Cyanobacterial Bloom Formation by Enhanced Ecological Adaptability and Competitive Advantage of Microcystis—Non-Negligible Role of Quorum Sensing
Abstract
1. Introduction
2. QS Discovery and Signaling Molecule Recognition in Microcystis
2.1. Cell Density-Dependent Regulatory Behaviors in Microcystis
2.2. Discovery and Recognition of QS Signaling Molecules in Microcystis
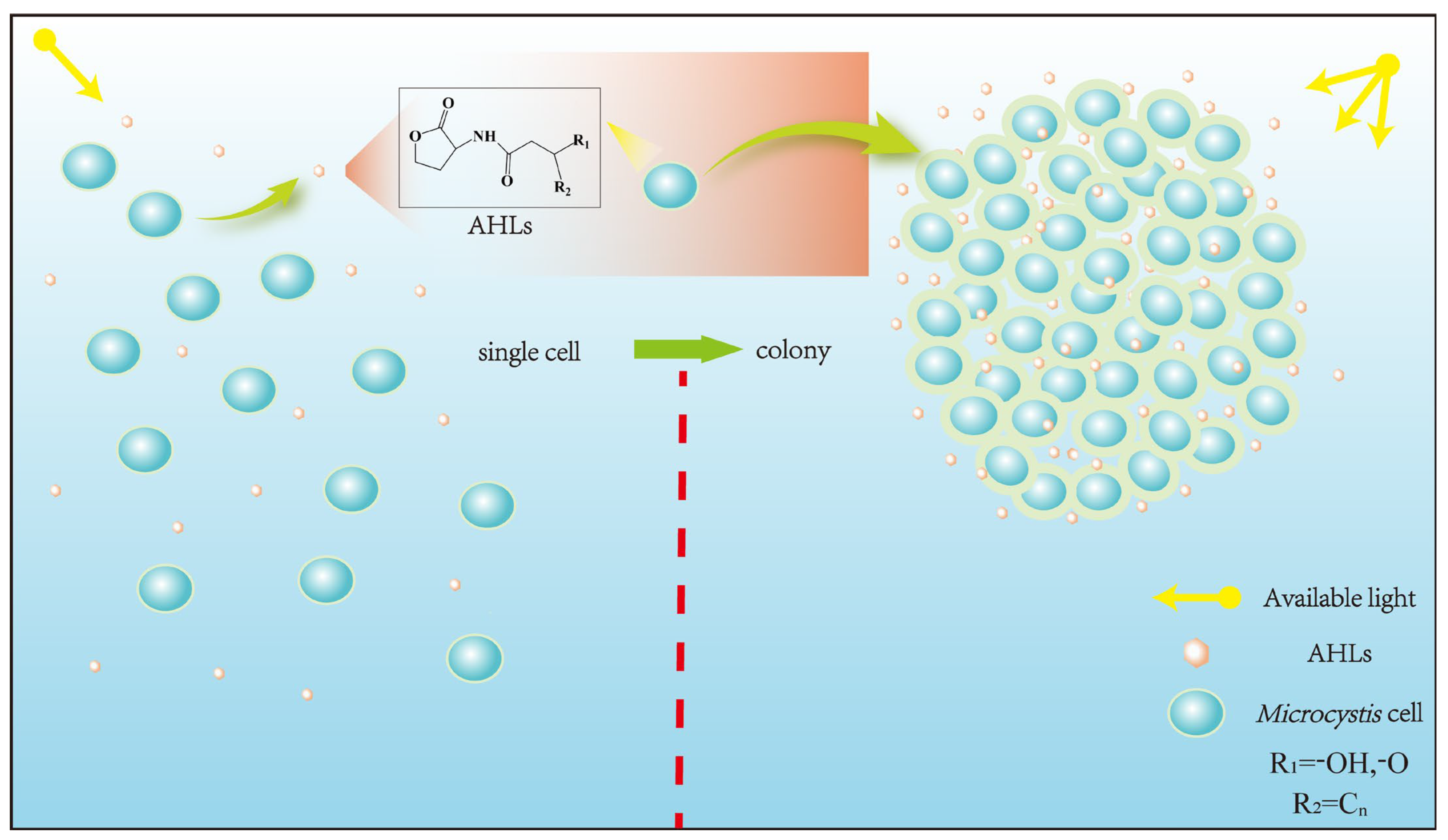
3. AHLs’ Roles in Regulating Physiology and Colony Formation of Microcystis
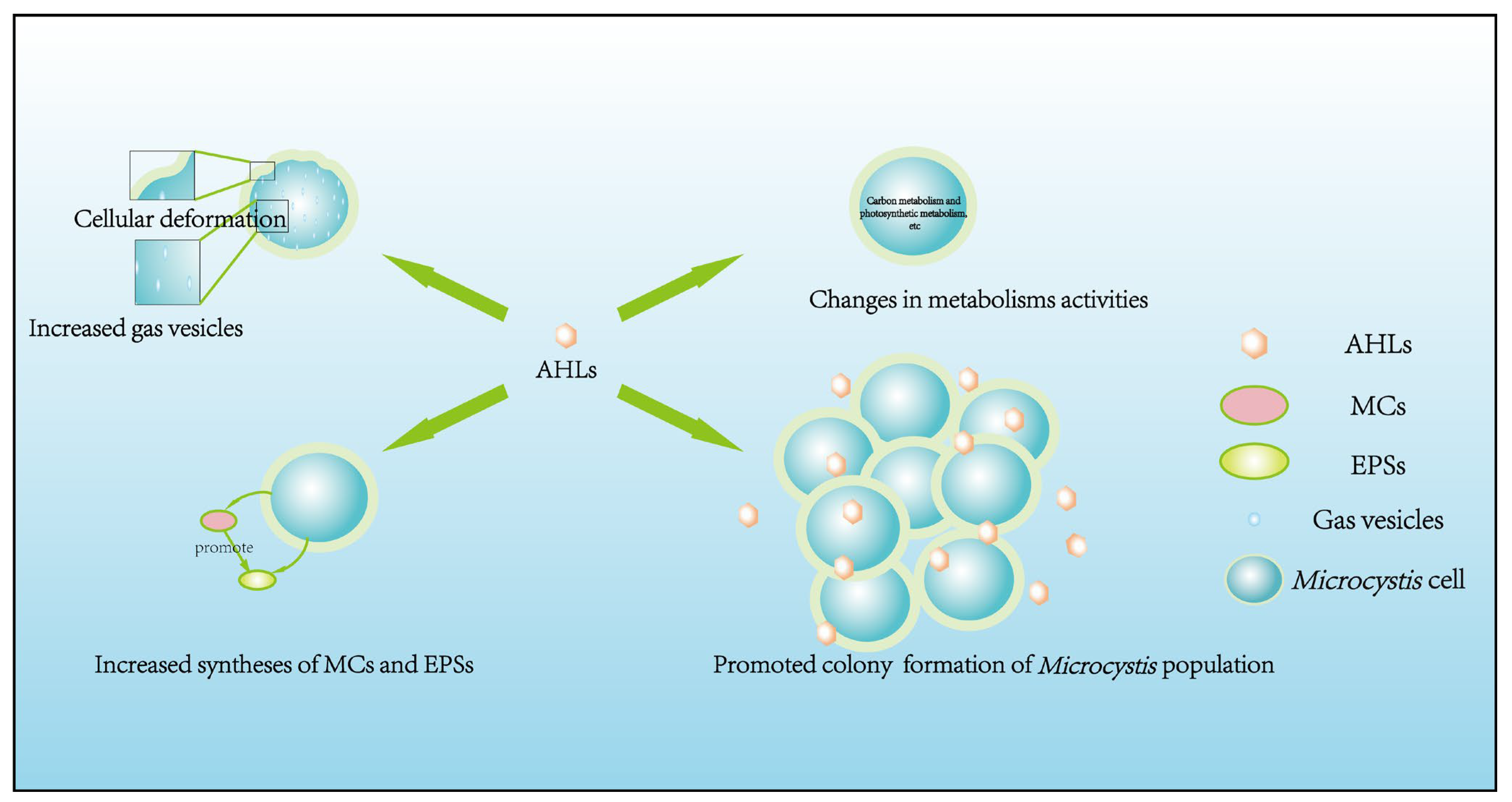
3.1. Regulatory Effects of Endogenous AHLs
3.1.1. Cellular Morphology
3.1.2. Cellular Physiological Adaptability
3.1.3. Nutrition and Energy Metabolism Activities
3.1.4. Colony Formation
3.2. Regulatory Effects of Exogenous AHLs
4. Genetic Information of AHL-Mediated QS in Microcystis
5. Quorum Quenching (QQ) of Microcystis and Application Implication
6. Future Perspectives
- (i)
- Elucidating the genetic mechanisms for QS and QQ of Microcystis, especially clarifying related gene functions and action pathways, using modern molecular biotechnologies and bioinformatics;
- (ii)
- Further identifying AHLs species produced by Microcystis and exploring their effects on the growth and physiology of many other bloom-forming algae;
- (iii)
- Exploring other QS-signaling molecules besides AHLs in Microcystis and whether some AHLs species produced by Microcystis can inhibit Microcystis’s QS, then comparing the molecular structure difference between QS-inducing and QS-inhibiting AHLs for Microcystis;
- (iv)
- Surveying the effects of diverse environmental factors on aqueous AHLs species distributions and AHLs’ effects.
