Evaluating the Safety of Bacillus cereus GW-01 Obtained from Sheep Rumen Chyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Information on Genomes
2.2. Prediction of Prophages, T3SS Effector Proteins, Virulence Factors, Antimicrobial Resistance Genes, and CRISPR Candidates
2.3. Phylogenetic and Pan Genome Analysis
2.4. RNA Sequencing and Transcriptomics Analysis
2.5. Data Analyses
3. Results
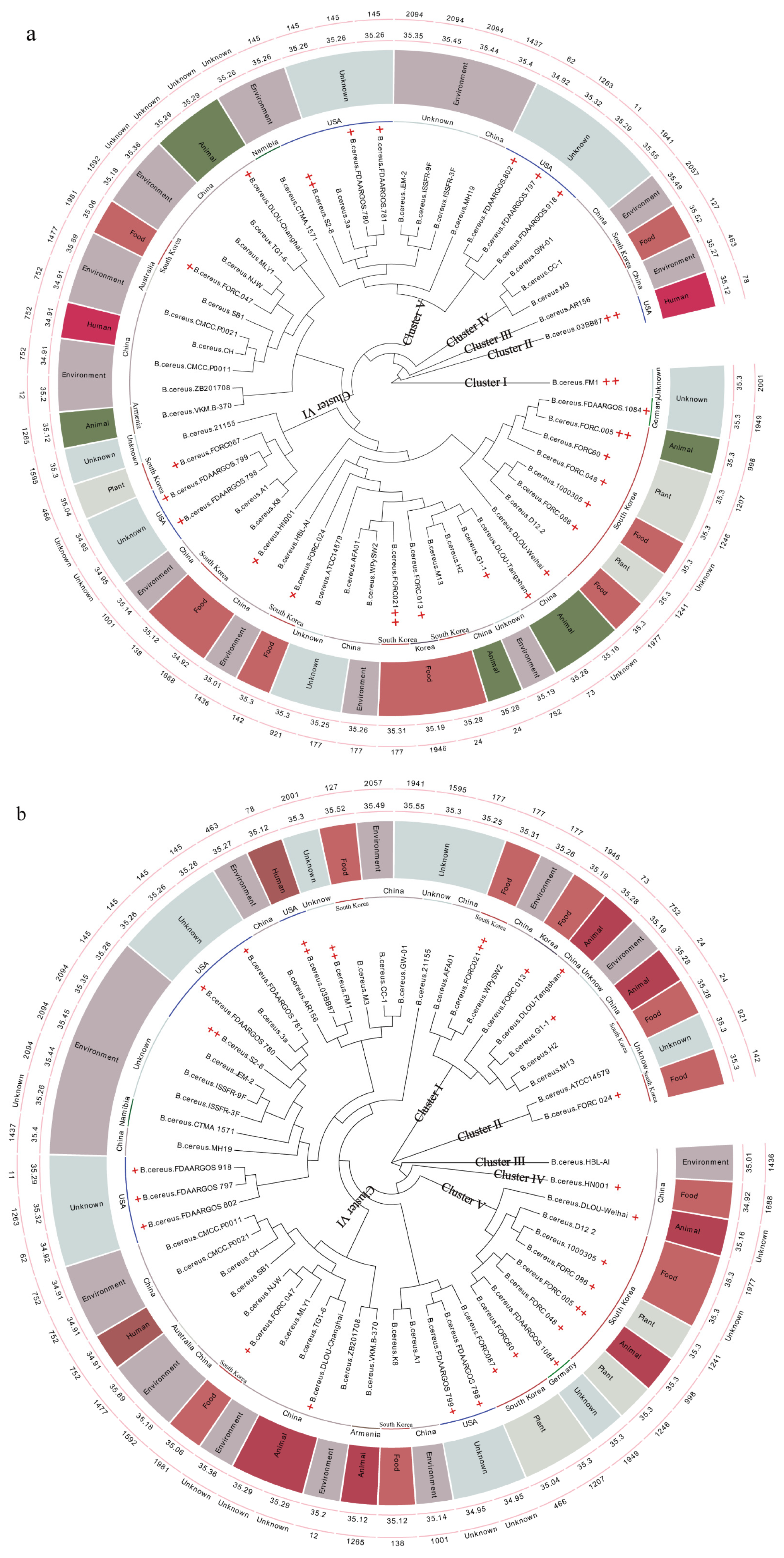
3.1. Phylogenetic Analysis of Bacillus cereus GW-01
3.2. Prophage, CRISPR, T3SS Effector Protein, VFDB, CARD, and Genomic Island Predictions
3.3. Secretion Pathway of Toxin in GW-01 Based on Transcriptome Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al Azad, S.; Moazzem Hossain, K.; Rahman, S.M.M.; Al Mazid, M.F.; Barai, P.; Gazi, M.S. In ovo inoculation of duck embryos with different strains of Bacillus cereus to analyse their synergistic post-hatch anti-allergic potentialities. Vet. Med. Sci. 2020, 6, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Anis Ahamed, N.; Panneerselvam, A.; Arif, I.A.; Syed Abuthakir, M.H.; Jeyam, M.; Ambikapathy, V.; Mostafa, A.A. Identification of potential drug targets in human pathogen Bacillus cereus and insight for finding inhibitor through subtractive proteome and molecular docking studies. J. Infect. Public Health 2021, 14, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Oh, D.H. Impact of the Isolation Source on the Biofilm Formation Characteristics of Bacillus cereus. J. Microbiol. Biotechnol. 2018, 28, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Scharek, L.; Altherr, B.J.; Tölke, C.; Schmidt, M.F.G. Influence of the probiotic Bacillus cereus var. toyoi on the intestinal immunity of piglets. Vet. Immunol. Immunopathol. 2007, 120, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.F.; Märtlbauer, E.; Dietrich, R.; Luo, H.L.; Ding, S.Y.; Zhu, K. Multifaceted toxin profile, an approach toward a better understanding of probiotic Bacillus cereus. Crit. Rev. Toxicol. 2019, 49, 342–356. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Thorsen, L.; Kpikpi, E.N.; Stuer-Lauridsen, B.; Cantor, M.D.; Nielsen, B.; Brockmann, E.; Derkx, P.M.F.; Jespersen, L. Characterization of Bacillus spp. strains for use as probiotic additives in pig feed. Appl. Microbiol. Biotechnol. 2014, 98, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Grutsch, A.A.; Nimmer, P.S.; Pittsley, R.H.; Kornilow, K.G.; McKillip, J.L. Molecular pathogenesis of Bacillus spp., with emphasis on the dairy industry. Fine Focus 2018, 4, 203–222. [Google Scholar] [CrossRef]
- Wang, S.; Duan, H.; Zhang, W.; Li, J.-W. Analysis of bacterial foodborne disease outbreaks in China between 1994 and 2005. FEMS Immunol. Med. Microbiol. 2007, 51, 8–13. [Google Scholar] [CrossRef]
- Ceuppens, S.; Boon, N.; Rajkovic, A.; Heyndrickx, M.; Van de Wiele, T.; Uyttendaele, M. Quantification methods for Bacillus cereus vegetative cells and spores in the gastrointestinal environment. J. Microbiol. Methods 2010, 83, 202–210. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Z.; Gu, X.; Zhao, J.; Guo, T.; Kong, J. Probiotic Bacillus subtilis LF11 Protects Intestinal Epithelium Against Salmonella Infection. Front. Cell. Infect. Microbiol. 2022, 12, 837886. [Google Scholar] [CrossRef]
