Recombinant Hemagglutinin Protein from H9N2 Avian Influenza Virus Exerts Good Immune Effects in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Virus and Mouse Strains
2.3. HA Gene Encoding and Plasmid Construction
2.4. SDS–PAGE and Western Blotting
2.5. Purification HA Protein
2.6. Hemagglutination Assay of HA Protein
2.7. HA Protein Acid Sensitivity
2.8. HA Protein Thermal Sensitivity
2.9. Animal Experiments and Safety Evaluation
2.10. Hemagglutination Inhibition Antibody Assays
2.11. IgG Antibody Assay
2.12. Mouse Splenocyte Supernatant Multicytokine Assay
2.13. Statistical Analysis
3. Results
3.1. HA Protein Successfully Expressed in Escherichia coli Cells
3.2. HA Protein Has Good Acid and Thermal Stability
3.3. The HA Protein Is Safe in Mice
3.4. High Levels of Hemagglutinin-Inhibiting Antibodies and IgG Antibodies
3.5. Effects of Multiple Cytokines on Mouse Spleen Cell Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parrish, C.R.; Kawaoka, Y. The origins of new pandemic viruses: The acquisition of new host ranges by canine parvovirus and influenza a viruses. Annu. Rev. Microbiol. 2005, 59, 553–586. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.; Lowen, A.C.; Mubareka, S.; Palese, P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS Pathog 2009, 5, e1000252. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Marshall, J.F.; Morrell, J.; Robb, D.; Mccauley, J.W.; Perez, D.R.; Parrish, C.R.; Murcia, P.R. Infection and pathogenesis of canine, equine, and human influenza viruses in canine tracheas. J. Virol. 2014, 88, 9208–9219. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.S.; Choi, J.Y.; Fieldhouse, J.K.; Borkenhagen, L.K.; Zemke, J.; Zhang, D.; Gray, G.C. The continual threat of influenza virus infections at the human—Animal interface. Evol. Med. Public Health 2018, 2018, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Listed, N.A. A revision of the system of nomenclature for influenza viruses: A WHO memorandum. B. World Health Organ. 1980, 58, 585–591. [Google Scholar]
- Guan, Y.; Shortridge, K.F.; Krauss, S.; Chin, P.S.; Dyrting, K.C.; Ellis, T.M.; Webster, R.G.; Peiris, M. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 2000, 74, 9372–9380. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, H.; Li, Q.; Huang, J.; Zhao, M.; Gu, X.; Jiang, K.; Wang, X.; Peng, D.; Liu, X. Enzootic genotype S of H9N2 avian influenza viruses donates internal genes to emerging zoonotic influenza viruses in China. Vet. Microbiol. 2014, 174, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Rahimirad, S.; Alizadeh, A.; Alizadeh, E.; Hosseini, S.M. The avian influenza H9N2 at avian-human interface: A possible risk for the future pandemics. J. Res. Med. Sci. 2016, 21, 51. [Google Scholar] [PubMed]