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, S.B. Chance and necessity: The evolution of morphological complexity and diversity. Nature 2001, 409, 1102–1109. [Google Scholar] [CrossRef]
- Li, B.Y.; Li, J.M.; An, G.Q.; Zhao, C.H.; Wang, C.Y. Long-term and strong suppression against Microcystis growth and microcystin-release by luteolin continuous-release microsphere: Optimal construction, characterization, effects and proteomic mechanisms. Water Res. 2021, 202, 117448. [Google Scholar] [CrossRef]
- Gagala, I.; Izydorczyk, K.; Jurczak, T.; Pawelczyk, J.; Dziadek, J.; Wojtal-Frankiewicz, A.; Jozwik, A.; Jaskulska, A.; Mankiewicz-Boczek, J. Role of environmental factors and toxic genotypes in the regulation of microcystins-producing cyanobacterial blooms. Microb. Ecol. 2014, 67, 465–479. [Google Scholar] [CrossRef]
- Zuo, J.; Chen, L.T.; Shan, K.; Hu, L.L.; Song, L.R.; Gan, N.Q. Assessment of different mcy genes for detecting the toxic to non-toxic Microcystis ratio in the field by multiplex qPCR. J. Oceanol. Limnol. 2018, 36, 1132–1144. [Google Scholar] [CrossRef]
- Song, W.J.; Wang, W.X.; Qiu, D.H.; Zheng, W.Z.; Li, X. Evaluating the effectiveness of various biochemical and molecular techniques to assess microcystin risk during the onset process of Microcystis blooms (delay-development stages). J. Clean. Prod. 2022, 369, 133335. [Google Scholar] [CrossRef]
- Guo, Z.H.; Li, J.M.; Luo, D.; Zhang, M.X. Novel ecological implications of non-toxic Microcystis towards toxic ecotype in population-promoting toxic ecotype dominance at various n levels and cooperative defense against luteolin-stress. FEMS Microbiol. Ecol. 2023, 99, 11–17. [Google Scholar] [CrossRef]
- Li, J.M.; Li, R.H.; Li, J. Current research scenario for microcystins biodegradation—A review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 2017, 595, 615–632. [Google Scholar] [CrossRef]
- Li, J.M.; Shimizu, K.; Zhou, Y.L.; Utsumi, M.; Sakharkar, M.K.; Zhang, Z.Y.; Sun, H.W.; Sugiura, N. Biodegradation of microcystins by bacterial communities co-existing with the flagellate Monas guttula and concurrent succession of community structures. J. Water Supply Res. Technol. AQUA 2011, 60, 352–363. [Google Scholar] [CrossRef]
- Shen, H.; Niu, Y.; Xie, P.; Tao, M.; Yang, X. Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshwater Biol. 2011, 56, 1065–1080. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Shiah, F.-K.; Chen, Y.-L. Importance of large colony formation in bloom-forming cyanobacteria to dominate in eutrophic ponds. Ann. Limnol.-Int. J. Lim 2011, 47, 167–173. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Mur, L.R.; Walsby, A.E. The ecophysiology of the harmful cyanobacterium Microcystis. In Harmful Cyanobacteria; Huisman, J., Matthijs, H.C.P., Visser, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 3, pp. 109–142. [Google Scholar]
- Bi, X.D.; Dai, W.; Zhang, S.L.; Xing, K.Z.; Guo, Y.J. Research progress on the competitive advantages and formation mechanism of Microcystis colony. Environ. Sci. Technol. 2014, 37, 41–44,65. [Google Scholar]
- Liu, Y.; Xu, Y.; Wang, Z.J.; Xiao, P.; Yu, G.L.; Wang, G.X.; Li, R.H. Dominance and succession of Microcystis genotypes and morphotypes in Lake Taihu, a large and shallow freshwater lake in China. Environ. Pollut. 2016, 219, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Lehman, P.W.; Kurobe, T.; Teh, S.J. Impact of extreme wet and dry years on the persistence of Microcystis harmful algal blooms in San Francisco Estuary. Quat. Int. 2022, 621, 16–25. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, G.J.; Qin, B.Q.; Zhang, G.S.; Wang, L.J.; Gao, Y.H.; Li, H.Y.; Chen, M.Y.; Zhong, C.N. Effect of the ingestion of metazooplankton on the formation of Microcystis blooms in summer in Lake Taihu. J. Lake Sci. 2013, 25, 398–405. [Google Scholar]