- Beecher, D.J.; Schoeni, J.L.; Wong, A.C. Enterotoxic activity of hemolysin BL from Bacillus cereus. Infect. Immun. 1995, 63, 4423–4428. [Google Scholar] [CrossRef]
- Jessberger, N.; Diedrich, R.; Janowski, R.; Niessing, D.; Märtlbauer, E. Presence and function of Hbl B’, the fourth protein component encoded by the hbl operon in Bacillus cereus. Virulence 2022, 13, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, R.; Moravek, M.; Bürk, C.; Granum Per, E.; Märtlbauer, E. Production and Characterization of Antibodies against Each of the Three Subunits of the Bacillus cereus Nonhemolytic Enterotoxin Complex. Appl. Environ. Microbiol. 2005, 71, 8214–8220. [Google Scholar] [CrossRef] [PubMed]
- Didier, A.; Dietrich, R.; Martlbauer, E. Antibody Binding Studies Reveal Conformational Flexibility of the Bacillus cereus Non-Hemolytic Enterotoxin (Nhe) A-Component. PLoS ONE 2016, 11, e0165135. [Google Scholar] [CrossRef] [PubMed]
- Fichant, A.; Lanceleur, R.; Hachfi, S.; Brun-Barale, A.; Blier, A.L.; Firmesse, O.; Gallet, A.; Fessard, V.; Bonis, M. New Approach Methods to Assess the Enteropathogenic Potential of Strains of the Bacillus cereus Group, including Bacillus thuringiensis. Foods 2024, 13, 1140. [Google Scholar] [CrossRef]
- Lund, T.; De Buyser, M.-L.; Granum, P.E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 2000, 38, 254–261. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- European Food Safety Authority. Opinion of the Scientific Panel on biological hazards (BIOHAZ) on Bacillus cereus and other Bacillus spp in foodstuffs. EFSA J. 2005, 3, 175. [Google Scholar] [CrossRef]
- Rajkovic, A.; Uyttendaele, M.; Ombregt, S.-A.; Jaaskelainen, E.; Salkinoja-Salonen, M.; Debevere, J. Influence of Type of Food on the Kinetics and Overall Production of Bacillus cereus Emetic Toxin. J. Food Prot. 2006, 69, 847–852. [Google Scholar] [CrossRef]
- Mascarenhas, L.R.D.; Vivoni, A.M.; Caetano, R.G.; Rusak, L.A.; Alvarenga, V.O.; Lacerda, I.C.A. Molecular characterization and toxigenic profiles of Bacillus cereus isolates from foodstuff and food poisoning outbreaks in Brazil. Braz. J. Microbiol. 2024, 55, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, G.Q.; Cui, Y.S.; Xiang, J.S.; Zhu, S.; Li, S.J.; Huang, J.Y.; Wang, Y.F.; Liu, Y.; Zhou, L. Genomic characterization of Bacillus cereus isolated from food poisoning cases revealed the mechanism of toxin production. Front. Microbiol. 2024, 14, 1238799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Hölzel, C.S.; Cui, Y.F.; Mayer, R.; Wang, Y.; Dietrich, R.; Didier, A.; Bassitta, R.; Märtlbauer, E.; Ding, S.Y. Probiotic Bacillus cereus Strains, a Potential Risk for Public Health in China. Front. Microbiol. 2016, 7, 718–719. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.R.; Chen, Y.S.; Sun, T.Y.; Wu, Y.T.; Su, Y.T.; Liu, C.Y.; Zhou, J.Y.; Deng, Y.Q.; Wen, J.K. Antimicrobial resistance, virulence characteristics and genotypes of Bacillus spp. from probiotic products of diverse origins. Food Res. Int. 2021, 139, 109949. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, W.Q.; Li, J.Z.; Wang, Z.H.; Xiao, D.; Wang, Y.F.; Ni, X.Q.; Zeng, D.; Zhang, D.M.; Jing, B.; et al. Screening of differentially expressed immune-related genes from spleen of broilers fed with probiotic Bacillus cereus PAS38 based on suppression subtractive hybridization. PLoS ONE 2019, 14, e0226829. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Z.; Yang, Q.; He, F.L.; Lan, R.T.; Hao, J.W.; Ni, P.; Liu, Y.; Li, R.J. National Safety Survey of Animal-use Commercial Probiotics and Their Spillover Effects From Farm to Humans: An Emerging Threat to Public Health. Clin. Infect. Dis. 2020, 70, 2386–2395. [Google Scholar] [CrossRef] [PubMed]
- Trapecar, M.; Leouffre, T.; Faure, M.; Jensen, H.E.; Granum, P.E.; Cencic, A.; Hardy, S.P. The use of a porcine intestinal cell model system for evaluating the food safety risk of Bacillus cereus probiotics and the implications for assessing enterotoxigenicity. Apmis 2011, 119, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, W.X.; Guo, H.; Yu, S.B.; Xue, L.; Chen, M.T.; Zhang, J.M.; Xu, Z.L.; Wu, Q.P.; Wang, J.N.; et al. The potential of lactose to inhibit cereulide biosynthesis of emetic Bacillus cereus in milk. Int. J. Food Microbiol. 2024, 411, 110517. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.D.; Burdock, G.A.; Jiménez, G.; Castillo, M. Literature review on the safety of Toyocerin®, a non-toxigenic and non-pathogenic Bacillus cereus var. toyoi preparation. Regul. Toxicol. Pharmacol. 2009, 55, 236–246. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Y.; Liu, X.; Xia, X.; Ding, S.; Zhu, K. Evaluation of the Toxicity and Toxicokinetics of Cereulide from an Emetic Bacillus cereus Strain of Milk Origin. Toxins 2016, 8, 156. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.T.; Liu, Y.T.; Jia, K.; Zhang, Z.; Dong, Q.L. Cereulide and Emetic Bacillus cereus: Characterizations, Impacts and Public Precautions. Foods 2023, 12, 833. [Google Scholar] [CrossRef] [PubMed]
- Durval, I.J.B.; Mendonça, A.H.R.; Rocha, I.V.; Luna, J.M.; Rufino, R.D.; Converti, A.; Sarubbo, L.A. Production, characterization, evaluation and toxicity assessment of a Bacillus cereus UCP 1615 biosurfactant for marine oil spills bioremediation. Mar. Pollut. Bull. 2020, 157, 111357. [Google Scholar] [CrossRef] [PubMed]
