Biological Characteristics, Artificial Domestication Conditions Optimization, and Bioactive Components of Beauveria caledonica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sample Collection
2.1.2. Culture Medium
2.2. Methods
2.2.1. Molecular Biological Identification of the Strain
2.2.2. Single-Factor Experiment
2.2.3. RSM Experiment
2.2.4. Determination of the Growth Rate and Biomass of Mycelia
2.2.5. Domestication Cultivation
2.2.6. Polysaccharide Detection in Fruiting Bodies
2.2.7. Adenosine and Cordycepin Detection in Fruiting Bodies
2.3. Data Analysis
3. Results
3.1. Molecular Analysis
3.2. Temperature Experiment
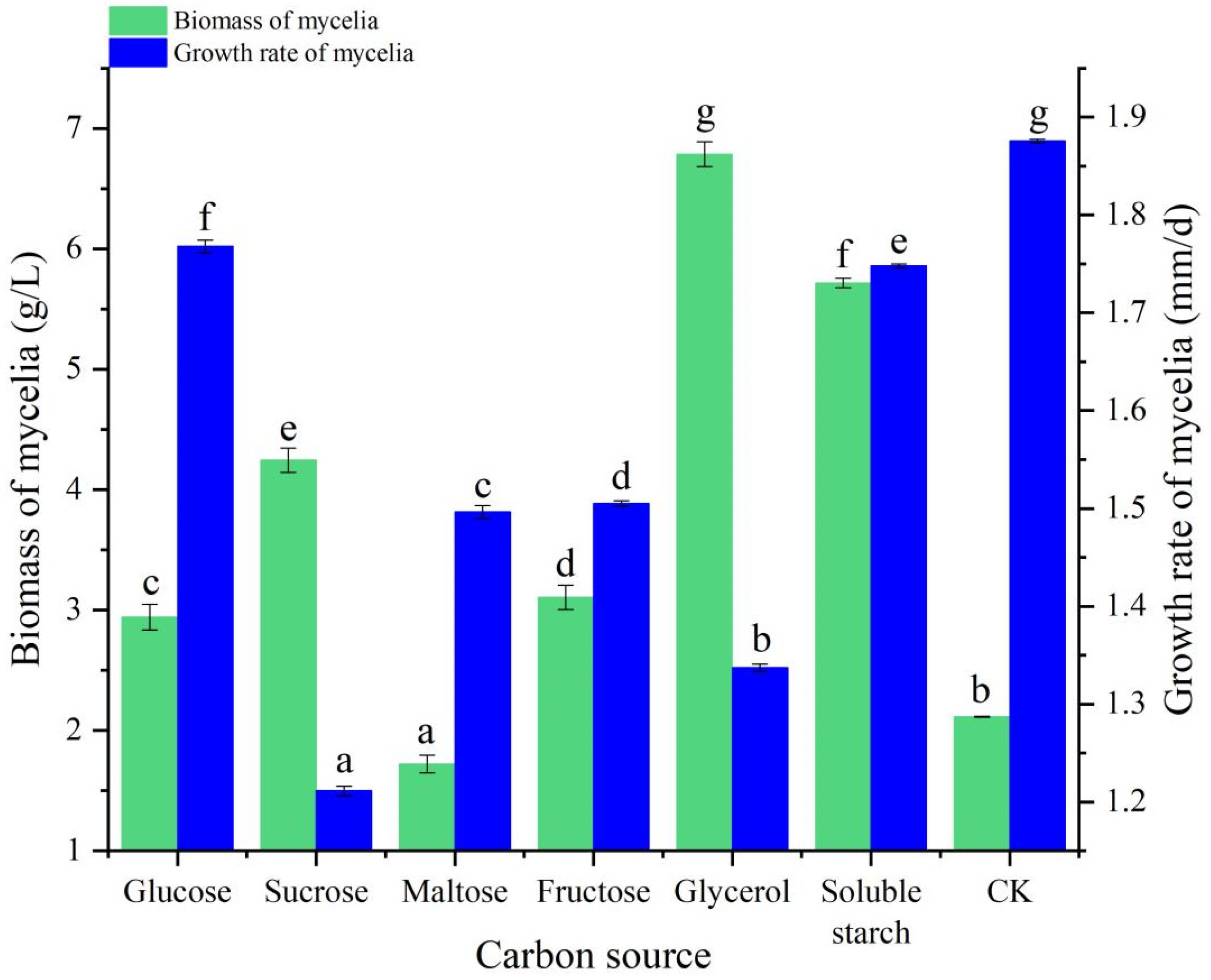
3.3. Carbon Source Experiment
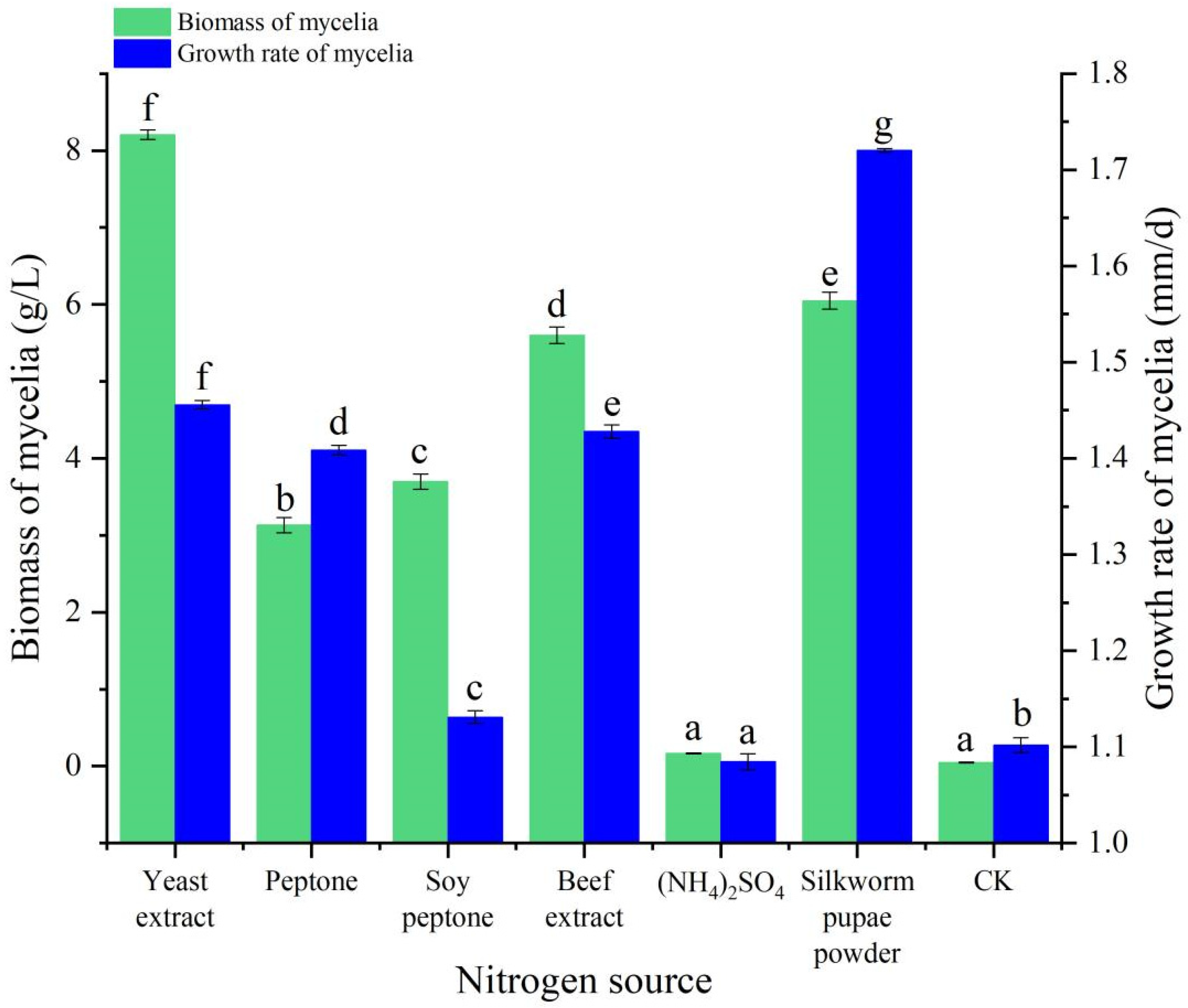
3.4. Nitrogen Source Experiment
3.5. pH Experiment
3.6. RSM Experiment
3.6.1. Statistical Analysis Using RSM
3.6.2. Analysis of Variance (ANOVA) of Regression Equations
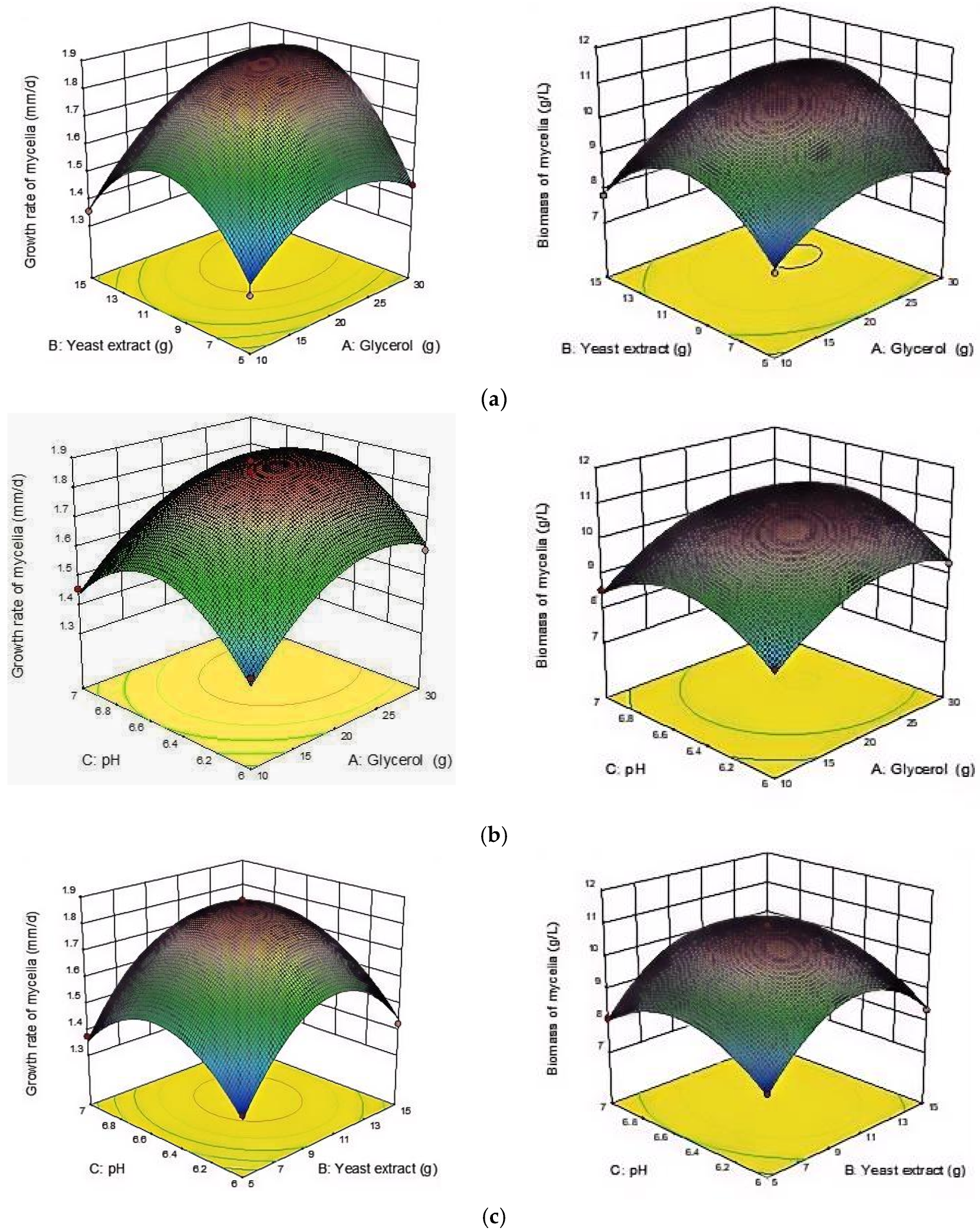
3.6.3. RSM Analysis of the Interaction of Factors
3.6.4. RSM Optimization Validation
3.7. Domestication Cultivation Experiment
3.8. Polysaccharide Content of Fruiting Bodies
3.9. Adenosine and Cordycepin Contents in Fruiting Bodies
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeon, S.H.; Kim, J.R.; Ahn, Y.J. Comparison of growth-inhibiting activities of Cordyceps militaris and Paecilomyces japonica cultured on Bombyx mori pupae towards human gastrointestinal bacteria. J. Sci. Food Agric. 2007, 87, 54–59. [Google Scholar] [CrossRef]
- Chen, W.H.; Liang, J.D.; Ren, X.X.; Zhao, J.H.; Han, Y.F.; Liang, Z.Q. Cryptic diversity of Isaria-like species in Guizhou, China. Life 2021, 11, 1093. [Google Scholar] [CrossRef]
- Kepler, R.; Ban, S.; Nakagiri, A.; Bischoff, J.; Hywel-Jones, N.; Owensby, C.A.; Spatafora, J.W. The phylogenetic placement of hypocrealean insect pathogens in the genus Polycephalomyces: An application of One Fungus One Name. Fungal Biol. 2013, 117, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Quandt, C.A.; Kepler, R.M.; Gams, W.; Araujo, J.P.M.; Ban, S.; Evans, H.C.; Hughes, D.; Humber, R.; Hywel-Jones, N.; Li, Z.; et al. Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 2014, 5, 121–134. [Google Scholar] [CrossRef]