- Van Le, V.; Srivastava, A.; Ko, S.-R.; Ahn, C.-Y.; Oh, H.-M. Microcystis colony formation: Extracellular polymeric substance, associated microorganisms, and its application. Bioresour. Technol. 2022, 360, 127610. [Google Scholar]
- Qiu, D.R. Biosynthesis pathway of extracellular polymeric substances and colonial formation of cyanobacteria underlying water blooms of Microcystis. Acta Hydrobiol. Sin. 2020, 44, 1008–1013. [Google Scholar]
- Wu, Q.Q.; Wu, K.; Ye, Y.W.; Dong, X.H.; Zhang, J.M. Quorum sensing and its roles in pathogenesis among animal-associated pathogens—A review. Acta Microbiol. Sin. 2009, 49, 853–858. [Google Scholar]
- Sanchez, S.; Ng, W.L. Motility control as a possible link between quorum sensing to surface attachment in vibrio species. Adv. Exp. Med. Biol. 2023, 1404, 65–75. [Google Scholar]
- Eickhoff, M.J.; Bassler, B.L. Snapshot: Bacterial quorum sensing. Cell 2018, 174, 1328–1328.e1. [Google Scholar] [CrossRef]
- Mangwani, N.; Dash, H.R.; Chauhan, A.; Das, S. Bacterial quorum sensing: Functional features and potential applications in biotechnology. J. Mol. Microbiol. Biotechnol. 2012, 22, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.A.; Giani, A. Cell density-dependent oligopeptide production in cyanobacterial strains. FEMS Microbiol. Ecol. 2014, 88, 175–183. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Waters, C.M.; Bossier, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Wood, S.A.; Rueckert, A.; Hamilton, D.P.; Cary, S.C.; Dietrich, D.R. Switching toxin production on and off: Intermittent microcystin synthesis in a Microcystis bloom. Environ. Microbiol. Rep. 2011, 3, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Dietrich, D.R.; Cary, S.C.; Hamilton, D.P. Increasing Microcystis cell density enhances microcystin synthesis: A mesocosm study. Inland Waters 2012, 2, 17–22. [Google Scholar] [CrossRef]
- Wang, S.L.; Ding, P.; Lu, S.Y.; Wu, P.; Wei, X.Q.; Huang, R.X.; Kai, T.H. Cell density-dependent regulation of microcystin synthetase genes (mcy) expression and microcystin-LR production in Microcystis aeruginosa that mimics quorum sensing. Ecotoxicol. Environ. Saf. 2021, 220, 112330. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.T. Preliminary Study on Signaling Molecules Regulating the Synthesis and Secretion of Microcystins in Microcystis Aeruginosa. Master’s Thesis, Northeast Normal University, Changchun, China, 2021. [Google Scholar]
- Dai, C.X.; Qu, Y.Y.; Wu, W.Z.; Li, S.Z.; Chen, Z.; Lian, S.Y.; Jing, J.W. Qsp: An open sequence database for quorum sensing related gene analysis with an automatic annotation pipeline. Water Res. 2023, 235, 119814. [Google Scholar] [CrossRef]
- Mi, J.Q.; Yu, Z.Y.; Yu, H.; Zhou, W.B. Quorum sensing systems in foodborne Salmonella spp. and corresponding control strategies using Quorum Sensing inhibitors for food storage. Trends Food Sci. Technol. 2024, 144, 104320. [Google Scholar] [CrossRef]
- Yi, L.; Dong, X.; Grenier, D.; Wang, K.C.; Wang, Y. Research progress of bacterial quorum sensing receptors: Classification, structure, function and characteristics. Sci. Total Environ. 2021, 763, 143031. [Google Scholar] [CrossRef]
