Lactobacillus crispatus-Mediated Gut–Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Probiotic Strain Preparation
2.3. Birds and Experiment Design
2.4. Sample Collection
2.5. Laying Performance
2.6. Egg Quality
2.7. Cecum and Oviduct Histopathology
2.8. Observation of Follicles
2.9. Serum and Uterus Inflammatory Index Detection
2.10. RNA Extraction and Real-Time Quantitative PCR
2.11. DNA Extractions and 16S rRNA Gene Sequencing of Cecal, Uterine, and Isthmic Microbiota
2.12. Analysis of Cecal, Uterine, and Isthmic Microbiota
2.13. Statistical Analysis
3. Results
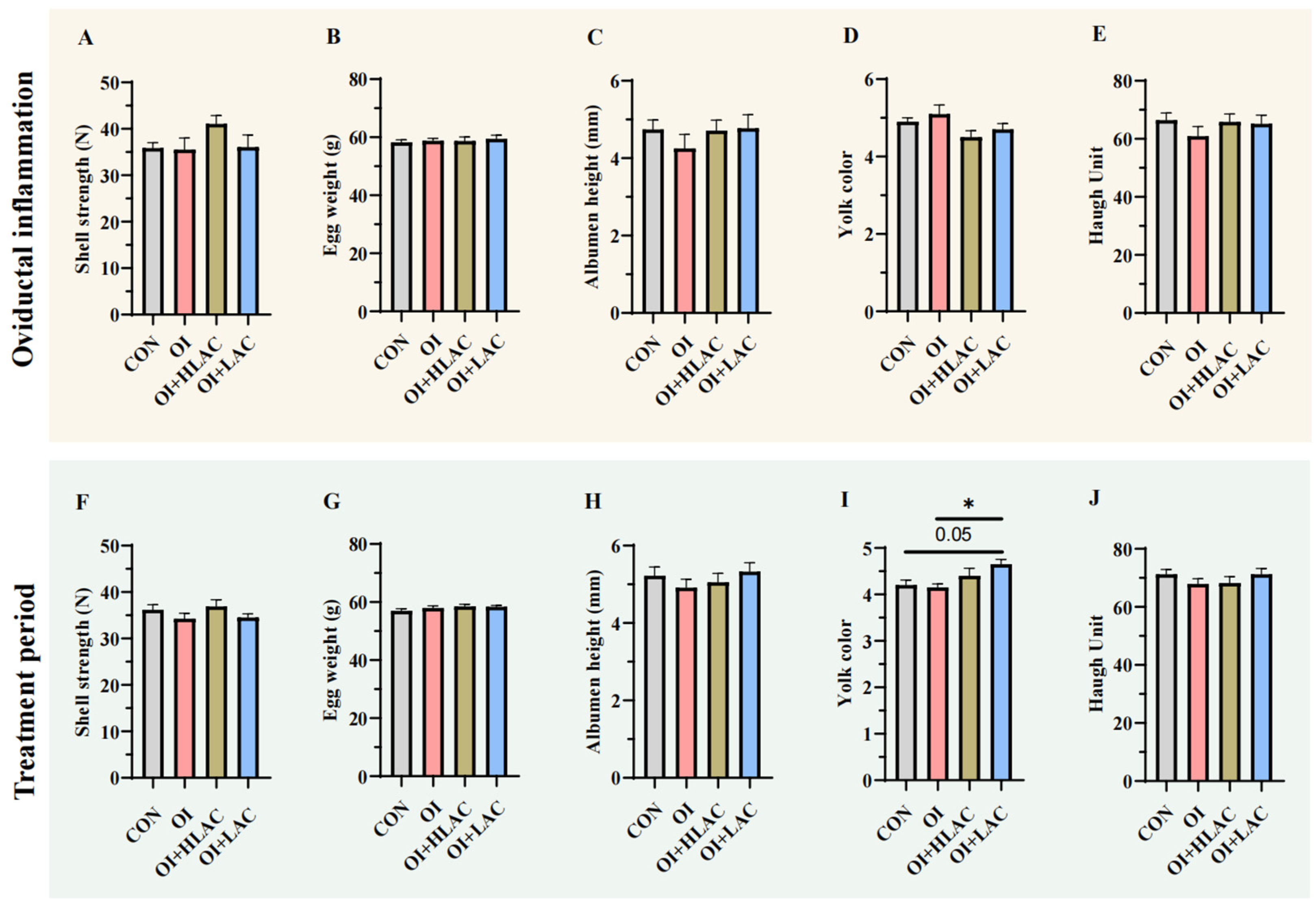
3.1. Effect of Lactobacillus crispatus on the Laying Performance of Laying Hens during Oviductal Inflammation
3.2. Effects of Lactobacillus crispatus on Laying Hen Egg Quality during Oviductal Inflammation
3.3. Effect of Lactobacillus crispatus on Laying Hen Ovarian Health during Oviductal Inflammation
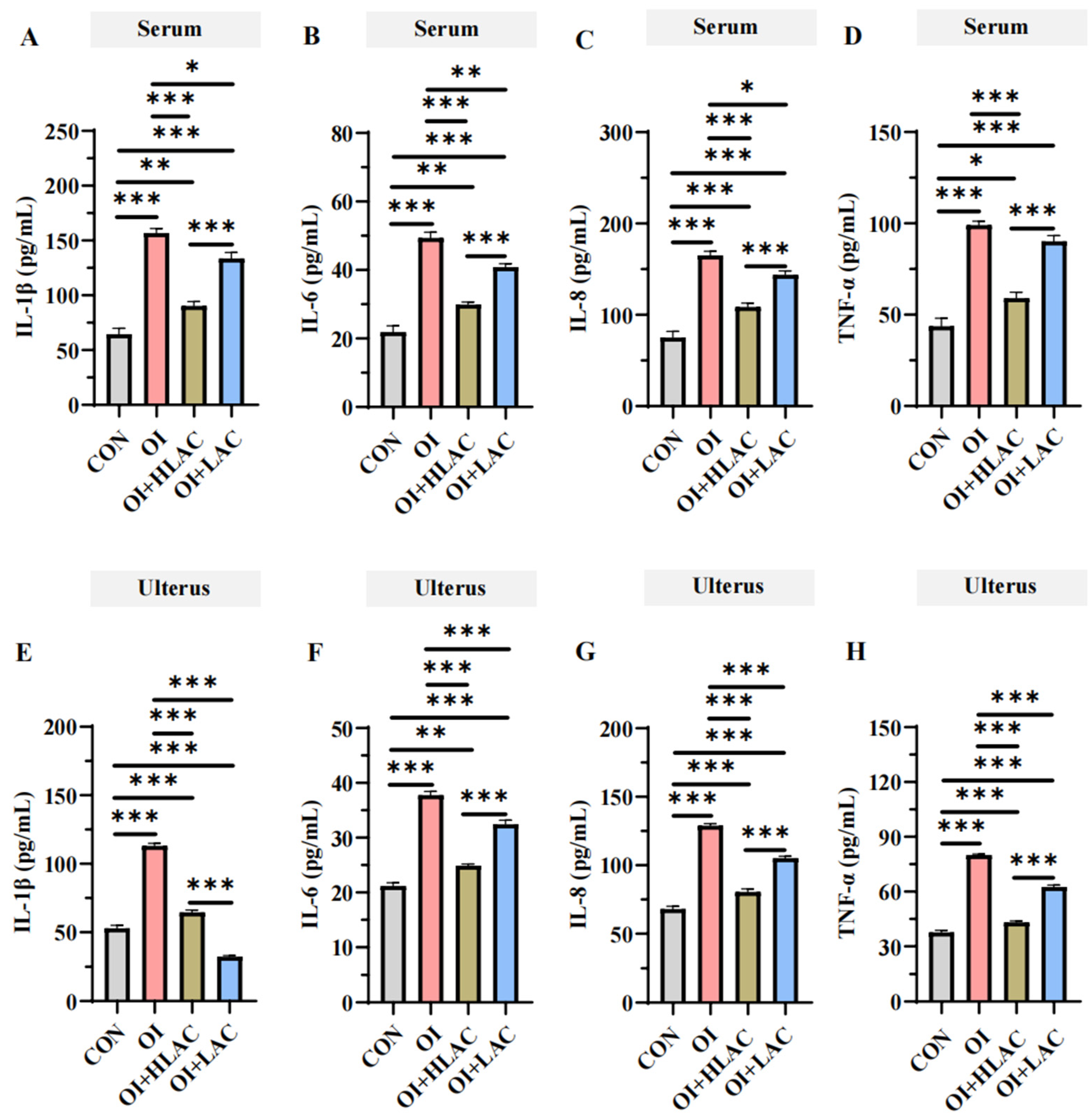
3.4. Effect of Lactobacillus crispatus on Inflammatory Cytokines in the Serum and Uterus of Laying Hens during Oviductal Inflammation
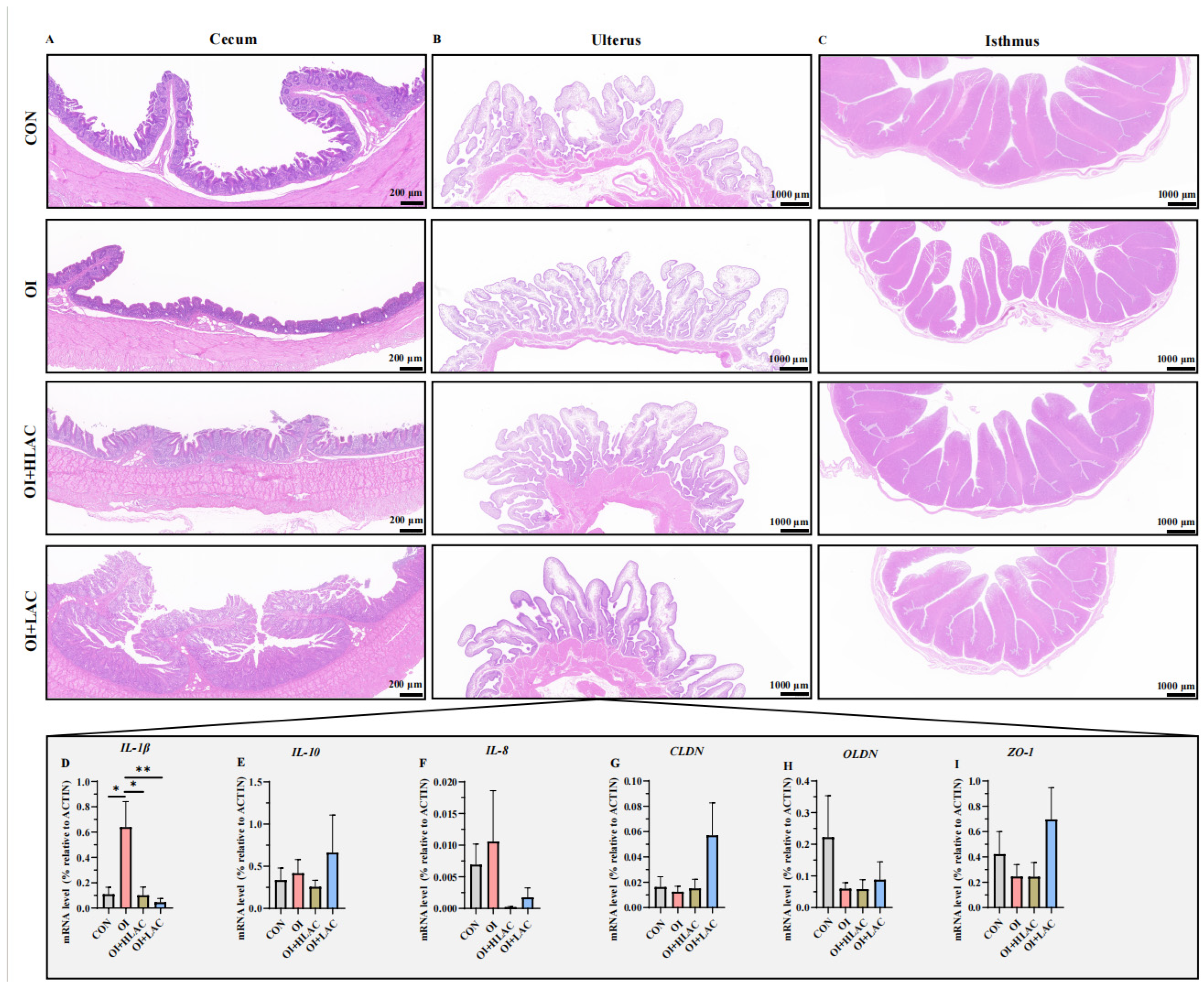
3.5. Effect of Lactobacillus crispatus on the Histological Morphology of Cecum, Isthmus, and Shell Gland Tissues and Inflammation-Related Gene Expression in the Shell Glands of Laying Hen Serum and Uterus during Oviductal Inflammation
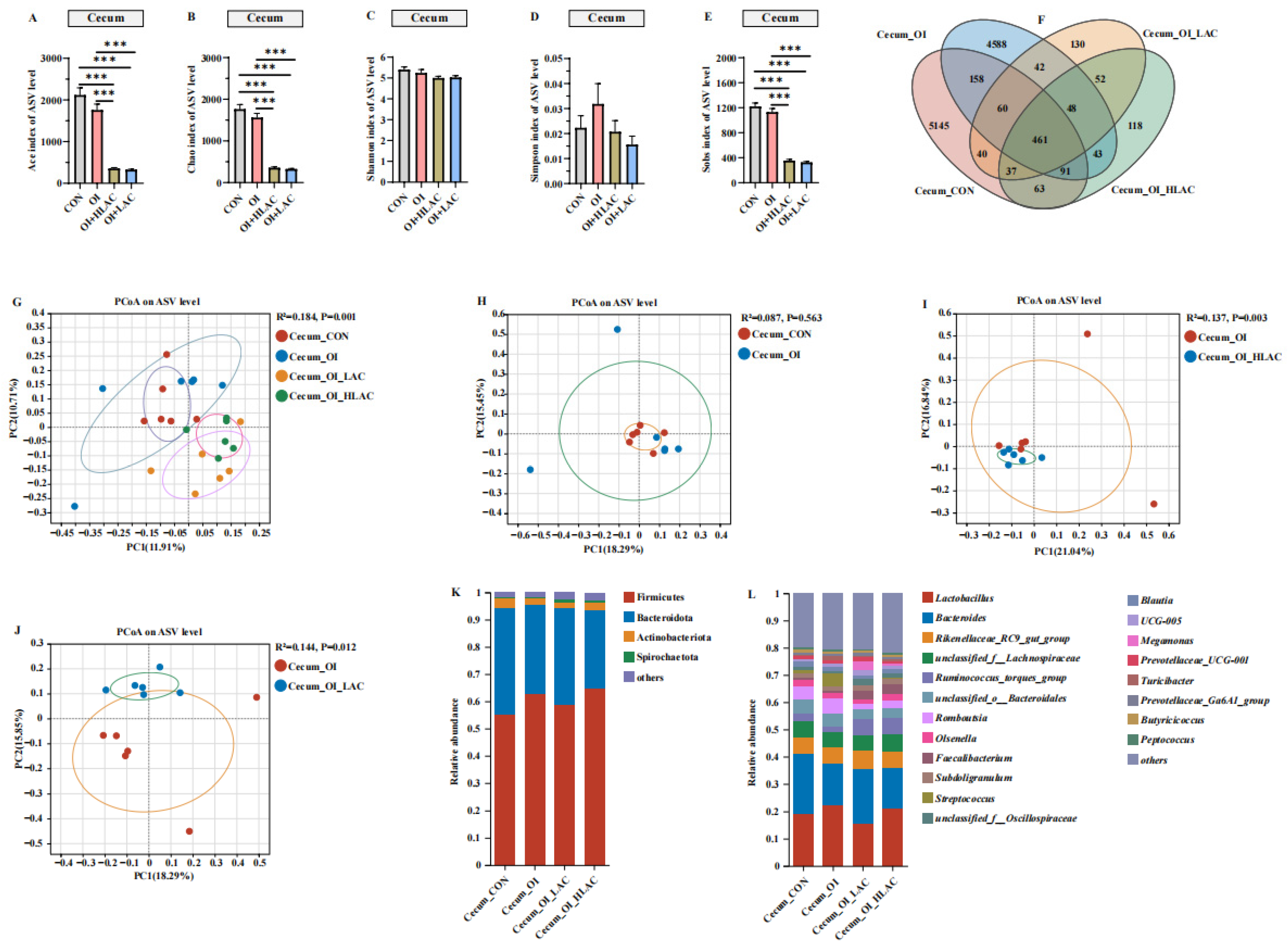
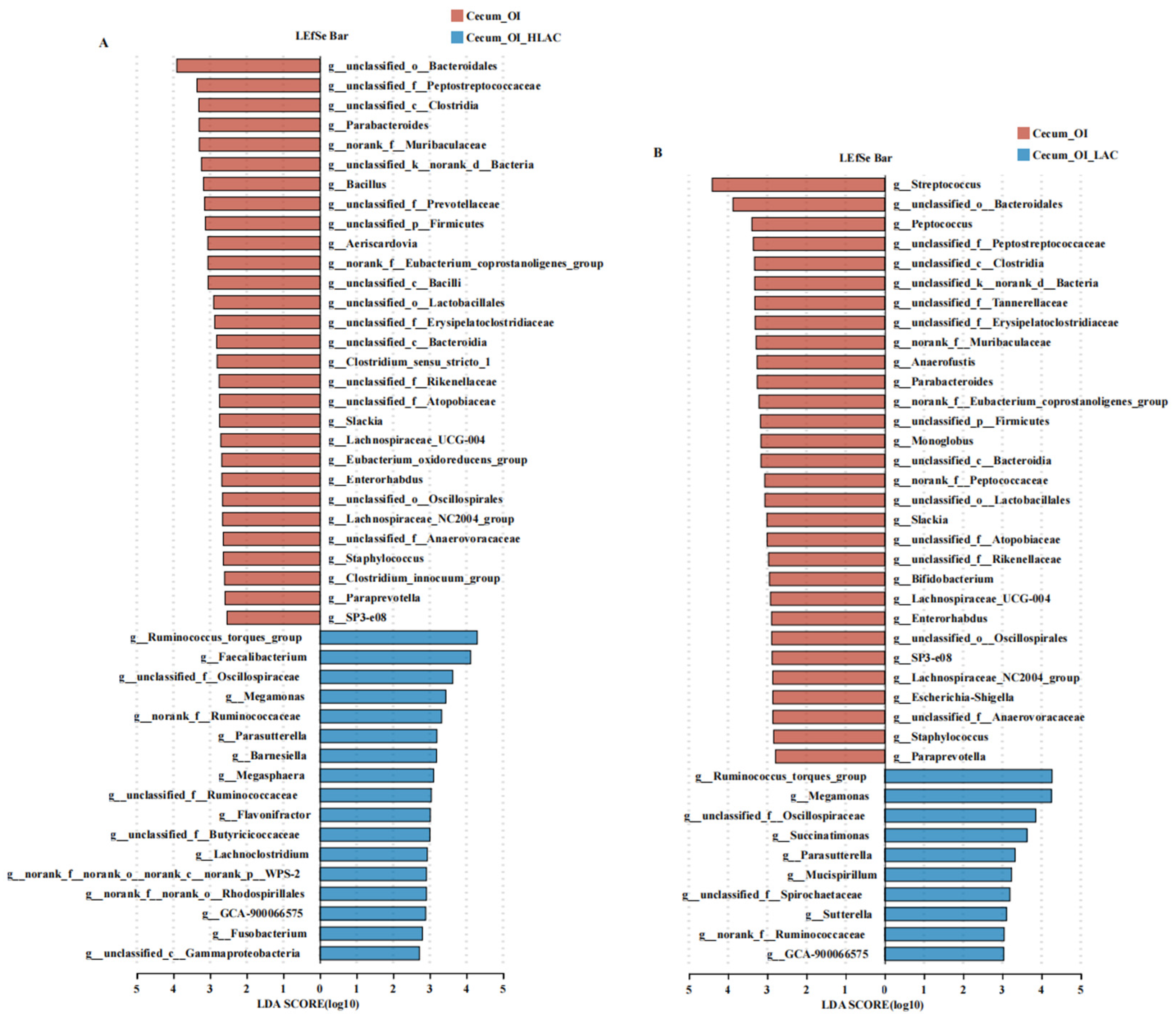
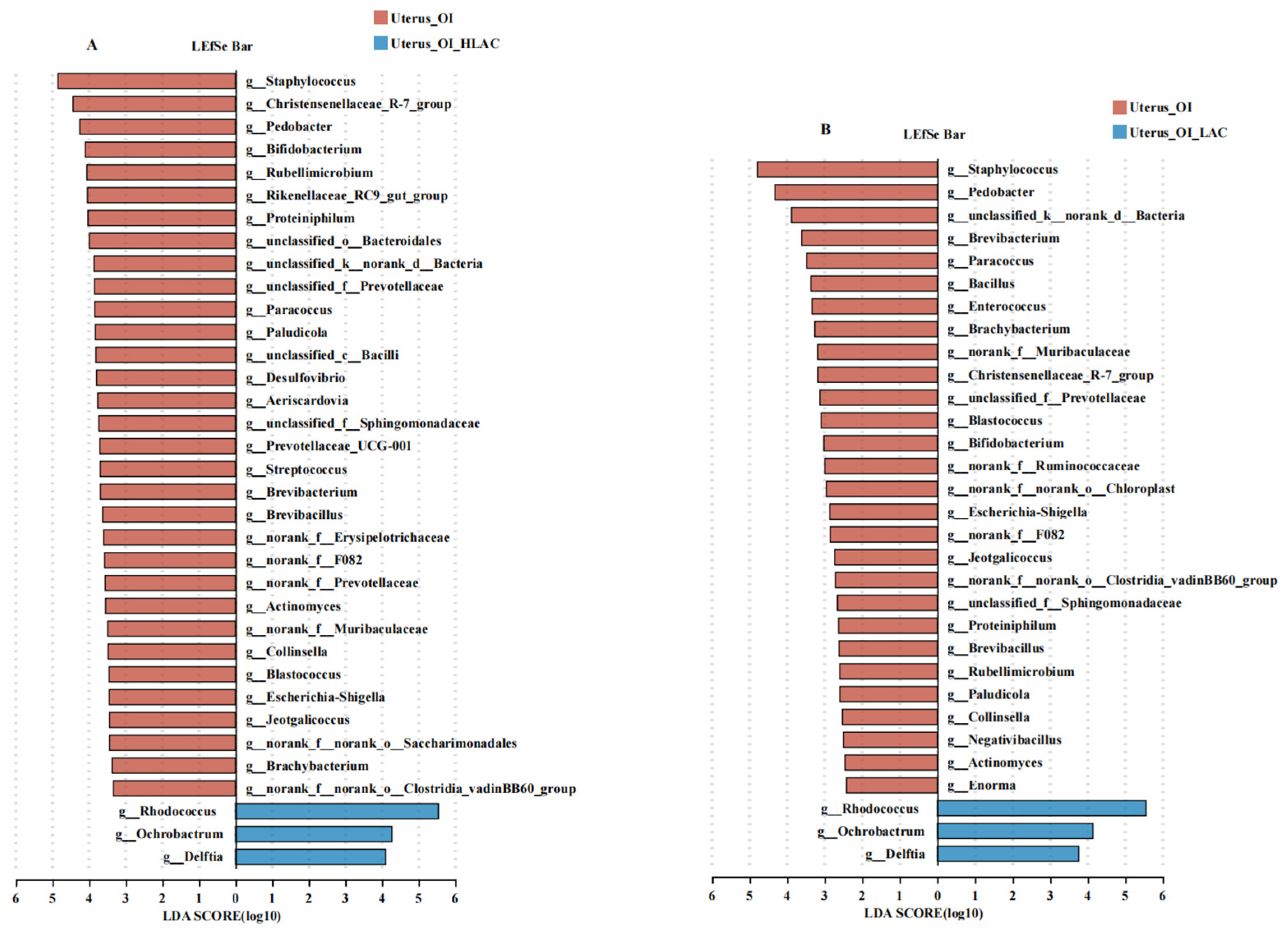
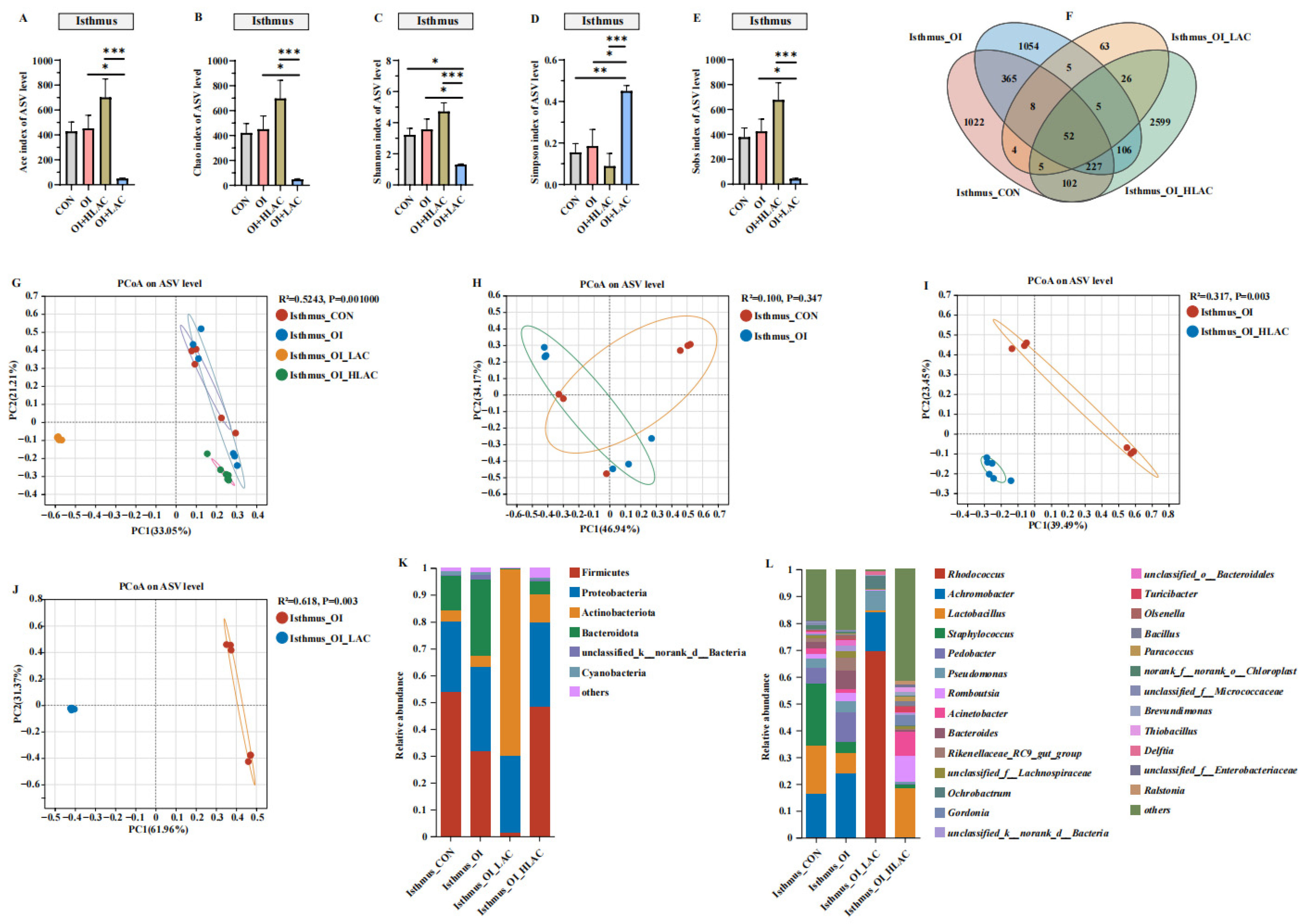
3.6. Effects of Lactobacillus crispatus on Cecal, Uterine, and Isthmic Microbiota in Laying Hens during Oviductal Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landman, W.J.; Heuvelink, A.; van Eck, J.H. Reproduction of the Escherichia coli peritonitis syndrome in laying hens. Avian Pathol. 2013, 42, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pors, S.E.; Christensen, J.P.; Bojesen, A.M.; Thøfner, I. Comparison and assessment of necropsy lesions in end-of-lay laying hens from different housing systems in Denmark. Poult. Sci. 2020, 99, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Quan, H.; Zhang, Y.; Li, Q.; Wang, Y.; Yuan, S.; Huang, S.; He, C. Co-Infection of Escherichia coli, Enterococcus faecalis and Chlamydia psittaci Contributes to Salpingitis of Laying Layers and Breeder Ducks. Pathogens 2021, 10, 755. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Li, Q.; Lan, F.; Li, X.; Li, G.; Yan, Y.; Wu, G.; Yang, N.; Sun, C. Microbiota continuum along the chicken oviduct and its association with host genetics and egg formation. Poult. Sci. 2021, 100, 101104. [Google Scholar] [CrossRef] [PubMed]
- Pors, S.E.; Olsen, R.H.; Christensen, J.P. Variations in virulence of avian pathogenic Escherichia coli demonstrated by the use of a new in vivo infection model. Vet. Microbiol. 2014, 170, 368–374. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, N.; Luo, M.; Gan, B.; Yao, Z.; Cao, X.; Wang, H.; Pan, K.; Shu, G.; Zeng, Y.; et al. Promotion of Egg Production Rate and Quality Using Limosilactobacillus oris BSLO 1801, a Potential Probiotic Screened from Feces of Laying Hens with Higher Egg Productive Performance. Probiotics Antimicrob. Proteins 2023, 15, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wang, Y.W.; Lu, Z.X.; Wang, W.W.; Miao, H.J.; Zhou, H.; Wang, L.; Li, A.K. Effects of dietary supplementation of microencapsulated Enterococcus fecalis and the extract of Camellia oleifera seed on laying performance, egg quality, serum biochemical parameters, and cecal microflora diversity in laying hens. Poult. Sci. 2019, 98, 2880–2887. [Google Scholar] [CrossRef]
- Chen, L.; Bai, X.; Wang, T.; Liu, J.; Miao, X.; Zeng, B.; Li, D. Gut Microbial Diversity Analysis of Different Native Chickens and Screening of Chicken-Derived Probiotics. Animals 2023, 13, 3672. [Google Scholar] [CrossRef]
- Jordan, F.T.W.; Williams, N.J.; Wattret, A.; Jones, T. Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock. Vet. Rec. 2005, 157, 573–577. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Consortium Human Microbiome Project. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lv, J.; Liu, Y.; Ma, H.; Chen, B.; Hao, K.; Feng, J.; Min, Y. Effects of Different Fermented Feeds on Production Performance, Cecal Microorganisms, and Intestinal Immunity of Laying Hens. Animals 2021, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Fan, S.; Fang, C.; Ge, L.; Lyu, J.; Huang, Z.; Zhao, S.; Zou, Y.; Huang, L.; Liu, X.; et al. Orally administrated Lactobacillus gasseri TM13 and Lactobacillus crispatus LG55 can restore the vaginal health of patients recovering from bacterial vaginosis. Front. Immunol. 