N-Acyl Homoserine Lactone Production by the Marine Isolate, Dasania marina
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
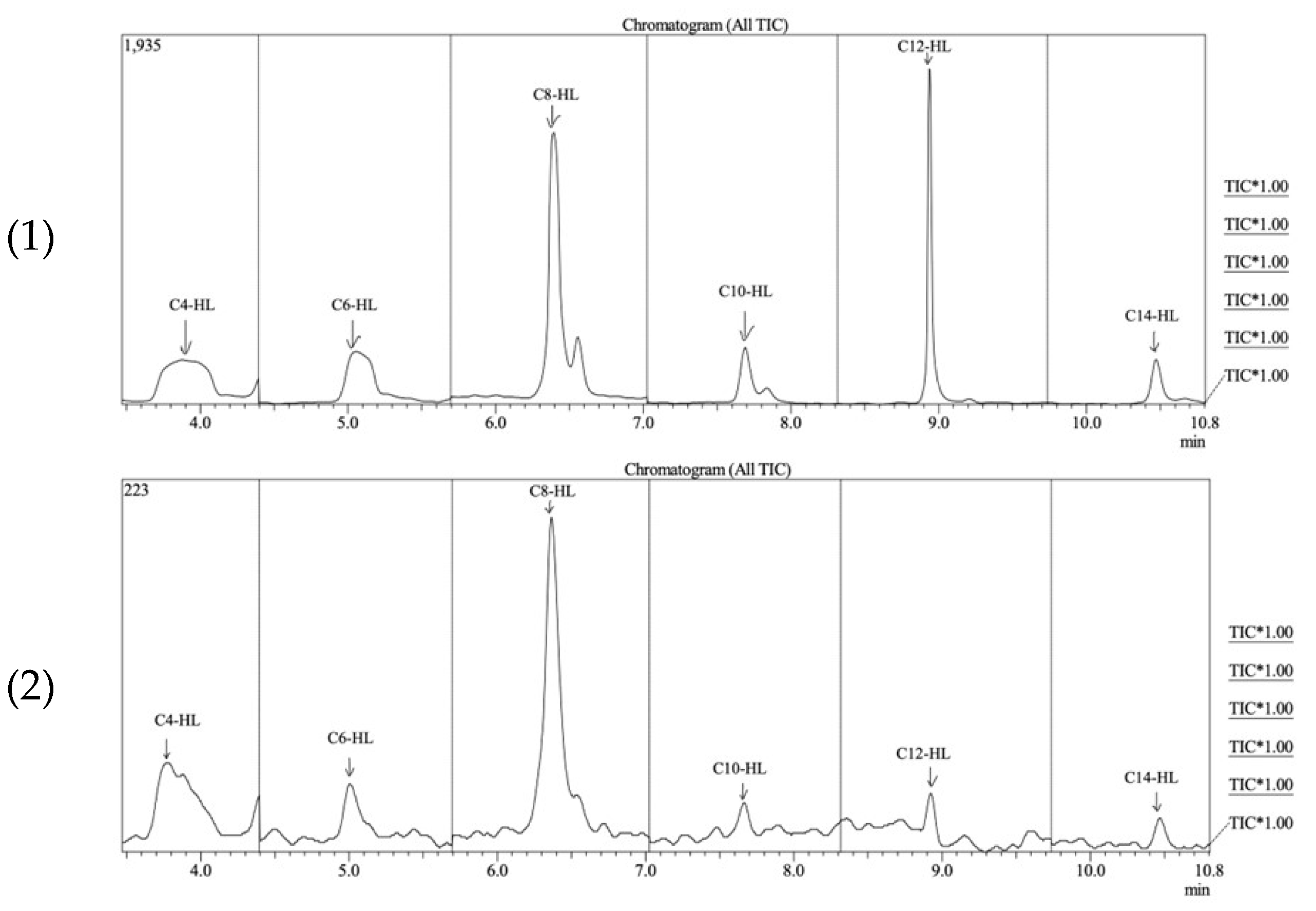
| Analyte | RT (min) | MRM Start Time (min) | MRM End Time (min) | Quantification | Qualification | ||
|---|---|---|---|---|---|---|---|
| MRM Transition | Collision Energy | MRM Transition | Collision Energy | ||||
| N-butyryl-l-Homoserine Lactone (C4-AHL) | 3.77 | 3.48 | 4.73 | 172.00 > 71.10 | 11 | 172.00 > 102.10 | 6 |
| N-hexanoyl-l-Homoserine Lactone (C6-AHL) | 5.03 | 4.73 | 5.71 | 200.00 > 102.10 | 4 | 200.00 > 99.10 | 7 |
| N-otonyl-l-Homoserine Lactone (C8-AHL) | 6.38 | 5.71 | 7.05 | 228.00 > 57.10 | 24 | 228.00 > 127.10 | 6 |
| N-decanoyl-Homoserine Lactone (C10-AHL) | 7.69 | 7.05 | 8.34 | 256.00 > 102.10 | 7 | 256.00 > 155.20 | 6 |
| N-dodecanoyl-Homoserine Lactone (C12-AHL) | 8.96 | 8.34 | 9.27 | 284.00 > 102.10 | 6 | 284.00 > 57.10 | 23 |
| N-tetradecanoyl-Homoserine Lactone (C14-AHL) | 10.51 | 9.27 | 10.80 | 312.00 > 102.10 | 6 | 312.00 > 57.10 | 23 |
| Compound | LOD (ppm) | LOQ (ppm) |
|---|---|---|
| C4-AHL | UD | UD |
| C6-AHL | 21.05 | 63.77 |
| C8-AHL | 20.95 | 63.48 |
| C10-AHL | 24.01 | 72.75 |
| C12-AHL | 29.21 | 88.52 |
| C14-AHL | UD | UD |
References
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Domzalski, A.; Perez, S.D.; Yoo, B.; Velasquez, A.; Vigo, V.; Pasolli, H.A.; Oldham, A.L.; Henderson, D.P.; Kawamura, A. Uncovering potential interspecies signaling factors in plant-derived mixed microbial culture. Bioorg. Med. Chem. 2021, 42, 116254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samreen Qais, F.A.; Ahmad, I. Anti-quorum sensing and biofilm inhibitory effect of some medicinal plants against gram-negative bacterial pathogens: In vitro and in silico investigations. Heliyon 2022, 