Initial Litter Chemistry and UV Radiation Drive Chemical Divergence in Litter during Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Solid-State 13C Cross-Polarization Magic-Angle Spinning Nuclear Magnetic Resonance Spectroscopy
2.4. Microbial Community Characterization and Bipartite Network Analysis
2.5. Extracellular Enzyme Activity Assays
2.6. Statistical Analyses
3. Results
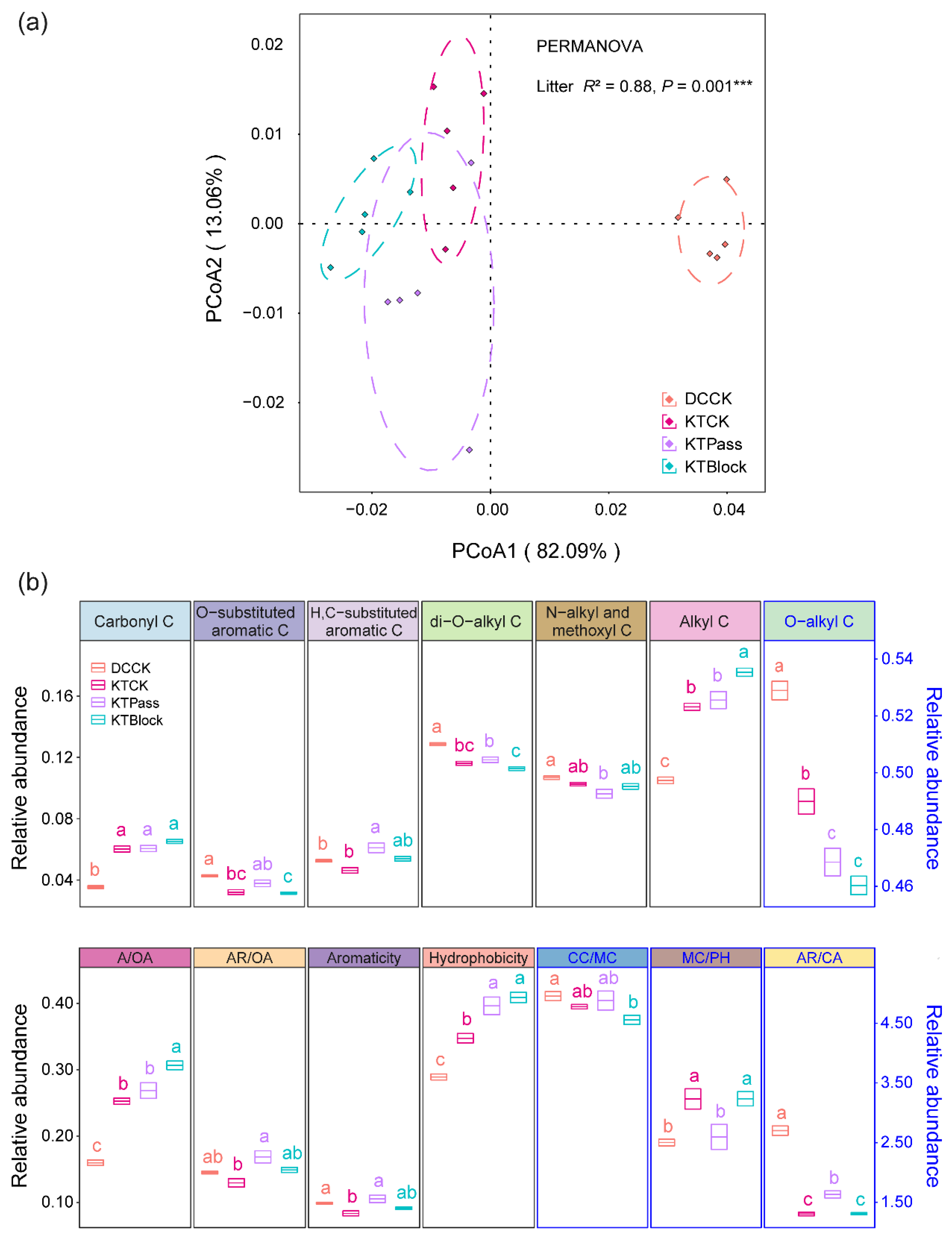
3.1. Effect of UV Radiation on Plants’ Chemical Traits during the Growth Phase
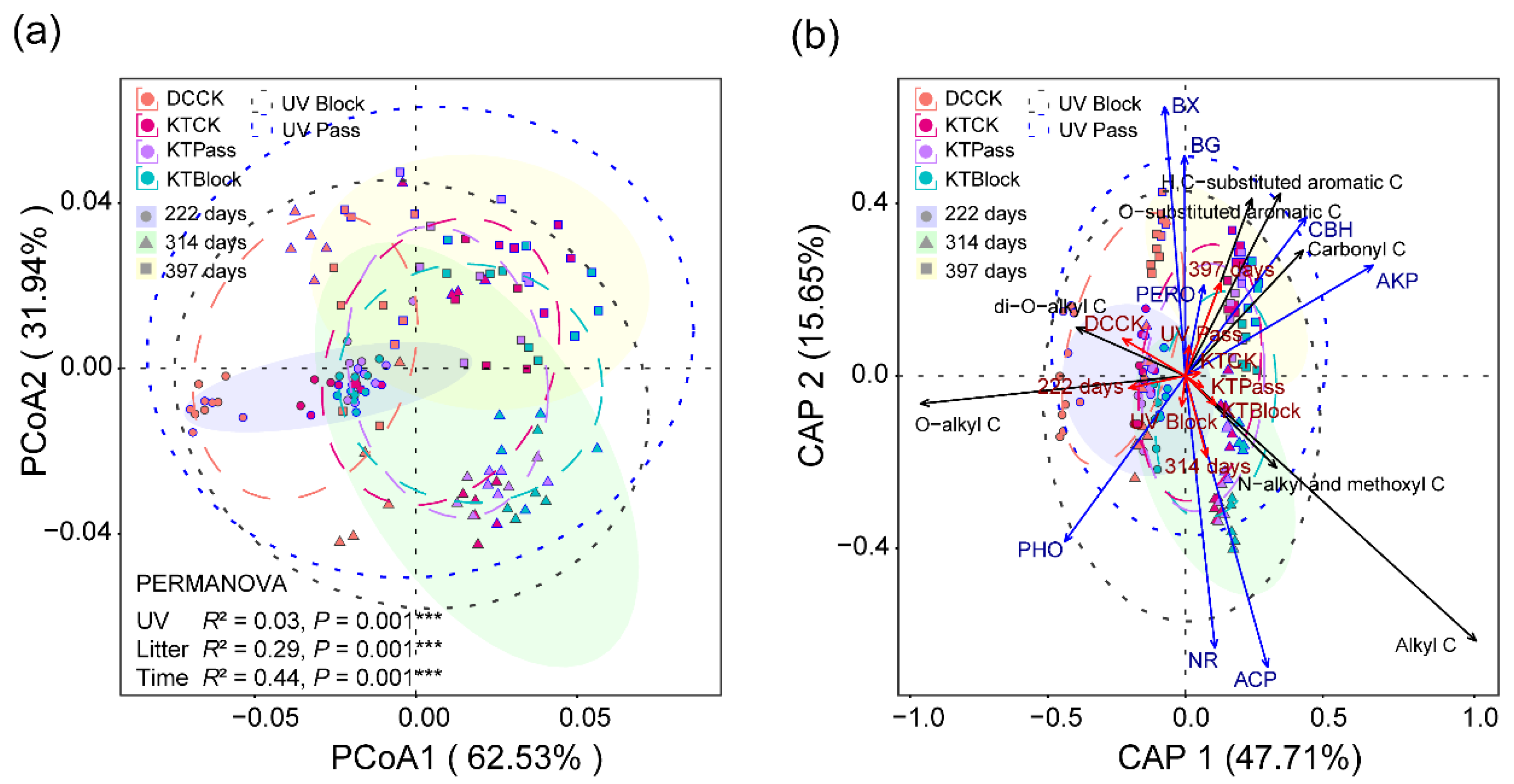
3.2. Effect of UV Radiation on Litter’s Chemical Traits during the Decomposition Phase
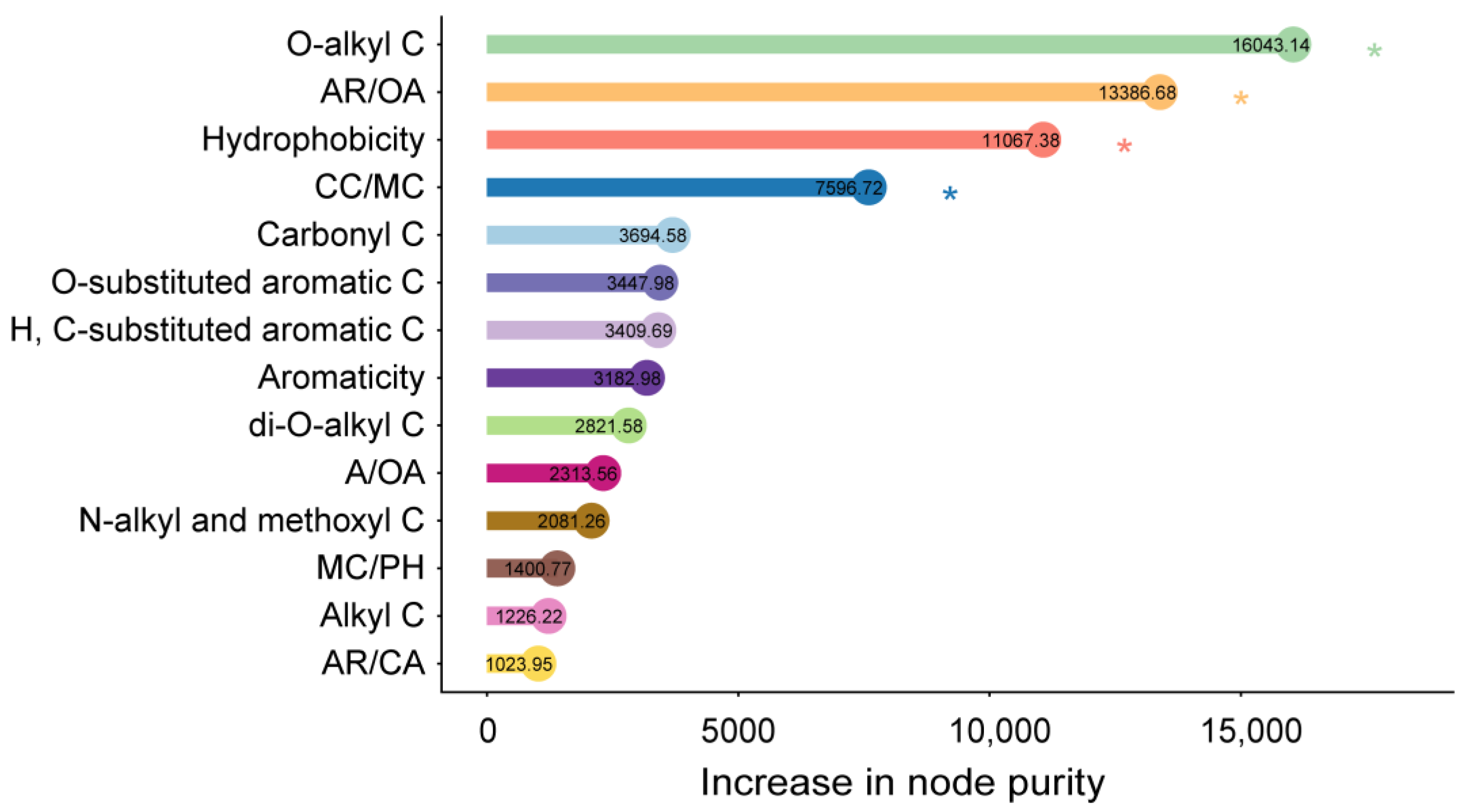
3.3. Relationships between Initial Litter Chemistry and Decay Rate
3.4. Relative Contribution of Litter’s Chemical Components as Predictors of Mass Loss
3.5. Relationships between Extracellular Enzyme Activity and Litter’s Chemical Complexity
3.6. Changes in Microbial Community Composition and Bipartite Networks
4. Discussion
4.1. Initial Chemical Traits Influence Litter Decomposition
4.2. Decay Time and Its Influence on Litter Decomposition
4.3. Microbial Communities and UV Radiation Influence Litter Decomposition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Austin, A.T.; Soledad Mendez, M.; Ballare, C.L. Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2016, 113, 4392–4397. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Veen, G.F.; Bonis, A.; Bradford, E.M.; Classen, A.T.; Cornelissen, J.H.C.; Crowther, T.W.; De Long, J.R.; Freschet, G.T.; Kardol, P.; et al. A test of the hierarchical model of litter decomposition. Nat. Ecol. Evol. 2017, 1, 1836–1845. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Garcia-Palacios, P.; McKie, B.G.; Handa, I.T.; Frainer, A.; Haettenschwiler, S. The importance of litter traits and decomposers for litter decomposition: A comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol. 2016, 30, 819–829. [Google Scholar] [CrossRef]
- Joly, F.-X.; Scherer-Lorenzen, M.; Haettenschwiler, S. Resolving the intricate role of climate in litter decomposition. Nat. Ecol. Evol. 2023, 7, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, C.; Mao, J.; Jiang, Y.; Bian, Q.; Liang, Y.; Chen, Y.; Sun, B. Microbial keystone taxa drive succession of plant residue chemistry. ISME J. 2023, 17, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Wickings, K.; Grandy, A.S.; Reed, S.C.; Cleveland, C.C. The origin of litter chemical complexity during decomposition. Ecol. Lett. 2012, 15, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Suseela, V.; Tharayil, N.; Xing, B.; Dukes, J.S. Warming alters potential enzyme activity but precipitation regulates chemical transformations in grass litter exposed to simulated climatic changes. Soil Biol. Biochem. 2014, 75, 102–112. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Z.; Fontaine, S.; Wang, W.; Luo, J.; Fan, J.; Ding, W. Dominant effects of organic carbon chemistry on decomposition dynamics of crop residues in a Mollisol. Soil Biol. Biochem. 2017, 115, 221–232. [Google Scholar] [CrossRef]
- Barnes, P.W.; Robson, T.M.; Zepp, R.G.; Bornman, J.F.; Jansen, M.A.K.; Ossola, R.; Wang, Q.W.; Robinson, S.A.; Foereid, B.; Klekociuk, A.R.; et al. Interactive effects of changes in UV radiation and climate on terrestrial ecosystems, biogeochemical cycles, and feedbacks to the climate system. Photochem. Photobiol. Sci. 2023, 22, 1049–1091. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef]
- Baker, N.R.; Allison, S.D. Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate. Ecology 2015, 96, 1994–2003. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; King, J.Y. Effects of UV exposure and litter position on decomposition in a California grassland. Ecosystems 2014, 17, 158–168. [Google Scholar] [CrossRef]
- Brandt, L.A.; Bohnet, C.; King, J.Y. Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems. J. Geophys. Res. Biogeosciences 2009, 114, G02004. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Wang, X.; Chen, Y. The interaction between abiotic photodegradation and microbial decomposition under ultraviolet radiation. Glob. Chang. Biol. 2015, 21, 2095–2104. [Google Scholar] [CrossRef]
- Henry, H.A.L.; Brizgys, K.; Field, C.B. Litter decomposition in a California annual grassland: Interactions between photodegradation and litter layer thickness. Ecosystems 2008, 11, 545–554. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballare, C.L. Dual role of lignin in plant litter decomposition in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef]
- Foereid, B.; Zarov, E.A.; Latysh, I.M.; Filippov, I.V.; Lapshina, E.D. Photo-exposure affects subsequent peat litter decomposition. Geoderma 2018, 315, 104–110. [Google Scholar] [CrossRef]
