Non-Linear Response of Alpha and Beta Diversity of Taxonomic and Functional Groups of Phytoplankton to Environmental Factors in Subtropical Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
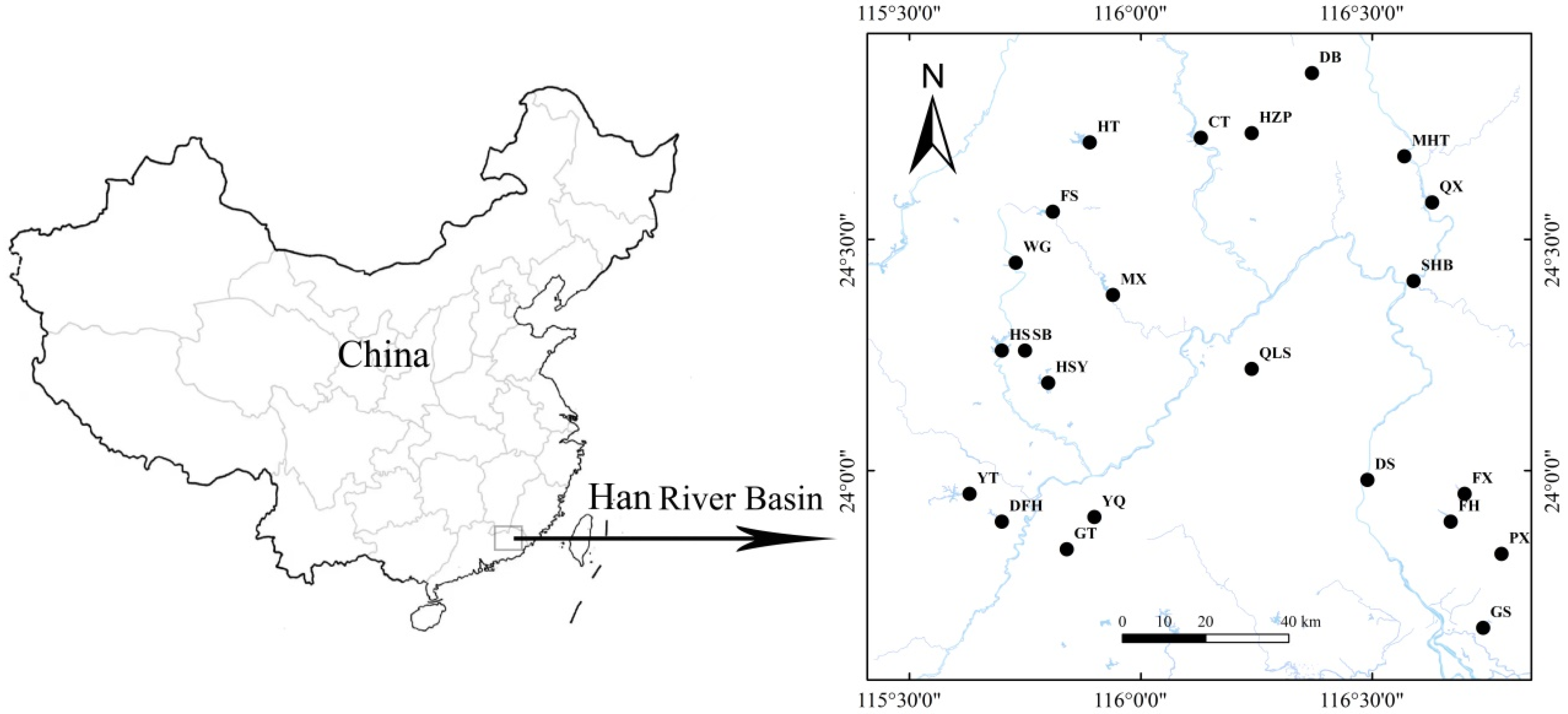
2.1. Study Area
2.2. Sampling and Analysis
2.3. Data Analysis
3. Results
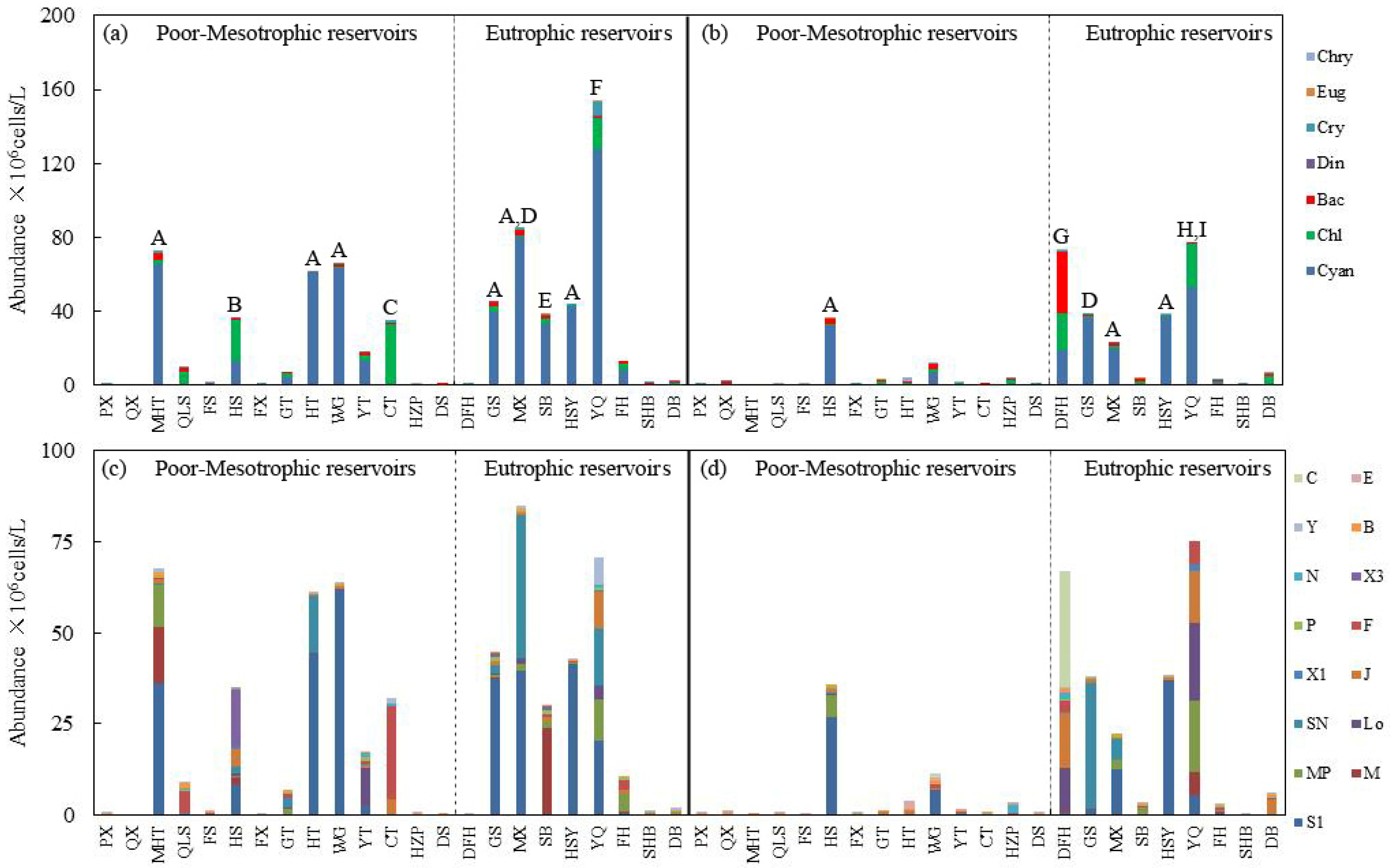
3.1. The Composition of Phytoplankton Species and Functional Groups
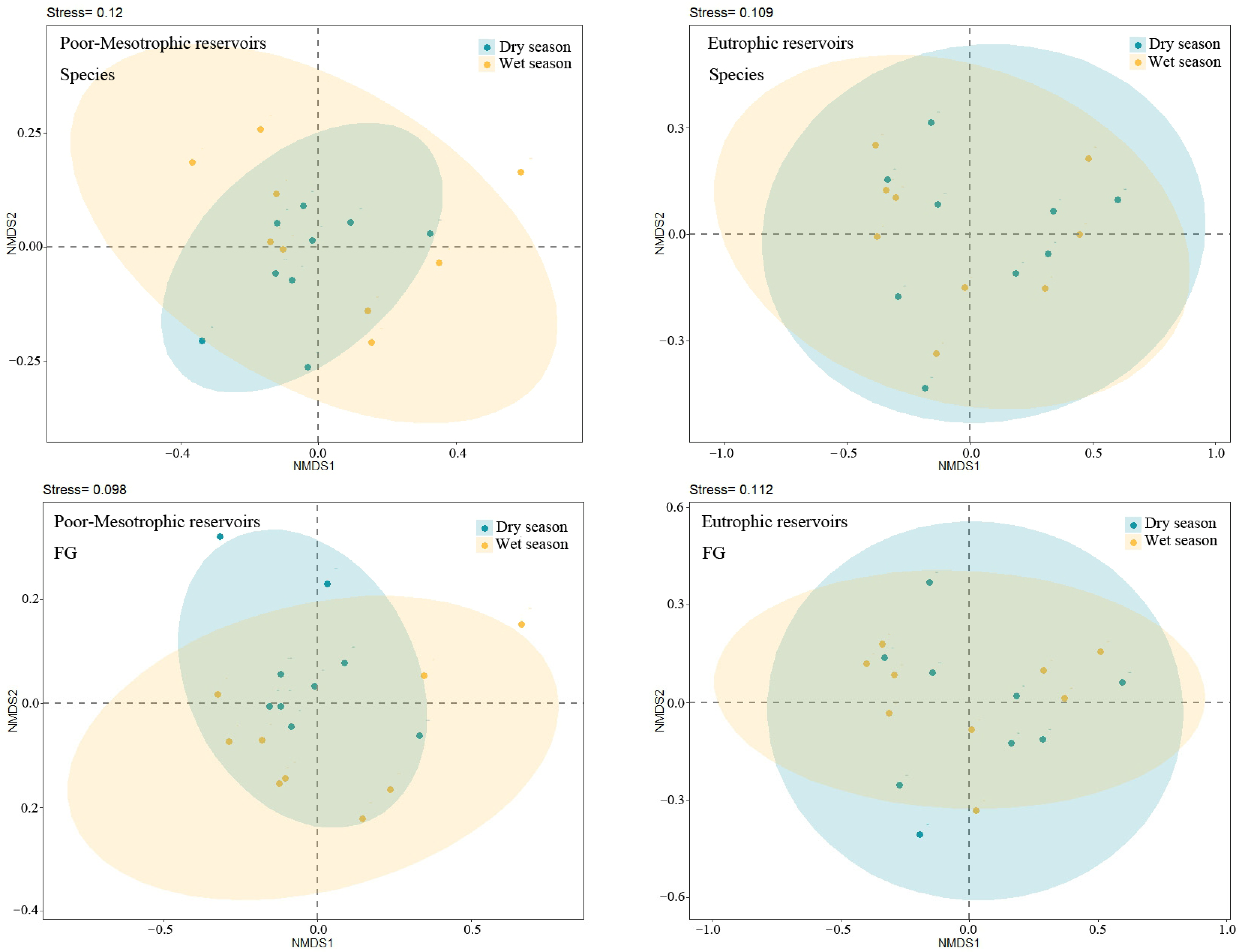
3.2. Alpha and Beta Diversity
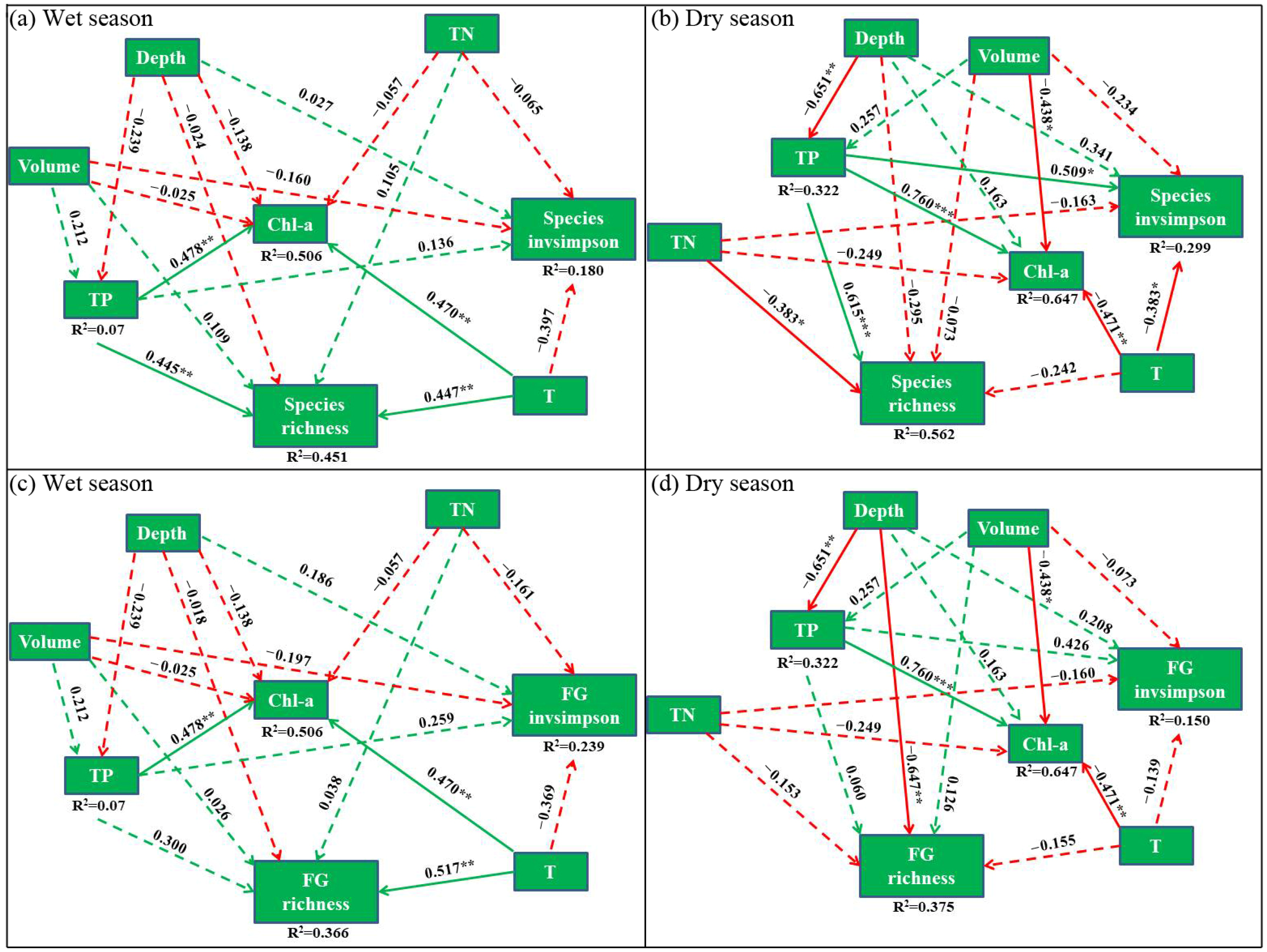
3.3. Environmental Variables Influencing Alpha and Beta Diversity
4. Discussion
4.1. Seasonal Dynamics of Phytoplankton Communities and Nutrient Levels
4.2. Non-Linear Responses of Species and Functional Group Beta Diversity