- Liu, L.L.; Zeng, X.Y.; Zheng, J.; Zou, Y.M.; Qiu, S.Y.; Dai, Y.F. AHL-mediated quorum sensing to regulate bacterial substance and energy metabolism: A review. Microbiol. Res. 2022, 262, 127102. [Google Scholar] [CrossRef] [PubMed]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Markus, V.; Paul, A.A.; Terali, K.; Ozer, N.; Marks, R.S.; Golberg, K.; Kushmaro, A. Conversations in the gut: The role of quorum sensing in normobiosis. Int. J. Mol. Sci. 2023, 24, 3722. [Google Scholar] [CrossRef]
- Kim, C.S.; Gatsios, A.; Cuesta, S.; Lam, Y.C.; Wei, Z.; Chen, H.W.; Russell, R.M.; Shine, E.; Wang, R.R.; Wyche, T.P.; et al. Characterization of autoinducer-3 structure and biosynthesis in E. coli. ACS Cent. Sci. 2020, 6, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Dow, L. How do quorum-sensing signals mediate algae-bacteria interactions? Microorganisms 2021, 9, 1391. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Shi, Y.H.; Zeng, G.M.; Gu, Y.L.; Chen, G.Q.; Shi, L.X.; Hu, Y.; Tang, S.; Zhou, J.X. Acyl-homoserine lactone-based quorum sensing and quorum quenching hold promise to determine the performance of biological wastewater treatments: An overview. Chemosphere 2016, 157, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Bhedi, C.D.; Prevatte, C.W.; Lookadoo, M.S.; Waikel, P.A.; Gillevet, P.M.; Sikaroodi, M.; Campagna, S.R.; Richardson, L.L. Elevated temperature enhances short- to medium-chain acyl homoserine lactone production by black band disease-associated vibrios. FEMS Microbiol. Ecol. 2017, 93, fix005. [Google Scholar] [CrossRef] [PubMed]
- Sharif, D.I.; Gallon, J.; Smith, C.J.; Dudley, E. Quorum sensing in cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium Gloeothece PCC6909. ISME J. 2008, 2, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.M.; Zhang, P.; Shen, F.; Zhou, C.X.; Liu, C.H. Does Microcystis aeruginosa have quorum sensing? FEMS Microbiol. Lett. 2012, 336, 38–44. [Google Scholar] [CrossRef]
- Yan, G.; Fu, L.; Ming, H.; Chen, C.L.; Zhou, D.D. Exploring an efficient and eco-friendly signaling molecule and its quorum quenching ability for controlling Microcystis blooms. Environ. Sci. Technol. 2023, 57, 16929–16939. [Google Scholar] [CrossRef]
- Xu, C.; Ni, L.X.; Li, S.Y.; Du, C.H.; Sang, W.L.; Jiang, Z.Y. Quorum sensing regulation in Microcystis aeruginosa: Insights into AHL-mediated physiological processes and MC-LR production. Sci. Total Environ. 2024, 919, 170867. [Google Scholar] [CrossRef] [PubMed]
- Lamas-Samanamud, G.; Reeves, T.; Tidwell, M.; Bohmann, J.; Lange, K.; Shipley, H. Changes in chemical structure of N-acyl nomoserine lactones and their effects on microcystin expression from Microcystis aeruginosa PCC7806. Environ. Eng. Sci. 2022, 39, 29–38. [Google Scholar] [CrossRef]
- Zhang, P. Preliminary Study on the Quorum Sensing of Microcystis aeruginosa. Master’s Thesis, Nanjing University, Nanjing, China, 2011. [Google Scholar]
- Xu, R.; Long, H.; Wang, Y.H.; Huang, K.Y. A new method for isolating gas vesicles from Microcystis for ultrasound contrast. Chin. J. Biotech. 2022, 38, 1589–1601. [Google Scholar]
- Utkilen, H.; Gjolme, N. Iron-stimulated toxin production in Microcystis-aeruginosa. Appl. Environ. Microbiol. 1995, 61, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Boerner, T.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef]
- Meissner, S.; Steinhauser, D.; Dittmann, E. Metabolomic analysis indicates a pivotal role of the hepatotoxin microcystin in high light adaptation of Microcystis. Environ. Microbiol. 2015, 17, 1497–1509. [Google Scholar] [CrossRef]