- Li, C.H.; Yang, S.S.; Deng, W.Q.; Lin, Q.Y. Cordyceps Sensu Lato and their domestication and cultivation in China. Acta Edulis Fungi 2023, 30, 113–148. (In Chinese) [Google Scholar]
- Xiao, Y.P.; Wang, Y.B.; Hyde, K.D.; Eleni, G.; Sun, J.Z.; Yang, Y.; Meng, J.; Yu, H.; Wen, T.C. Polycephalomycetaceae, a new family of clavicipitoid fungi segregates from Ophiocordycipitaceae. Fungal Divers. 2023, 120, 1–76. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Index Fungorum. Available online: https://www.indexfungorum.org/names/names.asp (accessed on 18 January 2024).
- Bussmann, R.W. Ophiocordyceps sinensis (Berk.) G.H. Sung, J.M. Sung, Hywel-Jones & Spatafora OPHIOCORDYCIPITACEAE. In Ethnobotany of the Himalayas. Ethnobotany of Mountain Regions; Kunwar, R.M., Sher, H., Bussmann, R.W., Eds.; Springer International: Cham, Switzerland, 2021; pp. 1393–1396. [Google Scholar]
- Zhou, X.W.; Li, L.J.; Tian, E.W. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit. Rev. Biotechnol. 2014, 34, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Luan, F.G.; Hywel-Jones, N.L.; Zhang, S.L.; Chen, M.J.; Huang, B.; Sun, C.S.; Chen, Z.A.; Li, C.R.; Tan, Y.J.; et al. Biodiversity of cordycipitoid fungi associated with Isaria cicadae Miquel Ⅱ: Teleomorph discovery and nomenclature of chanhua, an important medicinal fungus in China. Mycosystema 2021, 40, 95–107. (In Chinese) [Google Scholar]
- Basith, M.; Madelin, M.F. Studies on the production of perithecial stromata by Cordyceps militaris in artificial culture. Can. J. Bot. 1968, 46, 473–480. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Song, B.; Huang, H.; Li, T.H. Optimization of selected cultivation parameters for Cordyceps guangdongensis. Lett. Appl. Microbiol. 