Bacterial Resistances and Sensibilities in a Tertiary Care Hospital in Romania—A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacterial Identification and Antibiotic Testing
2.3. Culture Types and Identified Bacteria
2.4. Statistical Analysis
3. Results
3.1. Age Distribution
3.2. Culture and Bacteria Distribution
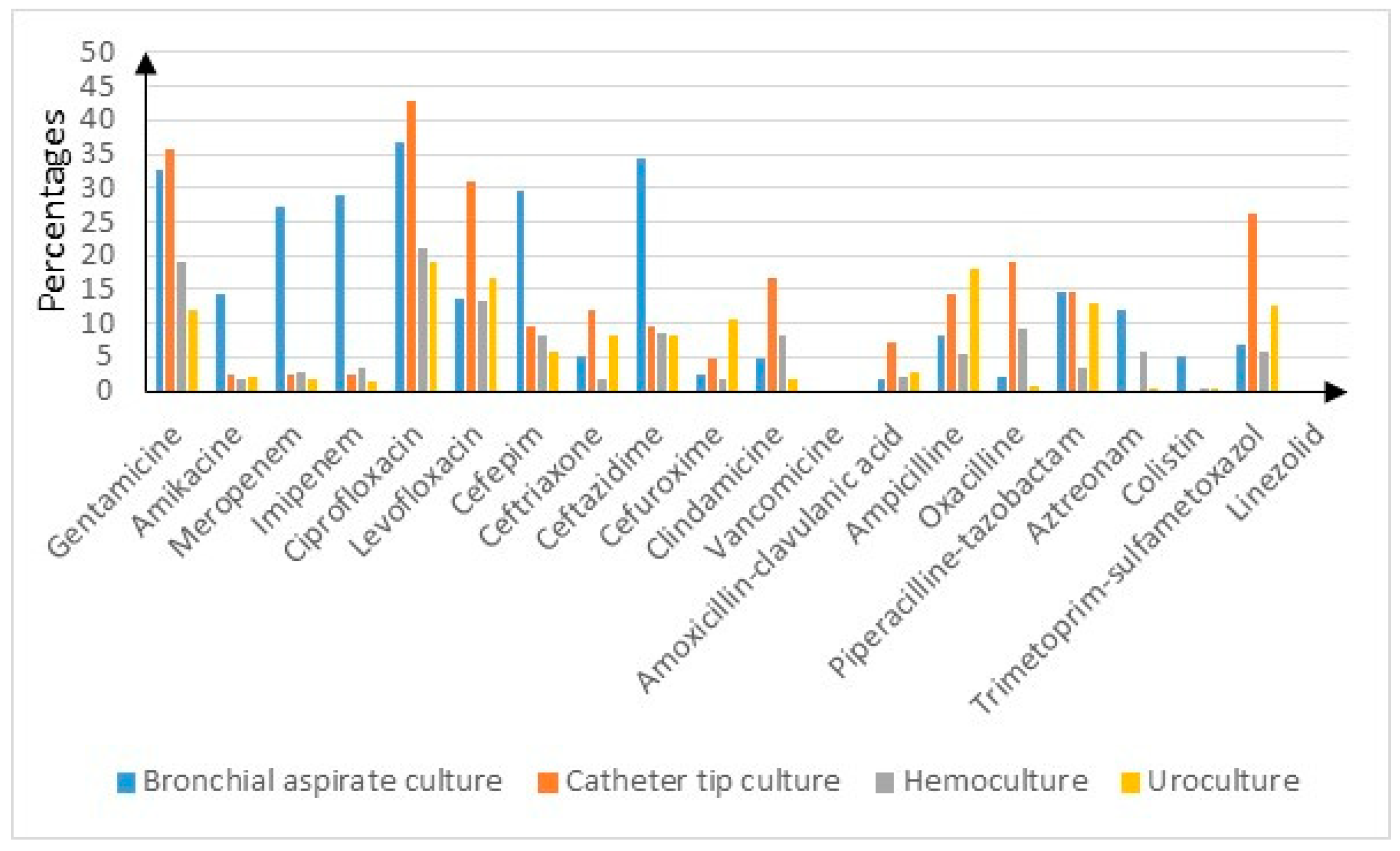
3.3. Resistance Rates and Sensibilities Stratified by Cultures
3.4. Resistance Rates and Sensibilities Stratified by Bacteria
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Thamlikitkul, V.; Hinjoy, S.; Day, N.P.; Peacock, S.J.; Limmathurotsakul, D.; Hospital, A.; et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife 2016, 5, e18082. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalimet, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Rao, P.C.; Dolecek, C.; Day, N.P.J.; Stergachis, A.; Lopez, A.D.; Murray, C.J.L. Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 2018, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Review on Antimicrobial Resistance; London: 2014. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 27 May 2024).