- Shen, Y.; Ke, C.; Li, Q.; Yuan, R.; Xiang, D.; Jia, W.; Yu, Y.; Liu, L.; Huang, C.; Qi, W.; et al. Novel reassortant avian influenza A(H5N6) viruses in humans, guangdong, china, 2015. Emerg. Infect. Dis. 2016, 22, 1507–1509. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.; Jiang, L.; Xiong, C.; Chen, Y.; Zhao, G.; Jiang, Q. The complexity of human infected AIV H5N6 isolated from China. BMC Infect. Dis. 2016, 16, 600. [Google Scholar] [CrossRef]
- Chen, L.J.; Lin, X.D.; Tian, J.H.; Liao, Y.; Ying, X.H.; Shao, J.W.; Yu, B.; Guo, J.J.; Wang, M.R.; Peng, Y.; et al. Diversity, evolution and population dynamics of avian influenza viruses circulating in the live poultry markets in China. Virology 2017, 505, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, H.; Li, L.; Liu, Y.; Zhang, W.; Gao, R.; Huang, W.; Luo, Q.; Gao, Y.; Luo, Q.; et al. Endemic variation of H9N2 avian influenza virus in china. Avian Dis. 2016, 60, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Chen, Y.; Ye, H.; Ding, S.; Zhang, T.; Liu, Y.; Li, H.; Huang, L.; Qi, W.; et al. Mutational antigenic landscape of prevailing H9N2 influenza virus hemagglutinin spectrum. Cell Rep. 2023, 42, 113409. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhao, Z.; Sang, J.; Jiang, W.; Chen, J.; Tang, T.; Li, Y.; Kan, Q.; Shao, H.; Zhang, J.; et al. Amino acid variation at hemagglutinin position 193 impacts the properties of H9N2 avian influenza virus. J. Virol. 2023, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Quan, K.; Chen, Z.; Hu, Q.; Nie, M.; Xu, N.; Gao, R.; Wang, X.; Qin, T.; Chen, S.; et al. The emergence of new antigen branches of H9N2 avian influenza virus in China due to antigenic drift on hemagglutinin through antibody escape at immunodominant sites. Emerg. Microbes Infect. 2023, 12, 2246582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Li, Y.; Wu, Z.; Ai, H.; Zhang, M.; Rong, L.; Blinov, M.L.; Tong, Q.; Liu, L.; et al. Mixed selling of different poultry species facilitates emergence of public-health-threating avian influenza viruses. Emerg. Microbes Infect. 2023, 12, 2214255. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, J.; Zhang, C.; Li, S.; Yuan, M.; Zhang, C.; Shen, C.; Yang, Y.; Fu, L.; Xu, G.; et al. Genetic, biological and epidemiological study on a cluster of H9N2 avian influenza virus infections among chickens, a pet cat, and humans at a backyard farm in Guangxi, China. Emerg. Microbes Infect. 2023, 12, 2143282. [Google Scholar] [CrossRef] [PubMed]
- Yi Peng Sun, J.L. H9N2 influenza virus in China: A cause of concern. Protein Cell 2015, 1, 18–25. [Google Scholar]
- Chivukula, S.; Plitnik, T.; Tibbitts, T.; Karve, S.; Dias, A.; Zhang, D.; Goldman, R.; Gopani, H.; Khanmohammed, A.; Sarode, A.; et al. Development of multivalent mRNA vaccine candidates for seasonal or pandemic influenza. Npj Vaccines 2021, 6, 153. [Google Scholar] [CrossRef]