2010, 51, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tibpromma, S.; Hughes, A.; Chethana, K.; Wijayawardene, N.; Dai, D.; Du, T.; Elgorban, A.; Stephenson, S.; Suwannarach, N.; et al. Culturable mycota on bats in central and southern Yunnan Province, China. Mycosphere 2023, 14, 497–662. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liang, Z.Q.; Whalley, A.J.; Yao, Y.J.; Liu, A.Y. Cordyceps brittlebankisoides, a new pathogen of grubs and its anamorph, Metarhizium anisopliae var. majus. J. Invertebr. Pathol. 2001, 78, 178–182. [Google Scholar] [CrossRef]
- Kepler, R.M.; Humber, R.A.; Bischoff, J.F.; Rehner, S.A. Clarification of generic and species boundaries for Metarhizium and related fungi through multigene phylogenetics. Mycologia 2014, 106, 811–829. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Liang, J.D.; Chen, W.H.; Han, Y.F.; Zou, X. Phenotypic polymorphism of the stilbellaceous entomogenous fungi in an ant nest of Ponera II. A cryptic new Tilachildium species, Tilachildium poneraticum. Microbiol. China 2016, 43, 379–385. [Google Scholar]
- Liang, J.D.; Chen, W.H.; Han, Y.F.; Liang, Z.Q. Tilachlidiopsis scarabaei L.S. Olive, a rare stilbellaceous entomogenous fungus in China. Microbiol. China 2014, 41, 1121–1124. [Google Scholar]
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D.J.J.O.E. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Imoulan, A.; Hussain, M.; Kirk, P.M.; Meziane, E.; Yao, Y.J. Entomopathogenic fungus Beauveria: Host specificity, ecology and significance of morpho-molecular characterization in accurate taxonomic classification. J. Asia-Pac. Entomol. 2017, 20, 1204–1212. [Google Scholar] [CrossRef]
- Huang, B.; Li, C.R.; Ding, D.G.; Fan, M.Z.; Li, Z.Z. A new recorded of Beauveria in China. Mycosystema 1997, 16, 317. [Google Scholar]
- Chen, W.H.; Han, Y.F.; Liang, Z.Q.; Jin, D.C. A new araneogenous fungus in the genus Beauveria from Guizhou, China. Phytotaxa 2017, 302, 57–64. [Google Scholar] [CrossRef]
- Chen, Z.H.; Chen, K.; Dai, Y.D.; Zheng, Y.; Wang, Y.B.; Yang, X.N.; Yu, H.; Yang, Y.M.; Xu, L. Beauveria species diversity in the Gaoligong Mountains of China. Mycol. Prog. 2019, 18, 933–943. [Google Scholar] [CrossRef]