- Guerreiro, M.A.; Kambach, S.; Stoll, R.; Brachmann, A.; Senker, J.; Begerow, D.; Persoh, D. Linking processes to community functions-insights into litter decomposition combining fungal metatranscriptomics and environmental NMR profiling. Mycol. Prog. 2023, 22, 10. [Google Scholar] [CrossRef]
- Wang, Q.; Pieriste, M.; Liu, C.; Kenta, T.; Robson, T.M.; Kurokawa, H. The contribution of photodegradation to litter decomposition in a temperate forest gap and understorey. New Phytol. 2021, 229, 2625–2636. [Google Scholar] [CrossRef]
- Pieriste, M.; Chauvat, M.; Kotilainen, T.K.; Jones, A.G.; Aubert, M.; Robson, T.M.; Forey, E. Solar UV-A radiation and blue light enhance tree leaf litter decomposition in a temperate forest. Oecologia 2019, 191, 191–203. [Google Scholar] [CrossRef]
- Marinho, O.A.; Martinelli, L.A.; Duarte-Neto, P.J.; Mazzi, E.A.; King, J.Y. Photodegradation influences litter decomposition rate in a humid tropical ecosystem, Brazil. Sci. Total Environ. 2020, 715, 136601. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, H.; Jiang, H.; Peng, C. Combination of nitrogen deposition and ultraviolet-B radiation decreased litter decomposition in subtropical China. Plant Soil 2014, 380, 349–359. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; Handa, I.T.; Haettenschwiler, S.; van Ruijven, J.; van Bodegom, P.M.; Aerts, R. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Zhou, Y.; Zheng, H.; Xu, Z.; Tan, B.; You, C.; Zhang, L.; Li, H.; Guo, L.; et al. Litter chemical traits strongly drove the carbon fractions loss during decomposition across an alpine treeline ecotone. Sci. Total Environ. 2021, 753, 142287. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Y.; Chen, L.; Jiang, L.; Hong, Y.; Zhu, J.; Liu, J.; Xu, D.; Kuang, K.; He, Z. Microclimate along an elevational gradient controls foliar litter cellulose and lignin degradation in a subtropical forest. Front. For. Glob. Chang. 2023, 6, 1134598. [Google Scholar] [CrossRef]
- Yi, B.; Lu, C.; Huang, W.; Yu, W.; Yang, J.; Howe, A.; Weintraub-Leff, S.R.; Hall, S.J. Resolving the influence of lignin on soil organic matter decomposition with mechanistic models and continental-scale data. Glob. Chang. Biol. 2023, 29, 5968–5980. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Wang, J.; Shi, Z.; Lu, L.; Guo, W.; Jia, H.; Cai, D. Dynamics and speciation of organic carbon during decomposition of leaf litter and fine roots in four subtropical plantations of China. For. Ecol. Manag. 2013, 300, 43–52. [Google Scholar] [CrossRef]