4.3. Limitations and Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Magurran, M.G. Biological Diversity: Frontiers in Measurement and Assessment; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Harrison, S.; Ross, S.J.; Lawton, J.H. Beta diversity on geographic gradients in Britain. J. Anim. Ecol. 1992, 61, 151–158. [Google Scholar] [CrossRef]
- Lennon, J.J.; Koleff, P.; GreenwooD, J.J.D.; Gaston, K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Stoll, S.; Jähnig, S.C.; Haase, P. Contrasting metacommunity structure and beta diversity in an aquatic-floodplain system. Oikos 2016, 125, 686–697. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Tuomisto, H.; Ruokolainen, K. Analyzing or explaining beta diversity? Understanding the targets of different methods of analysis. Ecology 2006, 87, 2697–2708. [Google Scholar] [CrossRef]
- Ferrier, S.; Manion, G.; Elith, J.; Richardson, K. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers. Distrib. 2007, 13, 252–264. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, M.; Yang, S.; Li, X.; Zhao, H.; Tang, J.; Jiang, G.; Li, Z.; Huang, Y.; Dong, K.; et al. Ecological stoichiometry influences phytoplankton alpha and beta diversity rather than the community stability in subtropical bay. Ecol. Evol. 2002, 12, e9301. [Google Scholar] [CrossRef]
- Hoyer, A.B.; Moreno-Ostos, E.; Vidal, J.; Blanco, J.M.; Palomino-Torres, R.L.; Basanta, A.; Escot, C.; Rueda, F.J. The influence of external perturbations on the function composition of phytoplankton in a Mediterranean Reservoir. Hydrobiologia 2009, 636, 49–64. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Xiao, L.-J.; Wang, T.; Hu, R.; Han, B.