- Li, Z.Z. Classification and identification of the Genus Beauveria. Res. Appl. Entomopathog. Fungi China 1990, 2, 89–95. [Google Scholar]
- Huang, B.; Chen, M.J.; Wang, S.B.; Li, Z.Z.; Fan, M.Z. A new record of Beauveria isolated from Scolytidae in China. Mycosystema 2007, 26, 139–142. [Google Scholar]
- Zhang, S.L.; He, L.M.; Chen, X.; Huang, B. Beauveria lii sp. nov. isolated from Henosepilachna vigintioctopunctata. Mycotaxon 2012, 121, 199–206. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, M.; Huang, Z.X.; Yang, G.M.; Han, Y.F.; Liang, J.D.; Liang, Z.Q. Beauveria majiangensis, a new entomopathogenic fungus from Guizhou, China. Phytotaxa 2018, 333, 243–250. [Google Scholar] [CrossRef]
- Guo, D.S.; Zhai, Y.Y.; Ren, G.W.; Cheng, J.L.; Liu, Y.; An, D.R. Advance of classification of genus Beauveria. Acta Agric. Boreali—Occident. Sin. 2019, 28, 497–509. (In Chinese) [Google Scholar]
- Imoulan, A.; Wu, H.J.; Lu, W.L.; Li, Y.; Li, B.B.; Yang, R.F.; Wang, W.J.; Wang, X.L.; Kirk, P.M.; Yao, Y.J. Beauveria medogensis sp. nov., a new fungus of the entomopathogenic genus from China. J. Invertebr. Pathol. 2016, 139, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, Q.; Wang, D.; Zou, W.Q.; Tang, D.X.; Hongthong, P.; Yu, H. Species diversity and virulence potential of the Beauveria bassiana complex and Beauveria scarabaeidicola complex. Front. Microbiol. 2022, 13, 841604. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.Q.; Liu, A.Y.; Feng, D.M. Some entomogeous fungi from Fanjing mountain preserve in China. Mycosystema 1993, 12, 110–117. (In Chinese) [Google Scholar]
- Chen, M.J.; Huang, B.; Li, Z.Z.; Spatafora, J.W. Morphological and genetic characterisation of Beauveria sinensis sp. Nov. from China. Mycotaxon 2013, 124, 301–308. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Liang, Z.Q.; Whalley, A.J.S.; Liu, A.Y.; Yao, Y.J. A new species of Beauveria, the anamorph of Cordyceps sobolifera. Fungal Divers. 2001, 7, 61–70. [Google Scholar]