- Wong, T.M.; Sullivan, J.H.; Eisenstein, E. Acclimation and Compensating Metabolite Responses to UV-B Radiation in Natural and Transgenic Populus spp. Defective in Lignin Biosynthesis. Metabolites 2022, 12, 767. [Google Scholar] [CrossRef]
- Fu, G.; Shen, Z.-X. Effects of enhanced UV-B radiation on plant physiology and growth on the Tibetan Plateau: A meta-analysis. Acta Physiol. Plant. 2017, 39, 85. [Google Scholar] [CrossRef]
- Parsons, S.A.; Congdon, R.A.; Lawler, I.R. Determinants of the pathways of litter chemical decomposition in a tropical region. New Phytol. 2014, 203, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Incerti, G.; Abd El-Gawad, A.M.; Cesarano, G.; Sarker, T.C.; Saulino, L.; Lanzotti, V.; Saracino, A.; Rego, F.C.; Mazzoleni, S. Comparing chemistry and bioactivity of burned vs. decomposed plant litter: Different pathways but same result? Ecology 2018, 99, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Anthony, M.A.; Crowther, T.W.; Maynard, D.S.; van den Hoogen, J.; Averill, C. Distinct Assembly Processes and Microbial Communities Constrain Soil Organic Carbon Formation. One Earth 2020, 2, 349–360. [Google Scholar] [CrossRef]
- Sokol, N.W.; Slessarev, E.; Marschmann, G.L.; Nicolas, A.; Blazewicz, S.J.; Brodie, E.L.; Firestone, M.K.; Foley, M.M.; Hestrin, R.; Hungate, B.A.; et al. Life and death in the soil microbiome: How ecological processes influence biogeochemistry. Nat. Rev. Microbiol. 2022, 20, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.M.; Peay, K.G.; Treseder, K.K. Litter chemistry influences decomposition through activity of specific microbial functional guilds. Ecol. Monogr. 2018, 88, 429–444. [Google Scholar] [CrossRef]
- Chen, Y.; Neilson, J.W.; Kushwaha, P.; Maier, R.M.; Barberan, A. Life-history strategies of soil microbial communities in an arid ecosystem. ISME J. 2021, 15, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Chang. Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S.X. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2023, 2, e66. [Google Scholar] [CrossRef]