8, e11113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escobar-Muciño, E.; Arenas-Hernández, M.M.P.; Luna-Guevara, M.L. Mechanisms of inhibition of quorum sensing as an alternative for the control of E. coli and Salmonella. Microorganisms 2022, 10, 884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miranda, S.W.; Asfahl, K.L.; Dandekar, A.A.; Greenberg, E.P. Pseudomonas aeruginosa quorum sensing. Adv. Exp. Med. Biol. 2022, 1386, 95–115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.K. Bacterial biofilm inhibitors: An overview. Ecotoxicol. Environ. Saf. 2023, 264, 115389. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.A.; Santos, L.; Vance, T.; Fileman, T.; Smith, D.; Bishop, J.D.D.; Viard, F.; Queirós, A.M.; Merino, G. Costs and benefits to European shipping of ballast-water and hull fouling treatment: Impacts of native and non-indigenous species. Mar. Policy 2016, 64, 148–155. [Google Scholar] [CrossRef]
- Heyer, A.; D’Souza, F.; Morales, C.F.L.; Ferrari, G.; Mol, J.M.C.; de Wit, J.H.W. Ship ballast tanks: A review from microbial corrosion and electrochemical point of view. Ocean Eng. 2013, 70, 188–200. [Google Scholar] [CrossRef]
- Malalasekara, L.; Henderson, D.P.; Oldham, A.L. A study of biofilm formation in marine bacteria isolated from ballast tank fluids. Trends Res. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hong, S.G.; Cho, H.H.; Cho, K.H.; Lee, H.K. Dasania marina gen. nov., sp. nov., of the order Pseudomonadales, isolated from Arctic marine sediment. J. Microbiol. 2007, 45, 505–509. [Google Scholar] [PubMed]