2023, 14, 1125239. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Ferrari, F.; Ciebiera, M.; Zgliczyńska, M.; Rapisarda, A.M.C.; Vecchio, G.M.; Pino, A.; Angelico, G.; Knafel, A.; Riemma, G.; et al. The Role of Genital Tract Microbiome in Fertility: A Systematic Review. Int. J. Mol. Sci. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.A.; Hillier, S.L. DNA fingerprinting of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J. Clin. Microbiol. 2003, 41, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4680–4687. [Google Scholar] [CrossRef]
- Hearps, A.C.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.A.; Gugasyan, R.; Anderson, D.J.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- Anton, L.; Sierra, L.J.; DeVine, A.; Barila, G.; Heiser, L.; Brown, A.G.; Elovitz, M.A. Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatus Contributes to Cervical Health. Front. Microbiol. 2018, 9, 2181. [Google Scholar] [CrossRef]
- NY/T 33-2004; Feeding Standard of Chicken. Chinese Academy of Agricultural Sciences: Beijing, China, 2004.
- Yang, X.; Xia, Y.H.; Wang, J.Y.; Li, Y.T.; Chang, Y.F.; Chang, H.T.; Liu, H.Y.; Chen, L.; Wang, C.Q. The role of GtxA during Gallibacterium anatis infection of primary chicken oviduct epithelial cells. Mol. Cell. Probes 2020, 53, 101641. [Google Scholar] [CrossRef]
- Song, L.; Tian, L.; Ma, Y.; Xie, Y.; Feng, H.; Qin, F.; Mo, L.; Lin, S.; Hou, L.; Wang, C. Protection of flavonoids from Smilax china L. rhizome on phenol mucilage-induced pelvic inflammation in rats by attenuating inflammation and fibrosis. J. Funct. Foods 2017, 28, 194–204. [Google Scholar] [CrossRef]
- Zhang, X.; He, M.; Lei, S.; Wu, B.; Tan, T.; Ouyang, H.; Xu, W.; Feng, Y. An integrative investigation of the therapeutic mechanism of Ainsliaea fragrans Champ. in cervicitis using liquid chromatography tandem mass spectrometry based on a rat plasma metabolomics strategy. J. Pharm. Biomed. Anal. 2018, 156, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef]
- Lv, J.; Guo, L.; Chen, B.; Hao, K.; Ma, H.; Liu, Y.; Min, Y. Effects of different probiotic fermented feeds on production performance and intestinal health of laying hens. Poult. Sci. 2022, 101, 101570. [Google Scholar] [CrossRef] [PubMed]