- Li, Z.; Zaiser, S.A.; Shang, P.; Heiden, D.L.; Hajovsky, H.; Katwal, P.; Devries, B.; Baker, J.; Richt, J.A.; Li, Y.; et al. A chimeric influenza hemagglutinin delivered by parainfluenza virus 5 vector induces broadly protective immunity against genetically divergent influenza a H1 viruses in swine. Vet. Microbiol. 2020, 250, 108859. [Google Scholar] [CrossRef]
- Kaugars, K.; Dardick, J.; de Oliveira, A.P.; Weiss, K.A.; Lukose, R.; Kim, J.; Leung, L.; Rajagopalan, S.; Wolin, S.; Akabas, L.; et al. A recombinant herpes virus expressing influenza hemagglutinin confers protection and induces antibody-dependent cellular cytotoxicity. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zheng, X.; Eschke, K.; Chaudhry, M.Z.; Bertoglio, F.; Tomic, A.; Krmpotic, A.; Hoffmann, M.; Bar-On, Y.; Boehme, J.; et al. MCMV-based vaccine vectors expressing full-length viral proteins provide long-term humoral immune protection upon a single-shot vaccination. Cell. Mol. Immunol. 2022, 19, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Boyoglu-Barnum, S.; Hutchinson, G.B.; Boyington, J.C.; Moin, S.M.; Gillespie, R.A.; Tsybovsky, Y.; Stephens, T.; Vaile, J.R.; Lederhofer, J.; Corbett, K.S.; et al. Glycan repositioning of influenza hemagglutinin stem facilitates the elicitation of protective cross-group antibody responses. Nat. Commun. 2020, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Nunez, I.A.; Huang, Y.; Ross, T.M. Next-Generation computationally designed influenza hemagglutinin vaccines protect against H5Nx virus infections. Pathogens 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Mohan, T.; Chang, T.Z.; Gonzalez, G.X.; Wang, Y.; Kwon, Y.; Kang, S.; Compans, R.W.; Champion, J.A.; Wang, B. Double-layered protein nanoparticles induce broad protection against divergent influenza a viruses. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Korenkov, D.; Isakova-Sivak, I.; Rudenko, L. Basics of CD8 T-cell immune responses after influenza infection and vaccination with inactivated or live attenuated influenza vaccine. Expert Rev. Vaccines 2018, 17, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, J.O.; Chang, J. Nucleoprotein vaccine induces cross-protective cytotoxic T lymphocytes against both lineages of influenza B virus. Clin. Exp. Vaccine Res. 2019, 8, 54–63. [Google Scholar] [CrossRef]
- Nelson, S.A.; Dileepan, T.; Rasley, A.; Jenkins, M.K.; Fischer, N.O.; Sant, A.J.; Lawrence Livermore National Lab; Llnl, L.C.U.S.; Schultz-Cherry, S. Intranasal nanoparticle vaccination elicits a persistent, polyfunctional CD4 t cell response in the murine lung specific for a highly conserved influenza virus antigen that is sufficient to mediate protection from influenza virus challenge. J. Virol. 2021, 95, e84121. [Google Scholar] [CrossRef]