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- National Office for Animal Health. National Office for Animal Health; Middlesex: 2016. NOAH Response to Final O’Neill AMR Review Report July 2016. Available online: https://www.noah.co.uk/wp-content/uploads/2016/07/FINAL-NOAH-response-to-final-O-Neill-review-25-07-16-cle.pdf (accessed on 27 June 2024).
- WHO Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 27 June 2024).
- US Centres for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019; US Department of Health and Human Services: Atlanta, GA, USA, 2019; Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 27 June 2024).
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization; Geneva: 2015. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 27 June 2024).
- Arbune, M.; Gurau, G.; Niculet, E.; Iancu, A.V.; Lupasteanu, G.; Fotea, S.; Vasile, M.C.; Tatu, A.L. Prevalence of Antibiotic Resistance of ESKAPE Pathogens over Five Years in an Infectious Diseases Hospital from South-East of Romania. Infect. Drug Resist. 2021, 14, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Ghenea, A.E.; Cioboată, R.; Drocaş, A.I.; Țieranu, E.N.; Vasile, C.M.; Moroşanu, A.; Țieranu, C.G.; Salan, A.-I.; Popescu, M.; Turculeanu, A.; et al. Prevalence and Antimicrobial Resistance of Klebsiella Strains Isolated from a County Hospital in Romania. Antibiotics 2021, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Barbu, I.C.; Gheorghe-Barbu, I.; Grigore, G.A.; Vrancianu, C.O.; Chifiriuc, M.C. Antimicrobial Resistance in Romania: Updates on Gram-Negative ESCAPE Pathogens in the Clinical, Veterinary, and Aquatic Sectors. Int. J. Mol. Sci. 2023, 24, 7892. [Google Scholar] [CrossRef]
- Szabó, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. An Overview of Healthcare Associated Infections and Their Detection Methods Caused by Pathogen Bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef] [PubMed]
- Chibelean, C.B.; Petca, R.-C.; Mareș, C.; Popescu, R.-I.; Enikő, B.; Mehedințu, C.; Petca, A. A Clinical Perspective on the Antimicrobial Resistance Spectrum of Uropathogens in a Romanian Male Population. Microorganisms 2020, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Petca, R.-C.; Mareș, C.; Petca, A.; Negoiță, S.; Popescu, R.-I.; Boț, M.; Barabás, E.; Chibelean, C.B. Spectrum and Antibiotic Resistance of Uropathogens in Romanian Females. Antibiotics 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Axente, C.; Licker, M.; Moldovan, R.; Hogea, E.; Muntean, D.; Horhat, F.; Bedreag, O.; Sandesc, D.; Papurica, M.; Dugaesescu, D.; et al. Antimicrobial consumption, costs and resistance patterns: A two year prospective study in a Romanian intensive care unit. BMC Infect. Dis. 2017, 17, 358. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Yende, S.; Linde-Zwirble, W.T.; Peck-Palmer, O.M.; Barnato, A.E.; Weissfeld, L.A.; Angus, D.C. Infection Rate and Acute Organ Dysfunction Risk as Explanations for Racial Differences in Severe Sepsis. JAMA 2010, 303, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Douglas, N.M.; Hennessy, J.N.; Currie, B.J.; Baird, R.W. Trends in Bacteremia Over 2 Decades in the Top End of the Northern Territory of Australia. Open Forum Infect. Dis. 2020, 7, ofaa472. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Jones, M.E.; Draghi, D.C.; Thornsberry, C.; Sahm, D.F.; Volturo, G.A. Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Ann. Clin. Microbiol. Antimicrob. 2004, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, L.; Wang, J. Distribution and Antibiotic Resistance Analysis of Blood Culture Pathogens in a Tertiary Care Hospital in China in the Past Four Years. Infect. Drug Resist. 2023, 16, 5463–5471. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and innovations in treating chronic and acute wound infections: From basic science to clinical practice. Burn. Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef]
- Tom, I.M.; Ibrahim, M.M.; Umoru, A.M.; Umar, J.B.; Bukar, M.A.; Haruna, A.B.; Aliyu, A. Infection of Wounds by Potential Bacterial Pathogens and Their Resistogram. Open Access Libr. J. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Ahmed, E.F.; Rasmi, A.H.; Darwish, A.M.A.; Gad, G.F.M. Prevalence and resistance profile of bacteria isolated from wound infections among a group of patients in upper Egypt: A descriptive cross-sectional study. BMC Res. Notes 2023, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, J.; Zhang, H.-Z.; Zhang, X.-Y.; Wang, Y.-M. Multidrug-resistant organisms in intensive care units and logistic analysis of risk factors. World J. Clin. Cases 2022, 10, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Wroblewska, M.; Rudnicka, J.; Marchel, H.; Luczak, M. Multidrug-resistant bacteria isolated from patients hospitalised in Intensive Care Units. Int. J. Antimicrob. Agents 2006, 27, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Ny, S.; Edquist, P.; Dumpis, U.; Gröndahl-Yli-Hannuksela, K.; Hermes, J.; Kling, A.-M.; Klingeberg, A.; Kozlov, R.; Källman, O.; Lis, D.O.; et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2019, 17, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Evans, T. Diagnosis and management of sepsis. Clin. Med. 2018, 18, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Draganescu, M.; Iancu, A.V.; Firescu, D.; Buzia, O.D.; Diaconu, C.; Rebegea, L. Trends in antimicrobials consumption and antimicrobial resistance in an infectious diseases hospital from the south-eastern region of Romania. Farmacia 2016, 64, 770–774. [Google Scholar]
- Tălăpan, D.; Sandu, A.-M.; Rafila, A. Antimicrobial Resistance of Staphylococcus aureus Isolated between 2017 and 2022 from Infections at a Tertiary Care Hospital in Romania. Antibiotics 2023, 12, 974. [Google Scholar] [CrossRef]
- Kaur, D.C.; Chate, S.S. Study of antibiotic resistance pattern in methicillin resistant Staphylococcus aureus with special reference to newer antibiotic. J. Glob. Infect. Dis. 2015, 7, 78–84. [Google Scholar] [CrossRef]
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- La Fauci, V.; Alessi, V. Antibiotic resistance: Where are we going. Ann Ig 2018, 30 (Suppl. S1), 52–57. [Google Scholar] [CrossRef]
- National Research Council, Committee on Drug Use in Food Animals. The Use of Drugs in Food Animals: Benefits and Risks; National Academies Press: Washington, DC, USA, 1999. [Google Scholar]