- Hencken, H. Chemical and physiological behavior of feed carotenoids and their effects on pigmentation. Poult. Sci. 1992, 71, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.E.; Johnson, E.J. Xanthophylls. Adv. Nutr. 2018, 9, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biol. Reprod. 2015, 93, 111. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, N.; Jiang, S.; Wang, X.; Yan, H.; Gao, C. Dietary replacement of soybean meal by fermented feedstuffs for aged laying hens: Effects on laying performance, egg quality, nutrient digestibility, intestinal health, follicle development, and biological parameters in a long-term feeding period. Poult. Sci. 2023, 102, 102478. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Wang, P.; Wang, F. Blockade of hypoxia-inducible factor-1α by YC-1 attenuates interferon-γ and tumor necrosis factor-α-induced intestinal epithelial barrier dysfunction. Cytokine 2011, 56, 581–588. [Google Scholar] [CrossRef]
- Stevenson, B.R.; Keon, B.H. The tight junction: Morphology to molecules. Annu. Rev. Cell Dev. Biol. 1998, 14, 89–109. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Ci, W.; Zheng, Y.; Han, X.; Huang, J.; Zhu, J. Effects of Dietary Supplementation with Lactobacillus acidophilus and Bacillus subtilis on Mucosal Immunity and Intestinal Barrier Are Associated with Its Modulation of Gut Metabolites and Microbiota in Late-Phase Laying Hens. Probiotics Antimicrob. Proteins 2023, 15, 912–924. [Google Scholar] [CrossRef]
- Song, D.; Li, A.; Chen, B.; Feng, J.; Duan, T.; Cheng, J.; Chen, L.; Wang, W.; Min, Y. Multi-omics analysis reveals the molecular regulatory network underlying the prevention of Lactiplantibacillus plantarum against LPS-induced salpingitis in laying hens. J. Anim. Sci. Biotechnol. 2023, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Iwabuchi, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Hachimura, S. Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS ONE 2018, 13, e0199018. [Google Scholar] [CrossRef]