-P.; Wang, S.; Qian, X.; Padisák, J. Succession of phytoplankton functional groups regulated by monsoonal hydrology in a large canyon-shaped reservoir. Water Res. 2011, 45, 5009–5019. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xiao, L.J. Functional Classification of Phytoplankton Assemblages in Reservoirs of Guangdong Province, South China. Tropical and Sub-Tropical Reservoir Limnology in China: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2012; pp. 59–70. [Google Scholar]
- Wu, N.; Wang, Y.; Wang, Y.; Sun, X.; Faber, C.; Fohrer, N. Environment regimes play an important role in structuring trait- and taxonomy-based temporal beta diversity of riverine diatoms. J. Ecol. 2022, 110, 1442–1454. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, R.; Lin, Q.; Hou, J.; Liu, Y.; Han, B.P.; Naselli-Flores, L. Spatial structure and β-diversity of phytoplankton in Tibetan Plateau lakes: Nestedness or replacement? Hydrobiologia 2018, 808, 301–314. [Google Scholar] [CrossRef]
- Hu, R.; Han, B.; Naselli-Flores, L. Comparing biological classifications of freshwater phytoplankton: A case study from South China. Hydrobiologia 2013, 701, 219–233. [Google Scholar] [CrossRef]
- Tadonleke, R.D.; Thouvenot, A.; Gilbert, D.; Sime-Ngando, T.; Debroas, D.; Devaux, J. Size-fractionated phytoplankton and relationships with Metazooplankton in a Newly Flooded Reservoir. Int. Rev. Hydrobiol. 2003, 88, 614–634. [Google Scholar] [CrossRef]
- Popovskaya, G.I. Ecological monitoring of phytoplankton in Lake Baikal. Aquat. Ecosyst. Health Manag. 2000, 3, 215–225. [Google Scholar] [CrossRef]
- EPBC. Standard Methods for the Monitoring and Analysis on Water and Wastewater, 4th ed.; Chinese Environmental Science Press: Beijing, China, 2002.
