossRef]
- Marcos Sánchez, F.; Albo Castaño, I.; Arbol Linde, F.; Celdrán Gil, J. Abscesos pulmonares por Prevotella oralis y Prevotella ruminicola en paciente VIH. An. Med. Interna 2003, 20, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Onorati, I.; Guiraudet, P.; Billard-Pomares, T.; Martinod, E. A recurrent lung abscess caused by delayed diagnosis of unique co-infection with Abiotrophia defectiva. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.M.; Abdel Aziz, K.K.; Amir, A.; Waseem, S. Complicated Management of Left-Sided Loculated Empyema Secondary to Streptococcus intermedius and Prevotella in a 53-Year-Old Male. Cureus 2023, 15, e47443. [Google Scholar] [CrossRef]
- Cobo, F.; Calatrava, E.; Rodríguez-Granger, J.; Sampedro, A.; Aliaga-Martínez, L.; Navarro-Marí, J.M. A rare case of pleural effusion due to Prevotella dentalis. Anaerobe 2018, 54, 144–145. [Google Scholar] [CrossRef]
- Arneitz, C.; Windhaber, J.; Castellani, C.; Kienesberger, B.; Klymiuk, I.; Fasching, G.; Till, H.; Singer, G. Cardiorespiratory performance capacity and airway microbiome in patients following primary repair of esophageal atresia. Pediatr. Res. 2021, 90, 66–73. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Li, J.; Tan, Y.; An, T.; Zhuo, M.; Pan, Z.; Ma, M.; Jia, B.; Zhang, H.; et al. Characterization of Lung and Oral Microbiomes in Lung Cancer Patients Using Culturomics and 16S rRNA Gene Sequencing. Microbiol. Spectr. 2023, 11, e0031423. [Google Scholar] [CrossRef]
- Asif, A.A.; Roy, M.; Ahmad, S. Rare case of Prevotella pleuritidis lung abscess. BMJ Case Rep. 2020, 13, e235960. [Google Scholar] [CrossRef]
- Sakamoto, M.; Ohkusu, K.; Masaki, T.; Kako, H.; Ezaki, T.; Benno, Y. Prevotella pleuritidis sp. nov., isolated from pleural fluid. Int. J. Syst. Evol. Microbiol. 2007, 57, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Kiral, E.; Oztunali, C.; Bozan, G.; Kilic, O.; Dinleyici, E.C. Severe Course of Lung Abscess due to Multidrug Resistant Proteus mirabilis and Escherichia coli. Pediatr. Infect. Dis. J. 2023, 42, e495–e497. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Saadaat, R.; Hamidi, H.; Haidary, A.M. Proteus mirabilis: A rare cause of pneumonia, radiologically mimicking malignancy of the lung. Clin. Case Rep. 2023, 11, e7937. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Kushima, H.; Koide, Y.; Kinoshita, Y. Pseudomonas fluorescens pneumonia. Int. J. Infect. Dis. 2024, 140, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiang, L.; Yin, Y.; Li, H.; Ma, D.; Qu, Y. Pneumonia caused by Pseudomonas fluorescens: A case report. BMC Pulm. Med. 2021, 21, 212. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Goto, T.; Hirotsu, Y.; Otake, S.; Oyama, T.; Amemiya, K.; Mochizuki, H.; Omata, M. Streptococcus australis and Ralstonia pickettii as Major Microbiota in Mesotheliomas. J. Pers. Med. 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhao, Z.; Dong, M. Lobar pneumonia caused by Ralstonia pickettii in a sixty-five-year-old Han Chinese man: A case report. J. Med. Case Rep. 2011, 5, 377. [Google Scholar] [CrossRef] [PubMed]
- Hiyamuta, H.; Tsuruta, N.; Matsuyama, T.; Satake, M.; Ohkusu, K.; Higuchi, K. First case report of respiratory infection with Rothia aeria. Nihon Kokyuki Gakkai Zasshi 2010, 48, 219–223. [Google Scholar] [PubMed]
- Sonehara, K.; Araki, T.; Hanaoka, M. Rothia aeria pneumonia in an immunocompetent patient: A novel case study. Respirol. Case Rep. 2021, 9, e0843. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.J.; Kaplan, M.H. Rothia dentocariosa pneumonia in an immunocompromised patient. Lung 1987, 165, 279–282. [Google Scholar] [CrossRef]
- Wallet, F.; Perez, T.; Roussel-Delvallez, M.; Wallaert, B.; Courcol, R. Rothia dentocariosa: Two new cases of pneumonia revealing lung cancer. Scand. J. Infect. Dis. 1997, 29, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Rigauts, C.; Aizawa, J.; Taylor, S.L.; Rogers, G.B.; Govaerts, M.; Cos, P.; Ostyn, L.; Sims, S.; Vandeplassche, E.; Sze, M.; et al. Rothia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease. Eur. Respir. J. 2022, 59, 2101293. [Google Scholar] [CrossRef]