- Campeotto, F.; Suau, A.; Kapel, N.; Magne, F.; Viallon, V.; Ferraris, L.; Waligora-Dupriet, A.J.; Soulaines, P.; Leroux, B.; Kalach, N.; et al. A fermented formula in pre-term infants: Clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 2011, 105, 1843–1851. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Sarrazin-Davila, L.E.; Servin, A.L. An experimental study and a randomized, double-blind, placebo-controlled clinical trial to evaluate the antisecretory activity of Lactobacillus acidophilus strain LB against nonrotavirus diarrhea. Pediatrics 2007, 120, e795–e803. [Google Scholar] [CrossRef]
- Li, P.; Wei, K.; He, X.; Zhang, L.; Liu, Z.; Wei, J.; Chen, X.; Wei, H.; Chen, T. Vaginal Probiotic Lactobacillus crispatus Seems to Inhibit Sperm Activity and Subsequently Reduces Pregnancies in Rat. Front. Cell. Dev. Biol. 2021, 9, 705690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, X.; Zhen, W.; Zeng, D.; Qu, L.; Wang, Z.; Ning, Z. Yeast Culture Improves Egg Quality and Reproductive Performance of Aged Breeder Layers by Regulating Gut Microbes. Front. Microbiol. 2021, 12, 633276. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Yan, H.; Ning, Z.; Wang, Z. Lactobacillus salivarius SNK-6 Activates Intestinal Mucosal Immune System by Regulating Cecal Microbial Community Structure in Laying Hens. Microorganisms 2022, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial regulation of organismal energy homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Xue, Z.; Sun, Z.; Zhang, M.; Wang, L.; Wang, G.; Wang, F.; Xu, J.; Cao, H.; et al. A phylo-functional core of gut microbiota in healthy young Chinese cohorts across lifestyles, geography and ethnicities. ISME J. 2015, 9, 1979–1990. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Liang, Y.; Zeng, W.; Hou, T.; Yang, H.; Wu, B.; Pan, R.; Huang, L. Gut microbiome and reproductive endocrine diseases: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1164186. [Google Scholar] [CrossRef]
- Han, Y.; Gong, Z.; Sun, G.; Xu, J.; Qi, C.; Sun, W.; Jiang, H.; Cao, P.; Ju, H. Dysbiosis of Gut Microbiota in Patients with Acute Myocardial Infarction. Front. Microbiol. 2021, 12, 680101. [Google Scholar] [CrossRef]
- Rossi, O.; van Berkel, L.A.; Chain, F.; Tanweer Khan, M.; Taverne, N.; Sokol, H.; Duncan, S.H.; Flint, H.J.; Harmsen, H.J.; Langella, P.; et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci. Rep. 2016, 6, 18507. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Ruesga-Gutierrez, E.; Ruvalcaba-Gomez, J.M.; Gomez-Godinez, L.J.; Villagran, Z.; Gomez-Rodriguez, V.M.; Heredia-Nava, D.; Ramirez-Vega, H.; Arteaga-Garibay, R.I. Allium-Based Phytobiotic for Laying Hens’ Supplementation: Effects on Productivity, Egg Quality, and Fecal Microbiota. Microorganisms 2022, 10, 117. [Google Scholar] [CrossRef]