- Kotta-Loizou, L.; Coutts, R.H.A. Studies on the virome of the entomopathogenic fungus Beauveria bassiana reveal novel dsRNA elements and mild hypervirulence. PLoS Pathog. 2017, 13, 1006183. [Google Scholar] [CrossRef]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 2375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, J.; Wang, H.; Bidochka, M.J.; Duan, M.; Xu, W.; Sui, L.; Ren, B.Z.; Li, Q.Y.; Zhang, Z.K. Fitness consequences of oviposition choice by an herbivorous insect on a host plant colonized by an endophytic entomopathogenic fungus. J. Pest. Sci. 2023, 96, 745–758. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, S.; Kim, J.C.; Lee, M.R.; Hossain, M.S.; Shin, T.S.; Kim, T.H.; Kim, J.S. Entomopathogeic Beauveria bassiana, granules to control soil-dwelling stage of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Biocontrol 2017, 62, 639–648. [Google Scholar] [CrossRef]
- Luo, H.Y. Studies on the Pathogenity of the Biocontrol Agent Beauveria bassiana to the Silkworm, Bombyx mori. Master’s Thesis, Guangxi Normal University, Guilin, China, 2009. (In Chinese). [Google Scholar]
- Jiang, X. Researches on the Separation, Purification and Pharmacological Effects of Active Components from Bombyx batryticatus. Ph.D. Thesis, Zhangjiang University, Hangzhou, China, 2013. (In Chinese). [Google Scholar]
- Pelizza, S.A.; Scorsetti, A.C.; Fogel, M.N.; Pacheco-Marino, S.G.; Stenglein, S.A.; Cabello, M.N.; Lange, C.E. Compatibility between entomopathogenic fungi and biorational insecticides in toxicity against Ronderosia bergi under laboratory conditions. BioControl 2015, 60, 81–91. [Google Scholar] [CrossRef]
- Xue, J.J.; Tang, Q.J.; Liu, Y.F.; Zhou, S.; Zhao, M.W.; Zhang, J.S. Comparison and optimization of the methods for analyzing the water soluble polysaccharides in Cordyceps militaris. Mycosystema. 2012, 31, 443–449. (In Chinese) [Google Scholar]