- Yang, J.; Deng, X.R.; Xian, Q.M.; Qian, X.; Li, A.M. Allelopathic effect of Microcystis aeruginosa on Microcystis wesenbergii: Microcystin-LR as a potential allelochemical. Hydrobiologia 2014, 727, 65–73. [Google Scholar] [CrossRef]
- Sedmak, B.; Elersek, T. Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microb. Ecol. 2006, 51, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.Q.; Xiao, Y.; Zhu, L.; Wu, Z.X.; Liu, J.; Hu, C.L.; Song, L.R. The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ. Microbiol. 2012, 14, 730–742. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Ihle, T.; Jähnichen, S.; Benndorf, J. Wax and wane of Microcystis (Cyanophyceae) and microcystins in lake sediments: A case study in Quitzdorf reservoir (Germany). J. Phycol. 2005, 41, 479–488. [Google Scholar] [CrossRef]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Boerner, T.; Dittmann, E.; Kaplan, A. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Xu, Y.; Kong, D.S.; Wang, J.; Shi, K.P.; Zhang, Y.; He, H.; Yang, S.G.; Ni, L.X.; Li, S.Y. Role of extracellular polymeric substances in resistance to allelochemical stress on Microcystis aeruginosa and its mechanism. J. Oceanol. Limnol. 2023, 41, 2219–2231. [Google Scholar] [CrossRef]
- Burkert, U.; Hyenstrand, P.; Drakare, S.; Blomqvist, P. Effects of the mixotrophic flagellate Ochromonas sp. on colony formation in Microcystis aeruginosa. Aquat. Ecol. 2001, 35, 11–17. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.X.; Shi, X.L.; Zhang, M.; Xing, P.; Cao, H.S. Changes in the morphology and polysaccharide content of Microcystis aeruginosa (Cyanobacteria) during flagellate grazing. J. Phycol. 2008, 44, 716–720. [Google Scholar] [CrossRef]
- Xu, C.; Ni, L.X.; Du, C.H.; Shi, J.H.; Ma, Y.S.; Li, S.Y.; Li, Y.P. Decoding Microcystis aeruginosa quorum sensing through AHL-mediated transcriptomic molecular regulation mechanisms. Sci. Total Environ. 2024, 926, 172101. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Zou, Y.M.; Zheng, J.; Qiu, S.Y.; Liu, L.L.; Wei, C.Y. Quorum sensing-mediated microbial interactions: Mechanisms, applications, challenges and perspectives. Microbiol. Res. 2023, 273, 127414. [Google Scholar] [CrossRef]
- Xu, H.C.; Lv, H.; Liu, X.; Wang, P.F.; Jiang, H.L. Electrolyte cations binding with extracellular polymeric substances enhanced Microcystis aggregation: Implication for Microcystis bloom formation in eutrophic freshwater lakes. Environ. Sci. Technol. 2016, 50, 9034–9043. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Xu, H.C.; Jiang, H.L.; Yu, G.H.; Yang, L.Y. Towards understanding the role of extracellular polymeric substances in cyanobacterial Microcystis aggregation and mucilaginous bloom formation. Chemosphere 2014, 117, 815–822. [Google Scholar] [CrossRef]
- Xue, H.F.; Zhou, Y.; Luo, K.; Chen, X.L. The influence of AHLs and its analog on the biosynthesis of UV-protectants in cyanobacteria. J. Yunnan Natl. Univ. 2009, 18, 135–138. [Google Scholar]
- Liao, L.; Chen, B.; Deng, K.K.; He, Q.; Lin, G.J.; Guo, J.S.; Yan, P. Effect of the N-hexanoyl-l-homoserine lactone on the carbon fixation capacity of the algae–bacteria system. Int. J. Environ. Res. Public Health 2023, 20, 5047. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L. Inhibition of AHLs Analog on the Growth of Bloom-Forming Cyanobacteria and Its Mathematic Model. Doctoral Dissertation, Yunnan University, Kunming, China, 2008. [Google Scholar]