- Su, Y.; Tian, S.; Li, D.; Zhu, W.; Wang, T.; Mishra, S.K.; Wei, R.; Xu, Z.; He, M.; Zhao, X.; et al. Association of female reproductive tract microbiota with egg production in layer chickens. GigaScience 2021, 10, giab067. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Dai, D.; Wang, J.; Zhang, H.; Wu, S.; Qi, G. Uterine microbial communities and their potential role in the regulation of epithelium cell cycle and apoptosis in aged hens. Microbiome 2023, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Kuyukina, M.S.; Ivshina, I.B.; Baeva, T.A.; Kochina, O.A.; Gein, S.V.; Chereshnev, V.A. Trehalolipid biosurfactants from nonpathogenic Rhodococcus actinobacteria with diverse immunomodulatory activities. New Biotechnol. 2015, 32, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Bitterman, P.; Abramowicz, J.S.; Dirks, A.L.; Bahr, J.M.; Hales, D.B.; Bradaric, M.J.; Edassery, S.L.; Rotmensch, J.; Luborsky, J.L. Histopathology of ovarian tumors in laying hens: A preclinical model of human ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2009, 19, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yan, W.; Wen, C.; Zheng, J.; Yang, N.; Sun, C. Enterotype identification and its influence on regulating the duodenum metabolism in chickens. Poult. Sci. 2020, 99, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, S.M.; Rahman, H.; Austin, D.A.; Austin, B. Properties of Probiotics Kocuria SM1 and Rhodococcus SM2 Isolated from Fish Guts. Probiotics Antimicrob. Proteins 2018, 10, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, S.M.; Abbass, A.; Tinsley, J.W.; Austin, B. Subcellular components of probiotics Kocuria SM1 and Rhodococcus SM2 induce protective immunity in rainbow trout (Oncorhynchus mykiss, Walbaum) against Vibrio anguillarum. Fish Shellfish. Immunol. 2011, 30, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Martínková, L.; Uhnáková, B.; Pátek, M.; Nesvera, J.; Kren, V. Biodegradation potential of the genus Rhodococcus. Environ. Int. 2009, 35, 162–177. [Google Scholar] [CrossRef]