- Noisumdaeng, P.; Roytrakul, T.; Prasertsopon, J.; Pooruk, P.; Lerdsamran, H.; Assanasen, S.; Kitphati, R.; Auewarakul, P.; Puthavathana, P. T cell mediated immunity against influenza H5N1 nucleoprotein, matrix and hemagglutinin derived epitopes in H5N1 survivors and non-H5N1 subjects. PeerJ 2021, 9, e11021. [Google Scholar] [CrossRef]
- Compans, R.W.; Cooper, M.; Koprowski, H. The Systemic and Mucosal Immune Response of Humans to Influenza a Virus; Springer: Berlin/Heidelberg, Germany, 1989; Volume 146, pp. 107–116. [Google Scholar]
- Xiong, F.; Zhang, C.; Shang, B.; Zheng, M.; Wang, Q.; Ding, Y.; Luo, J.; Li, X. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza a viruses. Emerg Microbes Infect 2023, 12, 2256422. [Google Scholar] [CrossRef]
- Hernandez, R.; Põder, J.; Laporte, K.M.; Malek, T.R. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat. Rev. Immunol. 2022, 22, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, G.; Vatrella, A.; Busceti, M.T.; Gallelli, L.; Preiano, M.; Lombardo, N.; Terracciano, R.; Maselli, R. Role of biologics in severe eosinophilic asthma - focus on reslizumab. Ther. Clin. Risk Manag. 2016, 12, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Lu, X.; Wang, S.; Hao, J.; Guo, D.; Wu, H.; Jiang, Y.; Sun, Y.; Wang, J.; Zhang, G.; et al. Type I interferon/STAT1 signaling regulates UBE2M-mediated antiviral innate immunity in a negative feedback manner. Cell Rep. 2023, 42, 112002. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.; Dietrich, D.; Martin, P.; Palmer, G.; Gabay, C. The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine 2015, 76, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.D.; Ong, J.; Dowling, J.K.; Mcauley, J.L.; Robertson, A.B.; Latz, E.; Drummond, G.R.; Cooper, M.A.; Hertzog, P.J.; Mansell, A. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza a virus infection via temporal inhibition. Sci. Rep. 2016, 6, 27912. [Google Scholar] [CrossRef] [PubMed]
- Novick, D.; Kim, S.; Kaplanski, G.; Dinarello, C.A. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 2013, 25, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bao, L.; Wang, L.; Li, F.; Wen, M.; Li, H.; Deng, W.; Zhang, X.; Cao, B. Anti-IFN-gamma therapy alleviates acute lung injury induced by severe influenza a (H1N1) pdm09 infection in mice. J. Microbiol. Immunol. Infection 2021, 54, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Bawazeer, A.O.; Rosli, S.; Harpur, C.M.; Docherty, C.A.; Mansell, A.; Tate, M.D. Interleukin-1β exacerbates disease and is a potential therapeutic target to reduce pulmonary inflammation during severe influenza a virus infection. Immunol. Cell Biol. 2021, 99, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Fritz, R.S.; Straus, S.E.; Gubareva, L.; Hayden, F.G. Symptom pathogenesis during acute influenza: Interleukin-6 and other cytokine responses. J. Med. Virol. 2001, 64, 262–268. [Google Scholar] [CrossRef]