- Herrera, N.; Echeverri, F. Evidence of quorum sensing in cyanobacteria by homoserine lactones: The origin of blooms. Water 2021, 13, 1831. [Google Scholar] [CrossRef]
- Rzymski, P.; Klimaszyk, P.; Jurczak, T.; Poniedzialek, B. Oxidative stress, programmed cell death and microcystin release in Microcystis aeruginosa in response to Daphnia grazers. Front. Microbiol. 2020, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Thornton, D. Diatom aggregation in the sea: Mechanisms and ecological implications. Eur. J. Phycol. 2002, 37, 149–161. [Google Scholar] [CrossRef]
- Romero, M.; Diggle, S.P.; Heeb, S.; Camara, M.; Otero, A. Quorum quenching activity in Anabaena sp. PCC 7120: Identification of Aiic, a novel AHL-acylase. FEMS Microbiol. Lett. 2008, 280, 73–80. [Google Scholar] [CrossRef]
- Lade, H.; Paul, D.; Kweon, J.H. N-acyl homoserine lactone-mediated quorum sensing with special reference to use of quorum quenching bacteria in membrane biofouling control. BioMed Res. Int. 2014, 2014, 162584. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Chen, X.L.; Wang, B.; Chen, S.N. The effects of exogenous AHLs on the growth and metabolism of blue-green algae. J. Yunnan Univ. Nat. Sci. Ed. 2007, 29, 303–307. [Google Scholar]
- Engebrecht, J.; Silverman, M. Identification of genes and gene-products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. USA 1984, 81, 4154–4158. [Google Scholar] [CrossRef]
- Piper, K.R.; Vonbodman, S.B.; Farrand, S.K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 1993, 362, 448–450. [Google Scholar] [CrossRef] [PubMed]
- Passador, L.; Cook, J.M.; Gambello, M.J.; Rust, L.; Iglewski, B.H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 1993, 260, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Bainton, N.J.; Bycroft, B.W.; Chhabra, S.R.; Stead, P.; Gledhill, L.; Hill, P.J.; Rees, C.E.D.; Winson, M.K.; Salmond, G.P.C.; Stewart, G.; et al. A general role for the lux autoinducer in bacterial cell signaling: Control of antibiotic biosynthesis in Erwinia. Gene 1992, 116, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Devine, J.H.; Baldvvin, T.O. Control of the lux regulon of Vibrio fischeri. J. Biolumin. Chemilumin. 1990, 5, 99–106. [Google Scholar] [CrossRef]
- Egland, K.A.; Greenberg, E.P. Quorum sensing in Vibrio fischeri: Elements of the luxl promoter. Mol. Microbiol. 1999, 31, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum sensing inhibitors: A bargain of effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Lamas-Samanamud, G.; Montante Iii, A.; Mertins, A.; Phan, D.; Loures, C.; Naves, F.; Reeves, T.; Shipley, H.J. The role of quorum sensing in the development of Microcystis aeruginosa blooms: Gene expression. Microorganisms 2023, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Lostroh, C.P.; Ogi, T.; Greenberg, E.P. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: A transcriptome analysis. J. Bacteriol. 2003, 185, 2066–2079. [Google Scholar] [CrossRef]
- Cui, T.Q.; Bai, F.L.; Li, J.R. Advance on quorum-sensing regulation and quenching mechanism of gram-negative bacteria mediated by AHLs. J. Chin. Inst. Food Sci. Technol. 2020, 20, 308–320. [Google Scholar]
- Parsek, M.R.; Val, D.L.; Hanzelka, B.L.; Cronan, J.E.; Greenberg, E.P. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 1999, 96, 4360–4365. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Jansson, J.K.; Remais, J.V.; Rich, V.I.; Singh, B.K.; Trivedi, P. Climate change microbiology—Problems and perspectives. Nat. Rev. Microbiol. 2019, 17, 391–396. [Google Scholar] [CrossRef] [PubMed]