- Duport, C.; Rousset, L.; Alpha-Bazin, B.; Armengaud, J. Bacillus cereus Decreases NHE and CLO Exotoxin Synthesis to Maintain Appropriate Proteome Dynamics During Growth at Low Temperature. Toxins 2020, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Tsigarida, E.; Robinson, T.; Calistri, P. The EFSA Scientific Panel on Biological Hazards first mandate: May 2003-May 2006. Insight into foodborne zoonoses. Trends Food Sci. Technol. 2009, 20, 188–193. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Y.; Gong, L.; Chen, X.; Xie, Q.; Jin, Y.; Du, J.; Wang, S.; Liu, G. Mechanism of β-cypermethrin metabolism by Bacillus cereus GW-01. Chem. Eng. J. 2022, 430, 132961. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, Y.; Si, C.; Du, J.; Xia, C.; Wang, Y.-n.; Liu, G.; Li, Q.; Zhao, J. Oral administration of Bacillus cereus GW-01 alleviates the accumulation and detrimental effects of β-cypermethrin in mice. Chemosphere 2023, 312, 137333. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Aziz, R.K.; Edwards, R.A. PhiSpy: A novel algorithm for finding prophages in bacterial genomes that combines similarity- and composition-based strategies. Nucleic Acids Res. 2012, 40, e126. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.; Wan, I.; Jones, S.J.; Brinkman, F.S.J.B. IslandPath: Aiding detection of genomic islands in prokaryotes. Bioinformatics 2003, 19, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Waack, S.; Keller, O.; Asper, R.; Brodag, T.; Damm, C.; Fricke, W.F.; Surovcik, K.; Meinicke, P.; Merkl, R. Score-based prediction of genomic islands in prokaryotic genomes using hidden Markov models. BMC Bioinform. 2006, 7, 142. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Hsiao, W.W.L.; Brinkman, F.S.L. Evaluation of genomic island predictors using a comparative genomics approach. BMC Bioinform. 2008, 9, 329. [Google Scholar] [CrossRef]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Simon Fraser University Research Computing Group; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S.L. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef]
- Castillo, D.; Christiansen, R.H.; Dalsgaard, I.; Madsen, L.; Espejo, R.; Middelboe, M. Comparative Genome Analysis Provides Insights into the Pathogenicity of Flavobacterium psychrophilum. PLoS ONE 2016, 11, e0152515. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.G.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Q.; Du, J.; Shao, Z. Genetic diversity and population structure of the Bacillus cereus group bacteria from diverse marine environments. Sci. Rep. 2017, 7, 689. [Google Scholar] [CrossRef]
- Tourasse, N.J.; Helgason, E.; Økstad, O.A.; Hegna, I.K.; KolstØ, A.B. The Bacillus cereus group: Novel aspects of population structure and genome dynamics. J. Appl. Microbiol. 2006, 101, 579–593. [Google Scholar] [CrossRef]
- Priest Fergus, G.; Barker, M.; Baillie Les, W.J.; Holmes Edward, C.; Maiden Martin, C.J. Population Structure and Evolution of the Bacillus cereus Group. J. Bacteriol. 2004, 186, 7959–7970. [Google Scholar] [CrossRef]