- Zhang, K.; Duan, J.; Zhao, S.; Zhang, J.; Keeble, J.; Liu, H. Evaluating the Ozone Valley over the Tibetan Plateau in CMIP6 Models. Adv. Atmos. Sci. 2022, 39, 1167–1183. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, H.; Zhang, C.; Cheng, Q.; Zheng, Y.; Wang, C.; Xiao, B.; Li, P.; Chen, C. Ambient ultraviolet radiation: A new factor affecting anaerobic fermentation of oat and subsequent methane emissions. Bioresour. Technol. 2022, 355, 127243. [Google Scholar] [CrossRef] [PubMed]
- Bornman, J.F.; Barnes, P.W.; Robson, T.M.; Robinson, S.A.; Jansen, M.A.K.; Ballare, C.L.; Flint, S.D. Linkages between stratospheric ozone, UV radiation and climate change and their implications for terrestrial ecosystems. Photochem. Photobiol. Sci. 2019, 18, 681–716. [Google Scholar] [CrossRef] [PubMed]
- Quan, Q.; Tian, D.; Luo, Y.; Zhang, F.; Crowthers, T.W.; Zhu, K.; Chen, H.Y.H.; Zhou, Q.; Niu, S. Water scaling of ecosystem carbon cycle feedback to climate warming. Sci. Adv. 2019, 5, eaav1131. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Wei, X.; Cao, R.; Wu, X.; Eisenhauer, N.; Sun, S. Effect of water table decline on the abundances of soil mites, springtails, and nematodes in the Zoige peatland of eastern Tibetan Plateau. Appl. Soil Ecol. 2018, 129, 77–83. [Google Scholar] [CrossRef]
- Cao, R.; Wei, X.; Yang, Y.; Xi, X.; Wu, X. The effect of water table decline on plant biomass and species composition in the Zoige peatland: A four-year in situ field experiment. Agric. Ecosyst. Environ. 2017, 247, 389–395. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Hobbie, S.E.; Milchunas, D.G.; Sinsabaugh, R.L. The role of photodegradation in surface litter decomposition across a grassland ecosystem precipitation gradient. Ecosystems 2010, 13, 765–781. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Giannino, F.; Mingo, A.; Lanzotti, V.; Mazzoleni, S. Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than C/N and Lignin/N ratios. Soil Biol. Biochem. 2013, 56, 40–48. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, J.; Ding, W.; Gunina, A.; Chen, Z.; Bol, R.; Luo, J.; Bolan, N. Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additions: Links to microbial community composition and activity. Geoderma 2017, 286, 116–124. [Google Scholar] [CrossRef]