- Hagau, N.; Slavcovici, A.; Gonganau, D.N.; Oltean, S.; Dirzu, D.S.; Brezoszki, E.S.; Maxim, M.; Ciuce, C.; Mlesnite, M.; Gavrus, R.L.; et al. Clinical aspects and cytokine response in severe H1N1 influenza a virus infection. Crit. Care 2010, 14, R203. [Google Scholar] [CrossRef]
- Oshansky, C.M.; Gartland, A.J.; Wong, S.S.; Jeevan, T.; Wang, D.; Roddam, P.L.; Caniza, M.A.; Hertz, T.; Devincenzo, J.P.; Webby, R.J.; et al. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am. J. Respir. Crit. Care Med. 2014, 189, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, R.; Chen, B.; Pan, Q.; Chen, Y.; Hong, J.; Zhang, L.; Liu, W.; Wang, S.; Chen, J.L. Influenza Virus-Induced robust expression of SOCS3 contributes to excessive production of IL-6. Front. Immunol. 2019, 10, 1843. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Karupiah, G. Targeting tumour necrosis factor to ameliorate viral pneumonia. FEBS J. 2022, 289, 883–900. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, O.H. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017, 469, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.A.; Szretter, K.J.; Katz, J.M.; Mizgerd, J.P.; Tumpey, T.M. Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. J. Infect. Dis. 2010, 202, 1161–1170. [Google Scholar] [CrossRef]
- Szretter, K.J.; Gangappa, S.; Lu, X.; Smith, C.; Shieh, W.J.; Zaki, S.R.; Sambhara, S.; Tumpey, T.M.; Katz, J.M. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 2007, 81, 2736–2744. [Google Scholar] [CrossRef]
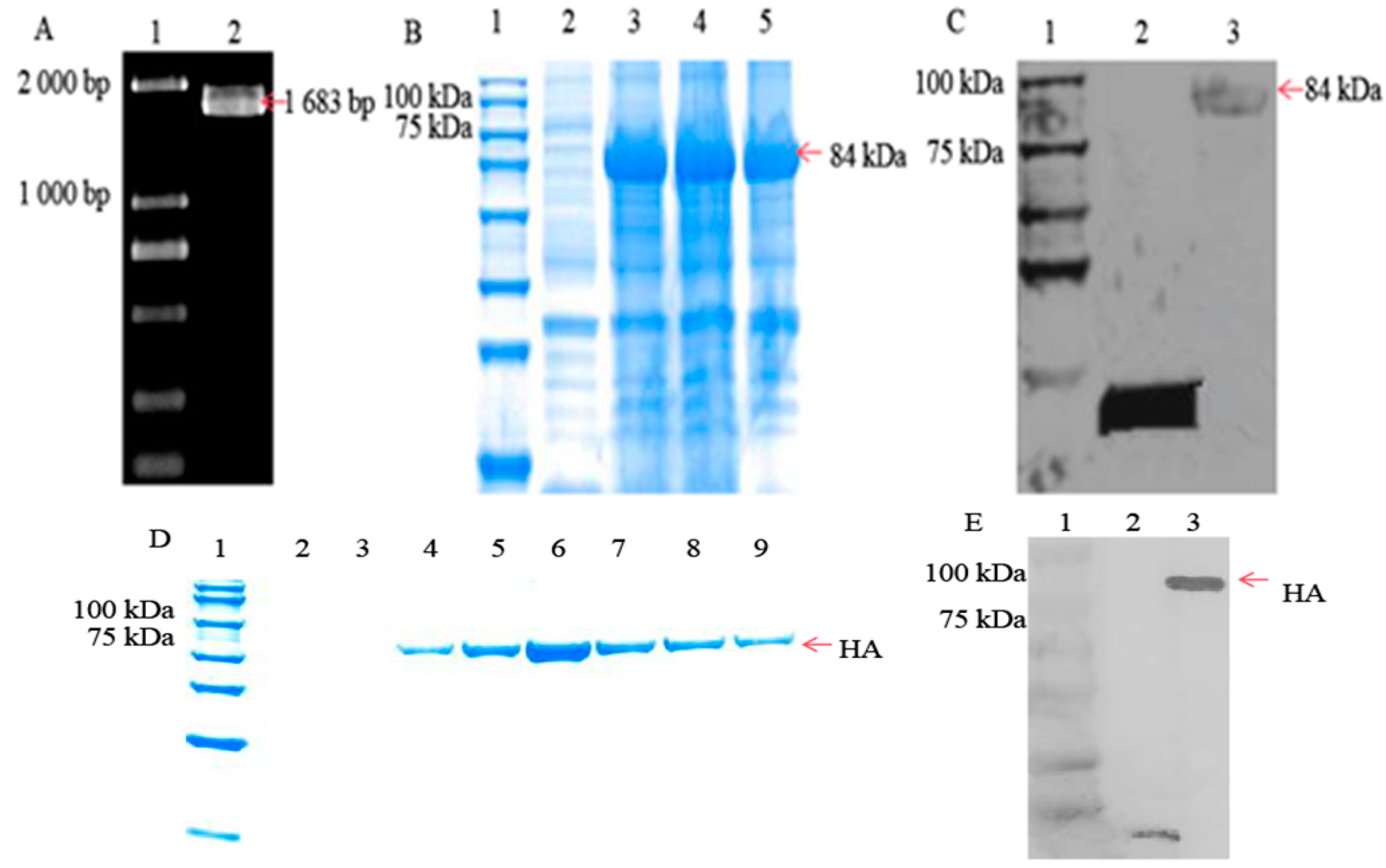
| Primer Name | Sequence (5′-3′) | Amplified Sequence Length |
|---|---|---|
| HA-U | ATAGGATCCATGGAGACAGTATCACTAATAACTA | 1683 bp |
| HA-D | ATAAGAATGCGGCCGCTTATATACAAATGTTGCATCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xie, Z.; Wei, Y.; Li, M.; Zhang, M.; Luo, S.; Xie, L. Recombinant Hemagglutinin Protein from H9N2 Avian Influenza Virus Exerts Good Immune Effects in Mice. Microorganisms 2024, 12, 1552. https://doi.org/10.3390/microorganisms12081552
Li X, Xie Z, Wei Y, Li M, Zhang M, Luo S, Xie L. Recombinant Hemagglutinin Protein from H9N2 Avian Influenza Virus Exerts Good Immune Effects in Mice. Microorganisms. 2024; 12(8):1552. https://doi.org/10.3390/microorganisms12081552
Chicago/Turabian StyleLi, Xiaofeng, Zhixun Xie, You Wei, Meng Li, Minxiu Zhang, Sisi Luo, and Liji Xie. 2024. "Recombinant Hemagglutinin Protein from H9N2 Avian Influenza Virus Exerts Good Immune Effects in Mice" Microorganisms 12, no. 8: 1552. https://doi.org/10.3390/microorganisms12081552