- APHA. Standard Methods for the Examination of Water and Wastewater; American Water Works Association and Water Pollution Control Federation: Washington, DC, USA, 1989. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Aizaki, M.; Otsuki, A.; Fukushima, T.; Hosomi, M.; Muraoka, K. Application of Carlson’s trophic state index to Japanese lakes and relationships between the index and other parameters. Proc.-Int. Assoc. Theor. Appl. Limnol. 1981, 23, 13–31. [Google Scholar] [CrossRef]
- Reynolds, C. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; pp. 24–28. [Google Scholar]
- Baselga, A.; Orme, D.; Villéger, S.; DeBortoli, J.; Leprieur, F.; Logez, M.; Henriques-Silva, R.; Mart, S.; Mart, R.; Gmez-Rodrguez, C.; et al. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components, R package version 1.5.6; Package ‘betapart’; R Core Team: Vienna, Austria, 2002. [Google Scholar]
- Arhonditsis, G.; Stow, C.; Steinberg, L.; Kenney, M.; Lathrop, R.; McBride, S.; Reckhow, K. Exploring ecological patterns with structural equation modeling and Bayesian analysis. Ecol. Model. 2006, 192, 385–409. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.M.; Olff, H.; Scheiner, S.M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 2010, 80, 67–87. [Google Scholar] [CrossRef]
- Shipley, B. Cause and Correlation in Biology: A User’s Guide to Path Analysis, Structural Equations, and Causal Inference in R; Cambridge University Press: New York, NY, USA, 2016. [Google Scholar]
- Xu, Y.; Xiang, Z.; Rizo, E.Z.; Naselli-Flores, L.; Han, B.-P. Combination of linear and nonlinear multivariate approaches effectively uncover responses of phytoplankton communities to environmental changes at regional scale. J. Environ. Manag. 2022, 305, 114399. [Google Scholar] [CrossRef] [PubMed]
- Nalewajko, C.; Murphy, T.P. Effects of temperature, and availability of nitrogen and phosphorus on the abundance of Anabaena and Microcystis in Lake Biwa, Japan: An experimental approach. Limnology 2001, 2, 45–48. [Google Scholar] [CrossRef]
- Znachor, P.; Zapomělová, E.; Řeháková, K.; Nedoma, J.; Šimek, K. The effect of extreme rainfall on summer succession and vertical distribution of phytoplankton in a lacustrine part of a eutrophic reservoir. Aquat. Sci. 2008, 70, 77–86. [Google Scholar] [CrossRef]
- Cupertino, A.; Gücker, B.; Von Rückert, G.; Figueredo, C.C. Phytoplankton assemblage composition as an environmental indicator in routine lentic monitoring: Taxonomic versus functional groups. Ecol. Indic. 2019, 101, 522–532. [Google Scholar] [CrossRef]
- Isvánovics, V.; Shafik, H.M.; Présing, M.; Juhos, S. Growth and phosphate uptake kinetics of the cyanobacterium, Cylindrospermopsis raciborskii (Cyanophyceae) in throughflow cultures. Freshw. Biol. 2000, 43, 257–275. [Google Scholar] [CrossRef]
- Ferber, L.R.; Levine, S.N.; Lini, A.; Livingston, G.P. Do cyanobacteria dominate in eutrophic lakes because they fix atmospheric nitrogen? Freshw. Biol. 2004, 49, 690–708. [Google Scholar] [CrossRef]
- Shen, H.; Song, L. Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia 2007, 592, 475–486. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, J.; Han, B.-P.; Naselli-Flores, L. The effects of absolute and relative nutrient concentrations (N/P) on phytoplankton in a subtropical reservoir. Ecol. Indic. 2020, 115, 106466. [Google Scholar] [CrossRef]
- Harpole, W.S.; Ngai, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B.; et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.A.; Goepfert, T.J.; Ritt, J.T. Some thoughts on the concept of colimitation: Three definitions and the importance of bioavailability. Limnol. Oceanogr. 2008, 53, 276–290. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.S.; Halsey, K.H. Phytoplankton community structuring and succession in a competition-neutral resource landscape. ISME Commun. 