- Bonanomi, G.; Incerti, G.; Barile, E.; Capodilupo, M.; Antignani, V.; Mingo, A.; Lanzotti, V.; Scala, F.; Mazzoleni, S. Phytotoxicity, not nitrogen immobilization, explains plant litter inhibitory effects: Evidence from solid-state 13C NMR spectroscopy. New Phytol. 2011, 191, 1018–1030. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Harmon, M.E.; Li, Y.; Cao, X.; Chappell, M.A.; Mao, J. Plant Species Rather Than Climate Greatly Alters the Temporal Pattern of Litter Chemical Composition During Long-Term Decomposition. Sci. Rep. 2015, 5, 15783. [Google Scholar] [CrossRef]
- Sarker, T.C.; Maisto, G.; De Marco, A.; Esposito, F.; Panico, S.C.; Alam, M.F.; Mazzoleni, S.; Bonanomi, G. Explaining trajectories of chemical changes during decomposition of tropical litter by 13C-CPMAS NMR, proximate and nutrients analysis. Plant Soil 2019, 436, 13–28. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C.; Berg, B.; McClaugherty, C. Initial litter chemical composition. In Plant Litter: Decomposition, Humus Formation, Carbon Sequestration; Springer: Cham, Switzerland, 2020; pp. 67–100. [Google Scholar]
- Sarker, T.C.; Incerti, G.; Spaccini, R.; Piccolo, A.; Mazzoleni, S.; Bonanomi, G. Linking organic matter chemistry with soil aggregate stability: Insight from 13C NMR spectroscopy. Soil Biol. Biochem. 2018, 117, 175–184. [Google Scholar] [CrossRef]
- Tian, K.; Kong, X.; Yuan, L.; Lin, H.; He, Z.; Yao, B.; Ji, Y.; Yang, J.; Sun, S.; Tian, X. Priming effect of litter mineralization: The role of root exudate depends on its interactions with litter quality and soil condition. Plant Soil 2019, 440, 457–471. [Google Scholar] [CrossRef]
- Mao, J.; Cao, X.; Olk, D.C.; Chu, W.; Schmidt-Rohr, K. Advanced solid-state NMR spectroscopy of natural organic matter. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 100, 17–51. [Google Scholar] [CrossRef] [PubMed]
- McKee, G.A.; Soong, J.L.; Calderon, F.; Borch, T.; Cotrufo, M.F. An integrated spectroscopic and wet chemical approach to investigate grass litter decomposition chemistry. Biogeochemistry 2016, 128, 107–123. [Google Scholar] [CrossRef]
- Hobbie, S.E. Nitrogen effects on decomposition: A five-year experiment in eight temperate sites. Ecology 2008, 89, 2633–2644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Cesarano, G.; La Storia, A.; Zotti, M.; Mazzoleni, S.; Incerti, G. Linking bacterial and eukaryotic microbiota to litter chemistry: Combining next generation sequencing with 13C CPMAS NMR spectroscopy. Soil Biol. Biochem. 2019, 129, 110–121. [Google Scholar] [CrossRef]