- Zou, G.; Li, B.; Wang, Y.; Yin, X.; Gong, M.; Shang, J.J.; Wei, Y.J.; Li, X.L.; Bao, D.P. Efficient conversion of spent mushroom substrate into a high value-added anticancer drug pentostatin with engineered Cordyceps militaris. Green. Chem. 2021, 23, 10030–10038. [Google Scholar] [CrossRef]
- Moura, G.M.; Marinho-Prado, J.S.; Assalin, M.R.; Martins, L.G.; Braga, E.S.; Tasic, L.; Dita, M.; Lopes, R.B. Natural occurrence of Beauveria caledonica, pathogenicity to Cosmopolites sordidus and antifungal activity against Fusarium oxysporum f. sp. cubense. Pest. Manag. sci. 2022, 78, 4458–4470. [Google Scholar]
- Namara, M.L.; Dolan, K.S.; Walsh, M.J.; Stephens, J.C.; Glare, T.R.; Kavanagh, K.; Griffin, C.T. Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence. Fungal Biol. 2019, 123, 601–610. [Google Scholar] [CrossRef]
- Tekpinar, A.D.; Kalmer, A. Utility of various molecular markers in fungal identification and phylogeny. Nova Hedwigia 2019, 109, 187–224. [Google Scholar] [CrossRef]
- Jian, J.H.; Guo, L.Q.; Ye, Z.W.; Liu, C.; Yin, L.; Lin, J.F. Research progress of Cordyceps militaris novel pigment. China Food. Addit 2015, 11, 137–140. (In Chinese) [Google Scholar]
- Li, C.R.; Nan, S.H.; Geng, D.G.; Fan, M.Z.; Li, Z.Z. Artificial culture of seventeen Cordyceps spp. Mycosystema 2006, 25, 639–645. (In Chinese) [Google Scholar]
- Yang, S.S.; Lin, Q.Y.; Zhu, L.N.; Bao, D.P.; Chen, M.J.; Li, C.H. Biological characteristics of Cordyceps tenuipes and the optimization of artificially domesticated conditions. Mycosystema 2021, 40, 1480–1497. (In Chinese) [Google Scholar]
- Cao, W.; Zhao, Y.; Yang, S.S.; Lin, Q.Y.; Deng, C.Y.; Li, C.H. Domestication and bioactive components of Polycephalomyces ramosus. Mycosystema 2024, 43, 133–145. (In Chinese) [Google Scholar]
- Zhang, J.X.; Wen, C.T.; Duan, Y.Q.; Zhang, H.H.; Ma, H.L. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 2019, 132, 906–914. [Google Scholar] [CrossRef]







| Independent Variables | Symbol | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Glycerol content (g/L) | A | 10 | 20 | 30 |
| Yeast extract content (g/L) | B | 5 | 10 | 15 |