- Gdoura-Ben Amor, M.; Siala, M.; Zayani, M.; Grosset, N.; Smaoui, S.; Messadi-Akrout, F.; Baron, F.; Jan, S.; Gautier, M.; Gdoura, R. Isolation, Identification, Prevalence, and Genetic Diversity of Bacillus cereus Group Bacteria From Different Foodstuffs in Tunisia. Front. Microbiol. 2018, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Chica, J.; Correa, M.M.; Aceves-Diez, A.E.; Castaneda-Sandoval, L.M. Genetic and toxigenic diversity of Bacillus cereus group isolated from powdered foods. J. Food Sci. Technol. 2021, 58, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Tsai, P.J.; Chen, Y.L.; Pranata, R.; Chen, R.J. Assessment of the Antibacterial Mechanism of Pterostilbene against Bacillus cereus through Apoptosis-like Cell Death and Evaluation of Its Beneficial Effects on the Gut Microbiota. J. Agric. Food Chem. 2021, 69, 12219–12229. [Google Scholar] [CrossRef]
- Baldwin, V.M. You Can’t B. cereus—A Review of Bacillus cereus Strains That Cause Anthrax-Like Disease. Front. Microbiol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus Food Infection as Multifactorial Process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef]
- Torres Manno, M.A.; Repizo, G.D.; Magni, C.; Dunlap, C.A.; Espariz, M. The assessment of leading traits in the taxonomy of the Bacillus cereus group. Antonie Van Leeuwenhoek 2020, 113, 2223–2242. [Google Scholar] [CrossRef]
- Böhm, M.-E.; Huptas, C.; Krey, V.M.; Scherer, S. Massive horizontal gene transfer, strictly vertical inheritance and ancient duplications differentially shape the evolution of Bacillus cereus enterotoxin operons hbl, cytK and nhe. BMC Evol. Biol. 2015, 15, 246. [Google Scholar] [CrossRef]
- Novichkov Pavel, S.; Wolf Yuri, I.; Dubchak, I.; Koonin Eugene, V. Trends in Prokaryotic Evolution Revealed by Comparison of Closely Related Bacterial and Archaeal Genomes. J. Bacteriol. 2009, 191, 65–73. [Google Scholar] [CrossRef]
- Kim, Y.; Koh, I.; Young Lim, M.; Chung, W.-H.; Rho, M. Pan-genome analysis of Bacillus for microbiome profiling. Sci. Rep. 2017, 7, 10984. [Google Scholar] [CrossRef]
- Guinebretière, M.-H.; Thompson, F.L.; Sorokin, A.; Normand, P.; Dawyndt, P.; Ehling-Schulz, M.; Svensson, B.; Sanchis, V.; Nguyen-The, C.; Heyndrickx, M.; et al. Ecological diversification in the Bacillus cereus Group. Environ. Microbiol. 2008, 10, 851–865. [Google Scholar] [CrossRef]
- Tourasse, N.J.; Økstad, O.A.; Kolstø, A.-B. HyperCAT: An extension of the SuperCAT database for global multi-scheme and multi-datatype phylogenetic analysis of the Bacillus cereus group population. Database 2010, 2010, baq017. [Google Scholar] [CrossRef] [PubMed]
- Drewnowska, J.M.; Swiecicka, I. Eco-Genetic Structure of Bacillus cereus sensu lato Populations from Different Environments in Northeastern Poland. PLoS ONE 2013, 8, e80175. [Google Scholar] [CrossRef] [PubMed]
- Didelot, X.; Barker, M.; Falush, D.; Priest, F.G. Evolution of pathogenicity in the Bacillus cereus group. Syst. Appl. Microbiol. 2009, 32, 81–90. [Google Scholar] [CrossRef]
- Schmidt, T.R.; Scott, E.J.; Dyer, D.W. Whole-genome phylogenies of the family Bacillaceae and expansion of the sigma factor gene family in the Bacillus cereus species-group. BMC Genom. 2011, 12, 430. [Google Scholar] [CrossRef]