- De Marco, A.; Spaccini, R.; Vittozzi, P.; Esposito, F.; Berg, B.; De Santo, A.V. Decomposition of black locust and black pine leaf litter in two coeval forest stands on Mount Vesuvius and dynamics of organic components assessed through proximate analysis and NMR spectroscopy. Soil Biol. Biochem. 2012, 51, 1–15. [Google Scholar] [CrossRef]
- Chavez-Vergara, B.; Merino, A.; Vazquez-Marrufo, G.; Garcia-Oliva, F. Organic matter dynamics and microbial activity during decomposition of forest floor under two native neotropical oak species in a temperate deciduous forest in Mexico. Geoderma 2014, 235, 133–145. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Castaneda-Gomez, L.; Lajtha, K.; Bowden, R.; Jauhar, F.N.M.; Jia, J.; Feng, X.; Simpson, M.J. Soil organic matter molecular composition with long-term detrital alterations is controlled by site-specific forest properties. Glob. Chang. Biol. 2023, 29, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Houfani, A.A.; Tlaskal, V.; Baldrian, P.; Hahnke, R.L.; Benallaoua, S. Actinobacterial Strains as Genomic Candidates for Characterization of Genes Encoding Enzymes in Bioconversion of Lignocellulose. Waste Biomass Valor. 2022, 13, 1523–1534. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Chen, Y.; Chen, B.; Guo, P.; Cui, Z. Metagenomic Insight into Lignocellulose Degradation of the Thermophilic Microbial Consortium TMC7. J. Microbiol. Biotechnol. 2021, 31, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mondejar, R.; Zuehlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberan, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, B.; Kong, X.; Tian, K.; Zeng, X.; Lu, W.; Pang, L.; Sun, S.; Tian, X. Initial Litter Chemistry and UV Radiation Drive Chemical Divergence in Litter during Decomposition. Microorganisms 2024, 12, 1535. https://doi.org/10.3390/microorganisms12081535
Yao B, Kong X, Tian K, Zeng X, Lu W, Pang L, Sun S, Tian X. Initial Litter Chemistry and UV Radiation Drive Chemical Divergence in Litter during Decomposition. Microorganisms. 2024; 12(8):1535. https://doi.org/10.3390/microorganisms12081535
Chicago/Turabian StyleYao, Bei, Xiangshi Kong, Kai Tian, Xiaoyi Zeng, Wenshuo Lu, Lu Pang, Shucun Sun, and Xingjun Tian. 2024. "Initial Litter Chemistry and UV Radiation Drive Chemical Divergence in Litter during Decomposition" Microorganisms 12, no. 8: 1535. https://doi.org/10.3390/microorganisms12081535