| Antibiotic Class | Antibiotic |
|---|---|
| Aminoglycozides | Gentamicin |
| Amikacin | |
| Carbapenems | Meropenem |
| Imipenem | |
| Fluoroqinolones | Ciprofloxacin |
| Levofloxacin | |
| Cephalosporins | Cefepim |
| Ceftriaxone | |
| Ceftazidime | |
| Cefuroxime | |
| Lincosamides | Clindamycin |
| Glicopeptides | Vancomycin |
| Penicilines | Amoxicillin–clavulanic acid |
| Ampicillin | |
| Oxacillin | |
| Piperacillin-tazobactam | |
| Monobactames | Aztreonam |
| Polimixines | Colistin |
| Sulfonamides | Trimethoprim-sulfamethoxazole |
| Oxazolidinones | Linezolid |
| Age Group | Number of Patients | Percentage (%) |
|---|---|---|
| 0–9 years | 249 | 6.97 |
| 10–19 years | 85 | 2.32 |
| 20–29 years | 227 | 6.36 |
| 30–39 years | 302 | 8.25 |
| 40–49 years | 313 | 8.55 |
| 50–59 years | 492 | 13.44 |
| 60–69 years | 862 | 23.56 |
| 70–79 years | 728 | 19.9 |
| 80–89 years | 343 | 9.37 |
| 90–100 years | 57 | 1.55 |
| Number of Cultures | Number of Identified Strains | PA | EN | SA | BC | KL | ST | AB | SM | PROV | PROT | CO | CI | EC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uroculture | 1343 | 1098 | 57 | 19 | 37 | 0 | 209 | 83 | 10 | 14 | 6 | 72 | 1 | 5 | 585 |
| Wound secretion culture | 738 | 885 | 121 | 10 | 313 | 17 | 93 | 16 | 27 | 21 | 6 | 91 | 1 | 10 | 84 |
| Blood culture | 360 | 381 | 29 | 25 | 189 | 2 | 28 | 12 | 14 | 1 | 1 | 7 | 4 | 1 | 51 |
| Bronchial aspirate culture | 229 | 235 | 28 | 16 | 37 | 0 | 43 | 11 | 65 | 1 | 2 | 9 | 3 | 2 | 18 |
| Cervical culture | 214 | 222 | 4 | 2 | 22 | 0 | 23 | 56 | 1 | 1 | 0 | 7 | 0 | 0 | 106 |
| Sputum culture | 125 | 122 | 26 | 7 | 27 | 0 | 32 | 11 | 2 | 0 | 0 | 3 | 0 | 0 | 14 |
| Abscess culture | 113 | 122 | 2 | 1 | 18 | 0 | 29 | 10 | 0 | 2 | 1 | 8 | 0 | 1 | 50 |
| Vaginal culture | 92 | 110 | 9 | 9 | 26 | 0 | 7 | 11 | 0 | 0 | 0 | 6 | 0 | 0 | 42 |
| Peritoneal liquid culture | 82 | 80 | 6 | 5 | 8 | 0 | 13 | 10 | 1 | 2 | 0 | 3 | 0 | 2 | 42 |
| Catheter tip culture | 42 | 44 | 4 | 5 | 21 | 1 | 4 | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 4 |
| Total | 3338 | 3299 | 286 | 99 | 698 | 20 | 481 | 221 | 123 | 42 | 16 | 207 | 9 | 21 | 996 |
| Percentages | 8.56 | 2.96 | 20.9 | 0.59 | 14.4 | 6.62 | 3.68 | 1.25 | 0.47 | 6.2 | 0.26 | 0.62 | 29.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisavu, L.; Chisavu, F.; Marc, L.; Mihaescu, A.; Bob, F.; Licker, M.; Ivan, V.; Schiller, A. Bacterial Resistances and Sensibilities in a Tertiary Care Hospital in Romania—A Retrospective Analysis. Microorganisms 2024, 12, 1517. https://doi.org/10.3390/microorganisms12081517
Chisavu L, Chisavu F, Marc L, Mihaescu A, Bob F, Licker M, Ivan V, Schiller A. Bacterial Resistances and Sensibilities in a Tertiary Care Hospital in Romania—A Retrospective Analysis. Microorganisms. 2024; 12(8):1517. https://doi.org/10.3390/microorganisms12081517
Chicago/Turabian StyleChisavu, Lazar, Flavia Chisavu, Luciana Marc, Adelina Mihaescu, Flaviu Bob, Monica Licker, Viviana Ivan, and Adalbert Schiller. 2024. "Bacterial Resistances and Sensibilities in a Tertiary Care Hospital in Romania—A Retrospective Analysis" Microorganisms 12, no. 8: 1517. https://doi.org/10.3390/microorganisms12081517