- Wu, W.; Huang, H.; Ling, Z.; Yu, Z.; Jiang, Y.; Liu, P.; Li, X. Genome sequencing reveals mechanisms for heavy metal resistance and polycyclic aromatic hydrocarbon degradation in Delftia lacustris strain LZ-C. Ecotoxicology 2016, 25, 234–247. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Guo, X.; Song, G.; Pang, S.; Fang, W.; Peng, Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients 2022, 14, 2760. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Meng, C.; Liu, Y.; Yi, Y.; Liang, A.; Zhang, Y.; Hao, Z. Bacillus licheniformis prevents and reduces anxiety-like and depression-like behaviours. Appl. Microbiol. Biotechnol. 2023, 107, 4355–4368. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Lerma, A.; García-Burgos, M.; Alférez, M.J.M.; Pérez-Carrasco, V.; Sanchez-Martin, V.; Linde-Rodríguez, Á.; Ortiz-González, M.; Soriano, M.; García-Salcedo, J.A.; López-Aliaga, I. Gut microbiome-short-chain fatty acids interplay in the context of iron deficiency anaemia. Eur. J. Nutr. 2022, 61, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Turton, K.; Parks, H.J.; Zarodkiewicz, P.; Hamad, M.A.; Dwane, R.; Parau, G.; Ingram, R.J.; Coll, R.C.; Bryant, C.E.; Valvano, M.A. The Achromobacter type 3 secretion system drives pyroptosis and immunopathology via independent activation of NLRC4 and NLRP3 inflammasomes. Cell Rep. 2023, 42, 113012. [Google Scholar] [CrossRef] [PubMed]
- Veschetti, L.; Boaretti, M.; Saitta, G.M.; Passarelli Mantovani, R.; Lleò, M.M.; Sandri, A.; Malerba, G. Achromobacter spp. prevalence and adaptation in cystic fibrosis lung infection. Microbiol. Res. 2022, 263, 127140. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Su, H.; Liu, D.; Wong, C.C.; Shang, H.; Fang, Y.; Zeng, X.; Chen, H.; Li, Y.; Huang, Z.; et al. Traditional Medicine Pien Tze Huang Suppresses Colorectal Tumorigenesis Through Restoring Gut Microbiota and Metabolites. Gastroenterology 2023, 165, 1404–1419. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Sun, X. Iron acquisition and regulation systems in Streptococcus species. Metallomics 2014, 6, 996–1003. [Google Scholar] [CrossRef]
- Sun, Q.; Yuan, C.; Zhou, S.; Lu, J.; Zeng, M.; Cai, X.; Song, H. Helicobacter pylori infection: A dynamic process from diagnosis to treatment. Front. Cell. Infect. Microbiol. 2023, 13, 1257817. [Google Scholar] [CrossRef]