2021, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.L.; Kemp, A.; Hickman, A.; Purdie, D. Environmental controls on the interannual variability in chlorophyll and phytoplankton community structure within the seasonal sub surface chlorophyll maximum in the Western English Channel. Cont. Shelf Res. 2024, 277, 105253. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xu, L.; Xie, S.; Feng, J. Interactive effects of temperature and nutrients on the phytoplankton community in an urban river in China. Environ. Monit. Assess. 2019, 191, 1–16. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.G., Jr.; Chave, J.; Terborgh, J.; Foster, R.B.; Núñez, V.P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Beta-diversity in tropical forest trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lindström, E.S.; Forslund, M.; Algesten, G.; Bergström, A.-K. External control of bacterial community structure in lakes. Limnol. Oceanogr. 2006, 51, 339–342. [Google Scholar] [CrossRef]
- de Moura, W.B.; da Silva, P.R.L.; Baumgartner, G.; Bueno, N.C.; Bortolini, J.C. Site contributions to phytoplankton beta diversity along two subtropical reservoirs. Aquat. Sci. 2022, 84, 59. [Google Scholar] [CrossRef]
- Rusanov, A.G.; Bíró, T.; Kiss, K.T.; Buczkó, K.; Grigorszky, I.; Hidas, A.; Duleba, M.; Trábert, Z.; Földi, A.; Ács, E. Relative importance of climate and spatial processes in shaping species composition, functional structure and beta diversity of phytoplankton in a large river. Sci. Total Environ. 2022, 807, 150891. [Google Scholar] [CrossRef]
- Lean, D.R.S.; Pick, F.R. Photosynthetic response of lake plankton to nutrient enrichment: A test for nutrient limitation. Limnology 1981, 26, 1001–1019. [Google Scholar] [CrossRef]
- O’Farrell, I.; Tezanos, P.P.; Izaguirre, I. Phytoplankton morphological response to the underwater light conditions in a vegetated wetland. Hydrobiologia 2007, 578, 65–77. [Google Scholar] [CrossRef]
- Padisák, J.; Naselli-Flores, L. Phytoplankton in extreme environments: Importance and consequences of habitat permanency. Hydrobiologia 2021, 848, 157–176. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Bisson, K.M. Neutral theory and plankton biodiversity. Annu. Rev. Mar. Sci. 2024, 16, 283–305. [Google Scholar] [CrossRef]
- Verreydt, D.; DeMeester, L.; Decaestecker, E.; Villena, M.J.; Gucht, K.V.D.; Vannormelingen, P.; Vyverman, W.; Declerck, S.A. Dispersal-mediated trophic interactions can generate apparent patterns of dispersal limitation in aquatic metacommunities. Ecol. Lett. 2012, 15, 218–226. [Google Scholar] [CrossRef]
- Bennett, J.R.; Cumming, B.F.; Ginn, B.K.; Smol, J.P. Broad-scale environmental response and niche conservatism in lacustrine diatom communities. Glob. Ecol. Biogeogr. 2010, 19, 724–732. [Google Scholar] [CrossRef]

| Environmental Variables | Wet Season Percent Deviance Explained | Dry Season Percent Deviance Explained | ||
|---|---|---|---|---|
| Species | FG | Species | FG | |
| T | 7.87 | 5.97 | / | / |
| TN | / | 6.48 | / | / |
| TP | 0.75 | 4.91 | 6.73 | 0.9 |
| PO4 | / | 1.65 | / | / |
| NO3-N | 3.25 | 2.54 | 2.22 | / |
| NH4-N | 2.7 | 1.48 | 2.62 | 0.54 |
| Geo | 0.57 | / | 5.14 | 0.36 |
| Cond | 4.88 | 0.47 | 4.06 | 0.47 |
| Total | 20.02 | 23.5 | 20.77 | 2.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Z.; Niu, H.; Tang, Q.; Hu, R. Non-Linear Response of Alpha and Beta Diversity of Taxonomic and Functional Groups of Phytoplankton to Environmental Factors in Subtropical Reservoirs. Microorganisms 2024, 12, 1547. https://doi.org/10.3390/microorganisms12081547
Xiang Z, Niu H, Tang Q, Hu R. Non-Linear Response of Alpha and Beta Diversity of Taxonomic and Functional Groups of Phytoplankton to Environmental Factors in Subtropical Reservoirs. Microorganisms. 2024; 12(8):1547. https://doi.org/10.3390/microorganisms12081547
Chicago/Turabian StyleXiang, Zhenlong, Haiyu Niu, Quehui Tang, and Ren Hu. 2024. "Non-Linear Response of Alpha and Beta Diversity of Taxonomic and Functional Groups of Phytoplankton to Environmental Factors in Subtropical Reservoirs" Microorganisms 12, no. 8: 1547. https://doi.org/10.3390/microorganisms12081547