- Yang, L.; Liu, T.; Liu, B.-C.; Liu, C.-T. Severe Pneumonia Advanced to Lung Abscess and Empyema Due to Rothia Mucilaginosa in an Immunocompetent Patient. Am. J. Med. Sci. 2020, 359, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Elston, H.R.; Magnuson, C.W. Lung abscess caused by nonchromogenic Serratia marcescens. Am. Rev. Respir. Dis. 1965, 92, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.D.; Godleski, J.J.; Balikian, J.P.; Herman, P.G. Pathologic patterns of Serratia marcescens pneumonia. Hum. Pathol. 1982, 13, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Roingeard, C.; Jaubert, J.; Guilleminault, L. A large and unusual lung abscess with positive culture to Slackia exigua. Int. J. Infect. Dis. 2015, 40, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Aggarwal, T.; Pannu, A.K. Staphylococcus aureus pyomyositis and septic lung emboli. CMAJ 2022, 194, E126. [Google Scholar] [CrossRef] [PubMed]
- Goerke, C.; Wolz, C. Adaptation of Staphylococcus aureus to the cystic fibrosis lung. Int. J. Med. Microbiol. 2010, 300, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Regard, L.; Martin, C.; Teillaud, J.-L.; Lafoeste, H.; Vicaire, H.; Ladjemi, M.Z.; Ollame-Omvane, E.; Sibéril, S.; Burgel, P.-R. Effective control of Staphylococcus aureus lung infection despite tertiary lymphoid structure disorganisation. Eur. Respir. J. 2021, 57, 2000768. [Google Scholar] [CrossRef]
- Dong, Y.; Glaser, K.; Schlegel, N.; Claus, H.; Speer, C.P. An underestimated pathogen: Staphylococcus epidermidis induces pro-inflammatory responses in human alveolar epithelial cells. Cytokine 2019, 123, 154761. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Han, Y.; Zhao, X.; Sun, Y. Characteristics of pathogenic microbes in lung microenvironment of lung cancer patients without respiratory infection. J. BUON 2021, 26, 1862–1870. [Google Scholar] [PubMed]
- Chou, D.-W.; Lee, C.-T. Primary lung abscess caused by Staphylococcus lugdunensis. J. Infect. Chemother. 2017, 23, 791–793. [Google Scholar] [CrossRef]
- Mbaebie, N.C.; Alarcon Velasco, S.V.; Touhey, J. Common variable immunodeficiency presenting in a man with recurrent pneumonia caused by Staphylococcus lugdunensis. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Obase, Y.; Higashiyama, Y.; Shimafuji, T.; Yamaguchi, Y.; Nakayama, H.; Mori, N.; Ishino, T.; Hirakata, Y.; Tomono, K.; Kohno, S. Case report: Lung abscess caused by Streptococcus agalactiae. Kansenshogaku Zasshi 1997, 71, 1085–1089. [Google Scholar] [CrossRef]
- Noguchi, S.; Yatera, K.; Kawanami, T.; Yamasaki, K.; Naito, K.; Akata, K.; Shimabukuro, I.; Ishimoto, H.; Yoshii, C.; Mukae, H. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 2015, 15, 133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Kim, E.; Cox, M.J.; Brodie, E.L.; Brown, R.; Wiener-Kronish, J.P.; Lynch, S.V. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS 2010, 14, 9–59. [Google Scholar] [CrossRef]
- Farooq, H.; Mohammad, T.; Farooq, A.; Mohammad, Q. Streptococcus gordonii Empyema: A Rare Presentation of Streptococcus gordonii Infection. Cureus 2019, 11, e4611. [Google Scholar] [CrossRef]
- Manasrah, N.; Nanja Reddy, S.; Al Sbihi, A.; Hafeez, W. Streptococcus intermedius: Unusual presentation and complication of lung abscess. BMJ Case Rep. 2021, 14, e245675. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Nelson, T.B. Streptococcus intermedius lung infection masquerading as malignancy. Clin. Case Rep. 2023, 11, e8018. [Google Scholar] [CrossRef]
- Inchaustegui, C.A.; Wang, K.Y.; Teniola, O.; de Rosen, V.L. Large septic pulmonary embolus complicating streptococcus mutans pulmonary valve endocarditis. J. Radiol. Case Rep. 2018, 12, 18–27. [Google Scholar] [CrossRef]
- Nishioka, K.; Kyo, M.; Nakaya, T.; Shime, N. Proteins produced by Streptococcus species in the lower respiratory tract can modify antiviral responses against influenza virus in respiratory epithelial cells. Microbes Infect. 2021, 23, 104764. [Google Scholar] [CrossRef] [PubMed]