- Kondrotaite, Z.; Valk, L.C.; Petriglieri, F.; Singleton, C.; Nierychlo, M.; Dueholm, M.K.D.; Nielsen, P.H. Diversity and Ecophysiology of the Genus OLB8 and Other Abundant Uncultured Saprospiraceae Genera in Global Wastewater Treatment Systems. Front. Microbiol. 2022, 13, 917553. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Ding, A.; Xie, E.; Tan, Q.; Xing, Y.; Wu, H.; Tian, Q.; Zhang, Y.; Zheng, L. Distinctive species interaction patterns under high nitrite stress shape inefficient denitrifying phosphorus removal performance. Bioresour. Technol. 2024, 394, 130269. [Google Scholar] [CrossRef] [PubMed]




| Ingredients | Percentage (%) |
|---|---|
| Corn | 65.05 |
| Soybean meal | 24.20 |
| Limestone | 8.20 |
| Dicalcium phosphate | 1.70 |
| Salt | 0.30 |
| DL-methionine | 0.12 |
| Choline chloride | 0.10 |
| Mineral premix 1 | 0.30 |
| Vitamin premix 2 | 0.03 |
| Total | 100 |
| Nutrient levels 3 | |
| Metabolizable energy (Mcal/kg) | 2.69 |
| Crude protein | 16.04 |
| Calcium | 3.60 |
| Non-Phosphorus | 0.39 |
| Methionine | 0.38 |
| Lysine | 0.78 |
| Threonine | 0.59 |
| Tryptophan | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, Q.; Mi, J.; Chen, H.; Yuan, T.; Wang, Y.; Zhao, L.; Ma, Q.; Huang, S. Lactobacillus crispatus-Mediated Gut–Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model. Microorganisms 2024, 12, 1559. https://doi.org/10.3390/microorganisms12081559
Li S, Wang Q, Mi J, Chen H, Yuan T, Wang Y, Zhao L, Ma Q, Huang S. Lactobacillus crispatus-Mediated Gut–Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model. Microorganisms. 2024; 12(8):1559. https://doi.org/10.3390/microorganisms12081559
Chicago/Turabian StyleLi, Shinuo, Qingfeng Wang, Jinqiu Mi, Haotian Chen, Tianhao Yuan, Yue Wang, Lihong Zhao, Qiugang Ma, and Shimeng Huang. 2024. "Lactobacillus crispatus-Mediated Gut–Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model" Microorganisms 12, no. 8: 1559. https://doi.org/10.3390/microorganisms12081559
APA StyleLi, S., Wang, Q., Mi, J., Chen, H., Yuan, T., Wang, Y., Zhao, L., Ma, Q., & Huang, S. (2024). Lactobacillus crispatus-Mediated Gut–Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model. Microorganisms, 12(8), 1559. https://doi.org/10.3390/microorganisms12081559