- Joelsson, A.C.; Zhu, J. LacZ-based detection of acyl-homoserince lactone quorum-sensing signals. Curr. Protoc. Microbiol. 2006, 3, 1C2.1–1C2.9. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Gao, P.; Chen, Y.C.; Shaw, P.D.; Farrand, S.K. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 2006, 11, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chai, Y.; Zhong, Z.; Li, S.; Winans, S.C. Agrobacterium bioassay strain for ultrasensitive detection of N-acylhomoserine lactone-type quorum-sensing molecules: Detection of autoinducers in Mesorhizobium huakuii. Appl. Environ. Microbiol. 2013, 69, 6949–6953. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Sá, M.C.A.; da Silva, W.M.; Rodrigues, C.C.S.; Rezende, C.P.; Marchioro, S.B.; Rocha Filho, J.T.R.; Sousa, T.J.; de Oliveira, H.P.; da Costa, M.M.; Figueiredo, H.C.P.; et al. Comparative proteomic analyses between biofilm-forming and non-biofilm-forming strains of Corynebacterium pseudotuberculosis isolated from goats. Front. Vet. Sci. 2021, 8, 614011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
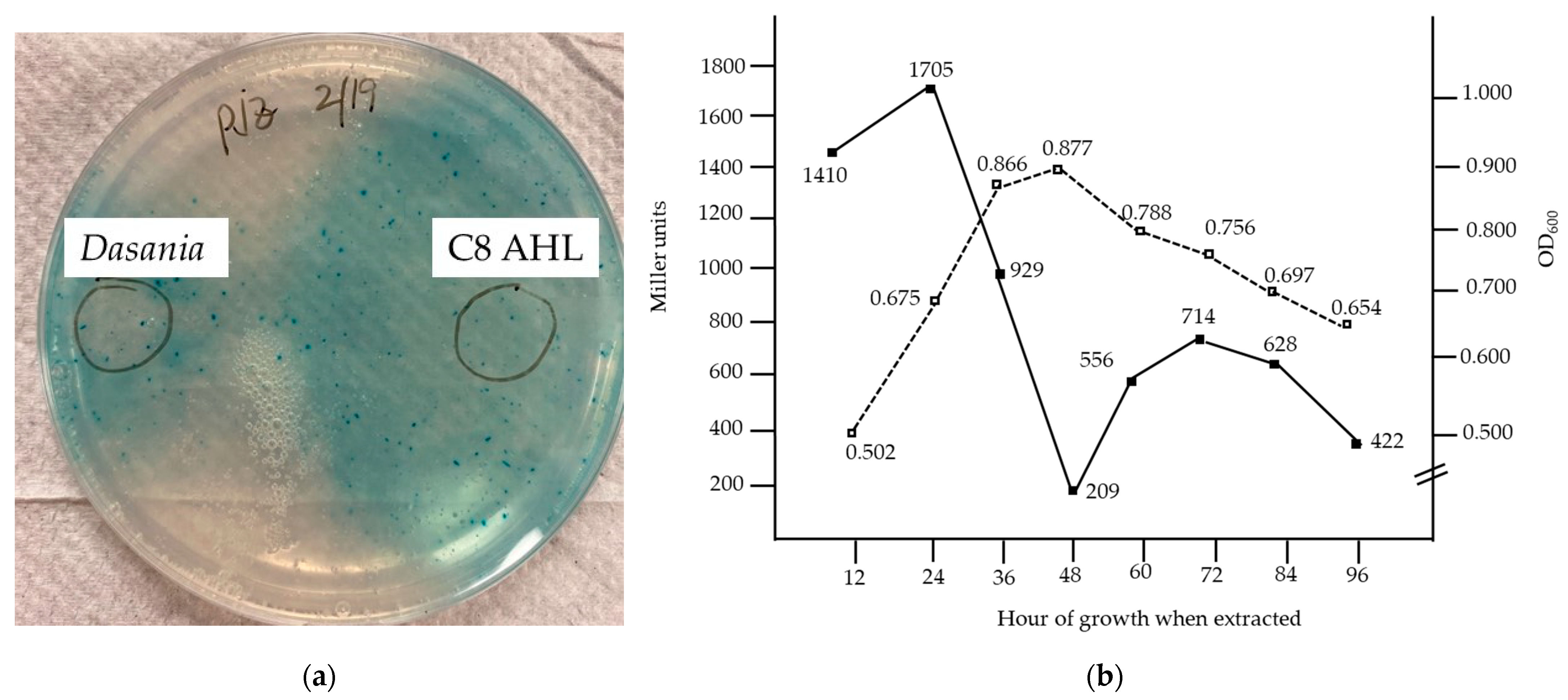

| Organism | C6 | C8 | C10 | C12 |
|---|---|---|---|---|
| D. marina (isolate SD1D) | UD | 4.46 | 5.12 | 8.72 |
| A. tagae (isolate SD5) | 5.94 | 4.50 | 5.09 | 8.82 |
| A. oceani (isolate SD8) | UD | 4.43 | UD | 8.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimiran, F.; David, S.; Birks, S.; Oldham, A.; Henderson, D. N-Acyl Homoserine Lactone Production by the Marine Isolate, Dasania marina. Microorganisms 2024, 12, 1496. https://doi.org/10.3390/microorganisms12071496
Alimiran F, David S, Birks S, Oldham A, Henderson D. N-Acyl Homoserine Lactone Production by the Marine Isolate, Dasania marina. Microorganisms. 2024; 12(7):1496. https://doi.org/10.3390/microorganisms12071496
Chicago/Turabian StyleAlimiran, Fnu, Samuel David, Scott Birks, Athenia Oldham, and Douglas Henderson. 2024. "N-Acyl Homoserine Lactone Production by the Marine Isolate, Dasania marina" Microorganisms 12, no. 7: 1496. https://doi.org/10.3390/microorganisms12071496