| Microcystis Strains | AHLs Homologs | Culture Media | Structure of AHLs Homologs | References |
|---|---|---|---|---|
| M. aeruginosa FACHB-905 | AHLs | BG-11 | Unknown | [28] |
| M. aeruginosa PCC-7820 | AHLs | BG-11 | Unknown | [41] |
| M. aeruginosa HB-836 | 3-OXO-C5-HSL | BG-11 |  | [42] |
| C6-HSL | BG-11 |  | ||
| 3-OXO-C7-HSL | BG-11 | Unknown | ||
| 3-OH-C4-HSL | BG-11 | 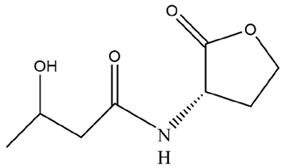 | ||
| M. aeruginosa FACHB-905 | AHLs(C13H19O8N) | BG-11 | Unknown | [43] |
| M. aeruginosa PCC7806 | C3-HSL | BG-11 | Unknown | [44] |
| C4-HSL | BG-11 | 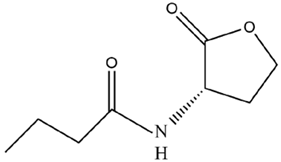 | ||
| M. aeruginosa PCC-7820 | C8-HSLs | Allen-BG11 |  | [45] |
| Microcystis Strains | Concentration | AHLs | Cell Growth | Cellular Morphology | Colony Formation | Chlorophyll a content | Photosynthesis | Superoxide Free Radical Content | Phycocyanin content | Intracellular Polysaccharide content | MCs content | EPSs content | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. aeruginosa HB909 | 0.2 μmol/L | 3-OH-C4-HSL | √ | √ | √ | × | [29] | ||||||
| C4-HSL | √ | √ | √ | × | √ | ||||||||
| C8-HSL | × | √ | - | √ | √ | ||||||||
| M. aeruginosa PCC-7820 | 0.02 μmol/L | AHLs (Unknown type) | √ | √ | [41] | ||||||||
| M. aeruginosa FACHB905 | √ | √ | √ | √ | √ | [43] | |||||||
| M. aeruginosa FACHB905 | 50 μmol/L | C4H7NO2·HBr | √ | [64] | |||||||||
| M. aeruginosa FACHB905 | 5 ng/L | C6-HSL | × | [65] | |||||||||
| 10 ng/L | √ | ||||||||||||
| 50 ng/L | × | ||||||||||||
| 500 ng/L | × | ||||||||||||
| 1000 ng/L | × | ||||||||||||
| M. aeruginosa FACHB905 | 5 μmol/L | C4H7NO2·HBr | - | [66] | |||||||||
| 10 μmol/L | × | ||||||||||||
| 20 μmol/L | × | ||||||||||||
| 40 μmol/L | × | ||||||||||||
| 50 μmol/L | × | × | √ | × | √ | ||||||||
| 60 μmol/L | × | ||||||||||||
| 80 μmol/L | × | ||||||||||||
| 100 μmol/L | × | ||||||||||||
| M. aeruginosa | 0.004 μmol/L | 3-oxo-C10-HSL | × | - | [67] | ||||||||
| C6-HSL | √ | √ | |||||||||||
| C10-HSL | × | - | |||||||||||
| C7-HSL | √ | √ | |||||||||||
| C12-HSL | √ | √ | |||||||||||
| C8-HSL | × | √ | |||||||||||
| 3-oxo-C8-HSL | √ | √ | |||||||||||
| C4-HSL | × | √ | |||||||||||
| M. aeruginosa FACHB905 | 10 μmol/L | C4H7NO2·HBr | - | √ | × | √ | [73] | ||||||
| 20 μmol/L | - | √ | × | √ | |||||||||
| 40 μmol/L | × | √ | × | √ | |||||||||
| 60 μmol/L | × | √ | × | √ | |||||||||
| 80 μmol/L | × | √ | × | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, J. Cyanobacterial Bloom Formation by Enhanced Ecological Adaptability and Competitive Advantage of Microcystis—Non-Negligible Role of Quorum Sensing. Microorganisms 2024, 12, 1489. https://doi.org/10.3390/microorganisms12071489
Zhang Z, Li J. Cyanobacterial Bloom Formation by Enhanced Ecological Adaptability and Competitive Advantage of Microcystis—Non-Negligible Role of Quorum Sensing. Microorganisms. 2024; 12(7):1489. https://doi.org/10.3390/microorganisms12071489
Chicago/Turabian StyleZhang, Ziqing, and Jieming Li. 2024. "Cyanobacterial Bloom Formation by Enhanced Ecological Adaptability and Competitive Advantage of Microcystis—Non-Negligible Role of Quorum Sensing" Microorganisms 12, no. 7: 1489. https://doi.org/10.3390/microorganisms12071489
APA StyleZhang, Z., & Li, J. (2024). Cyanobacterial Bloom Formation by Enhanced Ecological Adaptability and Competitive Advantage of Microcystis—Non-Negligible Role of Quorum Sensing. Microorganisms, 12(7), 1489. https://doi.org/10.3390/microorganisms12071489