- Zwick, M.E.; Joseph, S.J.; Didelot, X.; Chen, P.E.; Bishop-Lilly, K.A.; Stewart, A.C.; Willner, K.; Nolan, N.; Lentz, S.; Thomason, M.K.; et al. Genomic characterization of the Bacillus cereus sensu lato species: Backdrop to the evolution of Bacillus anthracis. Genome Res. 2012, 22, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, Q.; Göker, M.; Meier-Kolthoff, J.P.; Wang, M.; Sun, Y.; Wang, L.; Shao, Z. Genomic insights into the taxonomic status of the Bacillus cereus group. Sci. Rep. 2015, 5, 14082. [Google Scholar] [CrossRef] [PubMed]
- Jeßberger, N.; Krey, V.M.; Rademacher, C.; Böhm, M.-E.; Mohr, A.-K.; Ehling-Schulz, M.; Scherer, S.; Märtlbauer, E. From genome to toxicity: A combinatory approach highlights the complexity of enterotoxin production in Bacillus cereus. Front. Microbiol. 2015, 6, 560. [Google Scholar]
- Nevers, A.; Kranzler, M.; Perchat, S.; Gohar, M.; Sorokin, A.; Lereclus, D.; Ehling-Schulz, M.; Sanchis-Borja, V. Plasmid e Chromosome interplay in natural and non-natural hosts: Global transcription study of three Bacillu cereus group strains carrying pCER270 plasmid. Res. Microbiol. 2023, 174, 111357. [Google Scholar] [CrossRef]
- Ramarao, N.; Tran, S.L.; Marin, M.; Vidic, J. Advanced Methods for Detection of Bacillus cereus and Its Pathogenic Factors. Sensors 2020, 20, 2667. [Google Scholar] [CrossRef]
- Pena-Gonzalez, A.; Rodriguez, R.L.M.; Marston, C.K.; Gee, J.E.; Gulvik, C.A.; Kolton, C.B.; Saile, E.; Frace, M.; Hoffmaster, A.R.; Konstantinidis, K.T. Genomic Characterization and Copy Number Variation of Bacillus anthracis Plasmids pXO1 and pXO2 in a Historical Collection of 412 Strains. Msystems 2018, 3, e00065-18. [Google Scholar] [CrossRef]
- Zervas, A.; Aggerbeck, M.R.; Allaga, H.; Güzel, M.; Hendriks, M.; Jonuškienė, I.; Kedves, O.; Kupeli, A.; Lamovšek, J.; Mülner, P.; et al. Identification and Characterization of 33 Bacillus cereus sensu lato Isolates from Agricultural Fields from Eleven Widely Distributed Countries by Whole Genome Sequencing. Microorganisms 2020, 8, 2028. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Svensson, B.; Guinebretiere, M.-H.; Lindbäck, T.; Andersson, M.; Schulz, A.; Fricker, M.; Christiansson, A.; Granum, P.E.; Märtlbauer, E.; et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 2005, 151, 183–197. [Google Scholar] [CrossRef]
- Ramarao, N.; Sanchis, V. The Pore-Forming Haemolysins of Bacillus cereus: A Review. Toxins 2013, 5, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, D.W.; Glasset, B.; Dervyn, R.; Guérin, C.; Plancade, S.; Herbin, S.; Brisabois, A.; Nicolas, P.; Ramarao, N. New genetic biomarkers to differentiate non-pathogenic from clinically relevant Bacillus cereus strains. Clin. Microbiol. Infect. 2022, 28, 137.e1–137.e8. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, T.; Furukawa, N.; Aikawa, C.; Watanabe, T.; Haobam, B.; Kurokawa, K.; Maruyama, F.; Nakagawa, I. CRISPR Inhibition of Prophage Acquisition in Streptococcus pyogenes. PLoS ONE 2011, 6, e19543. [Google Scholar] [CrossRef]