| pH | C | 6.0 | 6.5 | 7.0 |
| Run | Coded Variable Levels | Response Value | |||
|---|---|---|---|---|---|
| A Glycerol Content(g) | B Yeast Extract Content(g) | C pH | Growth Rate of Mycelia (mm/d) | Biomass of Mycelia (g/L) | |
| 1 | 10 | 5 | 6.5 | 1.3006 | 7.6249 |
| 2 | 30 | 5 | 6.5 | 1.4537 | 8.5149 |
| 3 | 10 | 15 | 6.5 | 1.3555 | 7.799 |
| 4 | 30 | 15 | 6.5 | 1.7661 | 10.0495 |
| 5 | 10 | 10 | 6 | 1.3933 | 8.2417 |
| 6 | 30 | 10 | 6 | 1.5907 | 9.3202 |
| 7 | 10 | 10 | 7 | 1.4573 | 8.542 |
| 8 | 30 | 10 | 7 | 1.6432 | 9.8142 |
| 9 | 20 | 5 | 6 | 1.3231 | 7.7684 |
| 10 | 20 | 15 | 6 | 1.4233 | 8.3579 |
| 11 | 20 | 5 | 7 | 1.374 | 8.0837 |
| 12 | 20 | 15 | 7 | 1.5045 | 8.8608 |
| 13 | 20 | 10 | 6.5 | 1.8884 | 10.8245 |
| 14 | 20 | 10 | 6.5 | 1.844 | 10.7834 |
| 15 | 20 | 10 | 6.5 | 1.8359 | 10.9635 |
| 16 | 20 | 10 | 6.5 | 1.8537 | 10.8246 |
| 17 | 20 | 10 | 6.5 | 1.836 | 10.9647 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.73 | 9 | 0.082 | 68.49 | <0.0001 | ** |
| A | 0.11 | 1 | 0.11 | 94.12 | <0.0001 | ** |
| B | 0.045 | 1 | 0.045 | 37.53 | 0.0005 | ** |
| C | 7.725 × 10−3 | 1 | 7.725 × 10−3 | 6.49 | 0.0383 | * |
| AB | 0.017 | 1 | 0.017 | 13.92 | 0.0074 | ** |
| AC | 3.306 × 10−5 | 1 | 3.306 × 10−5 | 0.028 | 0.8724 | |
| BC | 2.295 × 10−4 | 1 | 2.295 × 10−4 | 0.19 | 0.6739 | |
| A2 | 0.075 | 1 | 0.075 | 63.35 | <0.0001 | ** |
| B2 | 0.26 | 1 | 0.26 | 218.77 | <0.0001 | ** |
| C2 | 0.16 | 1 | 0.16 | 136.66 | <0.0001 | ** |
| Residual | 8.337 × 10−3 | 7 | 1.191 × 10−3 | |||
| Lack of Fit | 6.431 × 10−3 | 3 | 2.144 × 10−3 | 4.50 | 0.0902 | |
| Pure Error | 1.906 × 10−3 | 4 | 4.766 × 10−4 | |||
| Cor Total | 0.74 | 16 | ||||
| R2 = 0.9888 RAdj2 = 0.9743 C.V./% = 2.19 | ||||||
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 25.33 | 9 | 2.81 | 161.52 | <0.0001 | ** |
| A | 3.77 | 1 | 3.77 | 216.28 | <0.0001 | ** |
| B | 1.18 | 1 | 1.18 | 67.83 | <0.0001 | ** |
| C | 0.33 | 1 | 0.33 | 18.65 | 0.0035 | ** |
| AB | 0.46 | 1 | 0.46 | 26.55 | 0.0013 | ** |