- Marquart, M.E. Pathogenicity and virulence of Streptococcus pneumoniae: Cutting to the chase on proteases. Virulence 2021, 12, 766–787. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Muhammad Haroon, D.; Badar, S.; Kaur, L.; Waqas, M.; Haider, F.; Syed, M.; Djekidel, K. Streptococcus pyogenes Pneumonia: A Rare and Severe Presentation in a Patient With Asthma. Cureus 2023, 15, e47182. [Google Scholar] [CrossRef] [PubMed]
- Lamoureux, C.; Guilloux, C.-A.; Beauruelle, C.; Gouriou, S.; Ramel, S.; Dirou, A.; Le Bihan, J.; Revert, K.; Ropars, T.; Lagrafeuille, R.; et al. An observational study of anaerobic bacteria in cystic fibrosis lung using culture dependant and independent approaches. Sci. Rep. 2021, 11, 6845. [Google Scholar] [CrossRef] [PubMed]
- Bosio, C.M.; Goodyear, A.W.; Dow, S.W. Early interaction of Yersinia pestis with APCs in the lung. J. Immunol. 2005, 175, 6750–6756. [Google Scholar] [CrossRef] [PubMed]
- Laws, T.R.; Davey, M.S.; Titball, R.W.; Lukaszewski, R. Neutrophils are important in early control of lung infection by Yersinia pestis. Microbes Infect. 2010, 12, 331–335. [Google Scholar] [CrossRef]
- Bittinger, K.; Charlson, E.S.; Loy, E.; Shirley, D.J.; Haas, A.R.; Laughlin, A.; Yi, Y.; Wu, G.D.; Lewis, J.D.; Frank, I.; et al. Improved characterization of medically relevant fungi in the human respiratory tract using next-generation sequencing. Genome Biol. 2014, 15, 487. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, H.C.; Gregory, C.; Brown, R.; Marchesi, J.R.; Hoogendoorn, B.; Matthews, I.P. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: A community based case control study. BMC Infect. Dis. 2013, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Lucht, L.; Tipton, L.; Rogers, M.B.; Fitch, A.; Kessinger, C.; Camp, D.; Kingsley, L.; Leo, N.; Greenblatt, R.M.; et al. Topographic diversity of the respiratory tract mycobiome and alteration in HIV and lung disease. Am. J. Respir. Crit. Care Med. 2015, 191, 932–942. [Google Scholar] [CrossRef]
- Willger, S.D.; Grim, S.L.; Dolben, E.L.; Shipunova, A.; Hampton, T.H.; Morrison, H.G.; Filkins, L.M.; O’Toole, G.A.; Moulton, L.A.; Ashare, A.; et al. Characterization and quantification of the fungal microbiome in serial samples from individuals with cystic fibrosis. Microbiome 2014, 2, 40. [Google Scholar] [CrossRef]


| Kingdom | Phylum/Division | Number of Organisms | Number of BGCs | BGCs/Organism | Average Sequence Input Size (Mbp) |
|---|---|---|---|---|---|
| Bacteria | Actinomycetota | 31 | 101 | 3.3 | 2.68 |
| Bacillota | 67 | 194 | 2.9 | 2.31 | |
| Bacteroidota | 33 | 58 | 1.8 | 3.17 | |
| Chlamydiota | 1 | - | - | 1.23 | |
| Fusobacteriota | 13 | 23 | 1.8 | 2.37 | |
| Mycoplasmatota | 2 | 0 | 0.0 | 0.82 | |
| Pseudomonadota | 79 | 537 | 6.8 | 4.04 | |
| Spirochaetota | 1 | 4 | 4.0 | 2.84 | |
| Total | 227 | 917 | 4.0 | 2.43 | |
| Fungi | Ascomycota | 47 | 693 | 14.7 | 21.00 |
| Dothideomycetes | 8 | 166 | 20.8 | 39.03 | |
| Eurotiomycetes | 7 | 334 | 47.7 | 32.25 | |
| Saccharomycetes | 26 | 39 | 1.5 | 12.97 | |
| Sordariomycetes | 3 | 108 | 36 | 36.5 | |
| Basidomycota | 45 | 220 | 4.9 | 38.36 | |
| Agaricomycetes | 29 | 195 | 6.7 | 42.71 | |
| Rozellomycota | 1 | - | - | - | |
| Total | 93 | 913 | 9.8 | 29.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semmler, F.; Regis Belisário-Ferrari, M.; Kulosa, M.; Kaysser, L. The Metabolic Potential of the Human Lung Microbiome. Microorganisms 2024, 12, 1448. https://doi.org/10.3390/microorganisms12071448
Semmler F, Regis Belisário-Ferrari M, Kulosa M, Kaysser L. The Metabolic Potential of the Human Lung Microbiome. Microorganisms. 2024; 12(7):1448. https://doi.org/10.3390/microorganisms12071448
Chicago/Turabian StyleSemmler, Florian, Matheus Regis Belisário-Ferrari, Maria Kulosa, and Leonard Kaysser. 2024. "The Metabolic Potential of the Human Lung Microbiome" Microorganisms 12, no. 7: 1448. https://doi.org/10.3390/microorganisms12071448
APA StyleSemmler, F., Regis Belisário-Ferrari, M., Kulosa, M., & Kaysser, L. (2024). The Metabolic Potential of the Human Lung Microbiome. Microorganisms, 12(7), 1448. https://doi.org/10.3390/microorganisms12071448