- Marraffini Luciano, A.; Sontheimer Erik, J. CRISPR Interference Limits Horizontal Gene Transfer in Staphylococci by Targeting DNA. Science 2008, 322, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Dobrindt, U.; Hochhut, B.; Hentschel, U.; Hacker, J. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2004, 2, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, M.; Dalmasso, G.; Beyrouthy, R.; Barnich, N.; Delmas, J.; Bonnet, R. Pathogenicity Factors of Genomic Islands in Intestinal and Extraintestinal Escherichia coli. Front. Microbiol. 2020, 11, 2065. [Google Scholar] [CrossRef]
- Duport, C.; Jobin, M.; Schmitt, P. Adaptation in Bacillus cereus: From Stress to Disease. Front. Microbiol. 2016, 7, 1550. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Guinebretiere, M.-H.; Monthán, A.; Berge, O.; Fricker, M.; Svensson, B. Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol. Lett. 2006, 260, 232–240. [Google Scholar] [CrossRef]
- Guinebretière, M.-H.; Broussolle, V.; Nguyen-The, C. Enterotoxigenic Profiles of Food-Poisoning and Food-Borne Bacillus cereus Strains. J. Clin. Microbiol. 2002, 40, 3053–3056. [Google Scholar] [CrossRef]
- Moravek, M.; Dietrich, R.; Buerk, C.; Broussolle, V.; Guinebretière, M.-H.; Granum, P.E.; Nguyen-the, C.; Märtlbauer, E. Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol. Lett. 2006, 257, 293–298. [Google Scholar] [CrossRef]
- Wehrle, E.; Moravek, M.; Dietrich, R.; Bürk, C.; Didier, A.; Märtlbauer, E. Comparison of multiplex PCR, enzyme immunoassay and cell culture methods for the detection of enterotoxinogenic Bacillus cereus. J. Microbiol. Methods 2009, 78, 265–270. [Google Scholar] [CrossRef]
- Fagerlund, A.; Lindbäck, T.; Granum, P.E. Bacillus cereus cytotoxins Hbl, Nhe and CytK are secreted via the Sec translocation pathway. BMC Microbiol. 2010, 10, 304. [Google Scholar] [CrossRef]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Ceragioli, M.; Beecher Douglas, J.; Senesi, S.; Wong Amy, C.L. Swarming Behavior of and Hemolysin BL Secretion by Bacillus cereus. Appl. Environ. Microbiol. 2007, 73, 4089–4093. [Google Scholar] [CrossRef]
- Ghelardi, E.; Celandroni, F.; Salvetti, S.; Beecher Douglas, J.; Gominet, M.; Lereclus, D.; Wong Amy, C.L.; Senesi, S. Requirement of flhA for Swarming Differentiation, Flagellin Export, and Secretion of Virulence-Associated Proteins in Bacillus thuringiensis. J. Bacteriol. 2002, 184, 6424–6433. [Google Scholar] [CrossRef]
- Fukumura, T.; Makino, F.; Dietsche, T.; Kinoshita, M.; Kato, T.; Wagner, S.; Namba, K.; Imada, K.; Minamino, T. Assembly and stoichiometry of the core structure of the bacterial flagellar type III export gate complex. PLoS Biol. 2017, 15, e2002281. [Google Scholar] [CrossRef]
- Minamino, T.; Macnab Robert, M. Components of the Salmonella Flagellar Export Apparatus and Classification of Export Substrates. J. Bacteriol. 1999, 181, 1388–1394. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Zhang, L.Y.; Wang, X.; Sun, F.L.; Kong, X.X.; Dong, H.S.; Xu, H. Two virulent sRNAs identified by genomic sequencing target the type III secretion system in rice bacterial blight pathogen. BMC Plant Biol. 2018, 18, 237. [Google Scholar] [CrossRef]