| AC | 9.38 × 10−3 | 1 | 9.38 × 10−3 | 0.54 | 0.4870 | |
| BC | 8.798 × 10−3 | 1 | 8.798 × 10−3 | 0.5 | 0.5004 | |
| A2 | 2.91 | 1 | 2.91 | 167.09 | <0.0001 | ** |
| B2 | 10.03 | 1 | 10.03 | 575.55 | <0.0001 | ** |
| C2 | 4.74 | 1 | 4.74 | 271.98 | <0.0001 | ** |
| Residual | 0.12 | 7 | 0.017 | |||
| Lack of Fit | 0.093 | 3 | 0.031 | 4.21 | 0.0992 | |
| Pure Error | 0.029 | 4 | 7.33 × 10−3 | |||
| Cor Total | 25.46 | 16 | ||||
| R2 = 0.9952 RAdj2 = 0.9890 C.V./% = 1.43 | ||||||
| Cultivation Medium | Average Yield (g) | Dry/Wet Weight Ratio | Biological Efficiency (%) |
|---|---|---|---|
| Wheat | 0.470 ± 0.047 | 0.403 ± 0.035 | 1.880 ± 0.188 |
| Rice | 0.529 ± 0.043 | 0.451 ± 0.034 | 2.115 ± 0.172 |
| Ingredient | Sample Concentration (μg/mL) | Standard Concentration (μg/mL) | Detect the Concentration (μg/mL) | Concentration Should be Measured (μg/mL) | Recovery (%) | Average Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| Adenosine | 32.076 | 20 | 26.869 | 26.038 | 96.907 | 98.175 | 0.955 |
| 32.108 | 26.738 | 26.054 | 97.441 | ||||
| 32.112 | 26.397 | 26.056 | 98.708 | ||||
| 32.078 | 26.331 | 26.039 | 98.893 | ||||
| 32.096 | 26.331 | 26.048 | 98.926 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Yu, C.; Zhao, Y.; Lin, Q.; Deng, C.; Li, C. Biological Characteristics, Artificial Domestication Conditions Optimization, and Bioactive Components of Beauveria caledonica. Microorganisms 2024, 12, 1554. https://doi.org/10.3390/microorganisms12081554
Cao W, Yu C, Zhao Y, Lin Q, Deng C, Li C. Biological Characteristics, Artificial Domestication Conditions Optimization, and Bioactive Components of Beauveria caledonica. Microorganisms. 2024; 12(8):1554. https://doi.org/10.3390/microorganisms12081554
Chicago/Turabian StyleCao, Wang, Changxia Yu, Yan Zhao, Qunying Lin, Chunying Deng, and Chuanhua Li. 2024. "Biological Characteristics, Artificial Domestication Conditions Optimization, and Bioactive Components of Beauveria caledonica" Microorganisms 12, no. 8: 1554. https://doi.org/10.3390/microorganisms12081554