- Hueck Christoph, J. Type III Protein Secretion Systems in Bacterial Pathogens of Animals and Plants. Microbiol. Mol. Biol. Rev. 1998, 62, 379–433. [Google Scholar] [CrossRef]
- Senesi, S.; Ghelardi, E. Production, Secretion and Biological Activity of Bacillus cereus Enterotoxins. Toxins 2010, 2, 1690–1703. [Google Scholar] [CrossRef]
- Salvetti, S.; Ghelardi, E.; Celandroni, F.; Ceragioli, M.; Giannessi, F.; Senesi, S. FlhF, a signal recognition particle-like GTPase, is involved in the regulation of flagellar arrangement, motility behaviour and protein secretion in Bacillus cereus. Microbiology 2007, 153, 2541–2552. [Google Scholar] [CrossRef]
- Tsirigotaki, A.; De Geyter, J.; Šoštaric, N.; Economou, A.; Karamanou, S. Protein export through the bacterial Sec pathway. Nat. Rev. Microbiol. 2017, 15, 21–36. [Google Scholar] [CrossRef]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins—The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Park, E.-j.; Hussain, M.S.; Wei, S.; Kwon, M.; Oh, D.-H. Genotypic and phenotypic characteristics of biofilm formation of emetic toxin producing Bacillus cereus strains. Food Control 2019, 96, 527–534. [Google Scholar] [CrossRef]
- Yan, F.; Yu, Y.; Wang, L.; Luo, Y.; Guo, J.-h.; Chai, Y. The comER Gene Plays an Important Role in Biofilm Formation and Sporulation in both Bacillus subtilis and Bacillus cereus. Front. Microbiol. 2016, 7, 1025. [Google Scholar] [CrossRef]
- Lindbäck, T.; Mols, M.; Basset, C.; Granum, P.E.; Kuipers, O.P.; Kovács, Á.T. CodY, a pleiotropic regulator, influences multicellular behaviour and efficient production of virulence factors in Bacillus cereus. Environ. Microbiol. 2012, 14, 2233–2246. [Google Scholar] [CrossRef]
- Hayrapetyan, H.; Tempelaars, M.; Nierop Groot, M.; Abee, T. Bacillus cereus ATCC 14579 RpoN (Sigma 54) Is a Pleiotropic Regulator of Growth, Carbohydrate Metabolism, Motility, Biofilm Formation and Toxin Production. PLoS ONE 2015, 10, e0134872. [Google Scholar] [CrossRef]
- Heilkenbrinker, U.; Dietrich, R.; Didier, A.; Zhu, K.; Lindbäck, T.; Granum, P.E.; Märtlbauer, E. Complex Formation between NheB and NheC Is Necessary to Induce Cytotoxic Activity by the Three-Component Bacillus cereus Nhe Enterotoxin. PLoS ONE 2013, 8, e63104. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Huang, X.; Qin, H.; Lei, Y.; Zhao, S.; Liu, S.; Liu, G.; Zhao, J. Evaluating the Safety of Bacillus cereus GW-01 Obtained from Sheep Rumen Chyme. Microorganisms 2024, 12, 1457. https://doi.org/10.3390/microorganisms12071457
Xu B, Huang X, Qin H, Lei Y, Zhao S, Liu S, Liu G, Zhao J. Evaluating the Safety of Bacillus cereus GW-01 Obtained from Sheep Rumen Chyme. Microorganisms. 2024; 12(7):1457. https://doi.org/10.3390/microorganisms12071457
Chicago/Turabian StyleXu, Bowen, Xinyi Huang, Haixiong Qin, Ying Lei, Sijia Zhao, Shan Liu, Gang Liu, and Jiayuan Zhao. 2024. "Evaluating the Safety of Bacillus cereus GW-01 Obtained from Sheep Rumen Chyme" Microorganisms 12, no. 7: 1457. https://doi.org/10.3390/microorganisms12071457





