How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health?
Abstract
1. Introduction
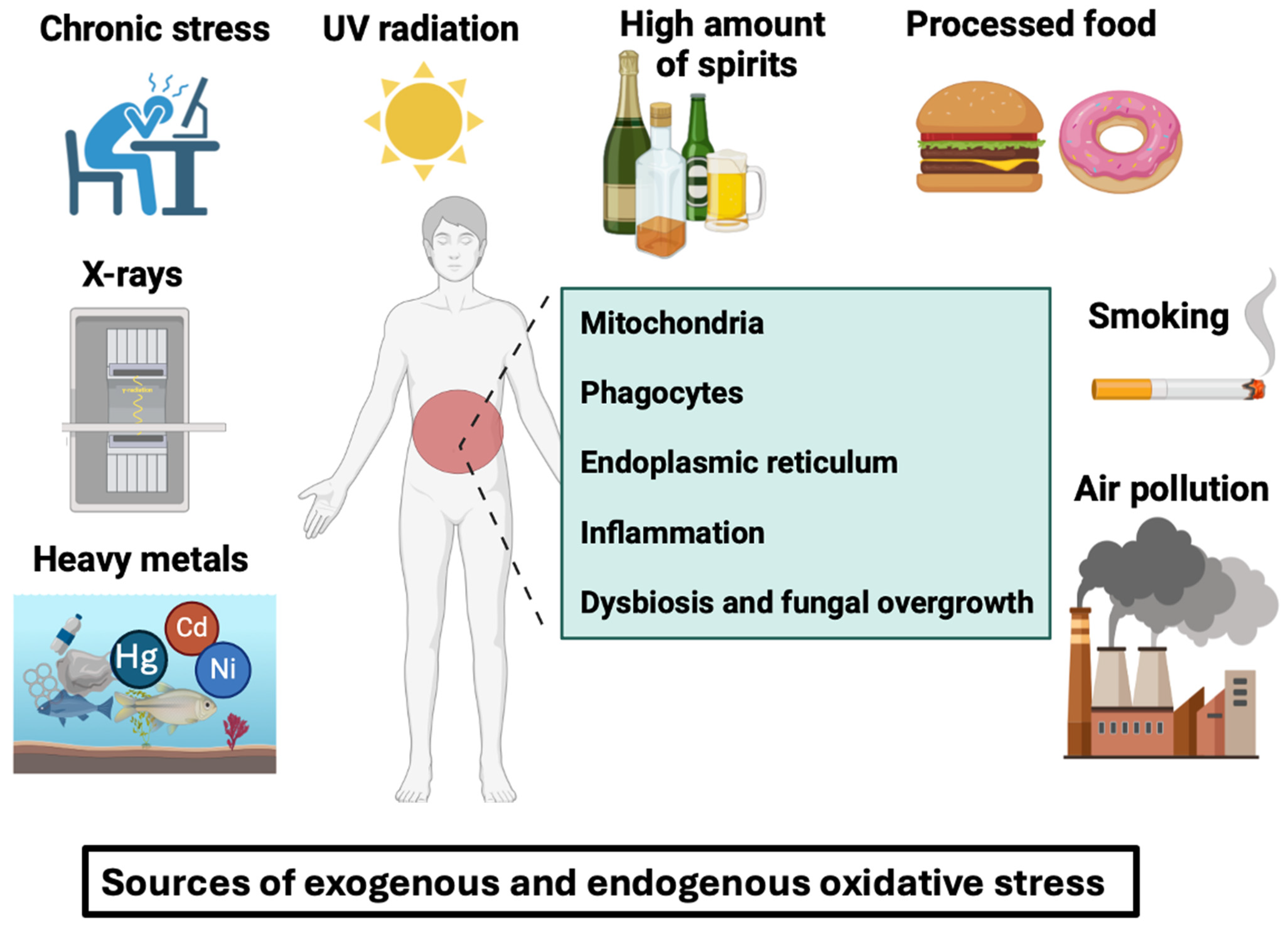
2. An Overview of Adverse Factors That Trigger Oxidative Stress, with a Special Focus on ROS Generation in IBD
3. Overview of Oxidative Stress Inhibition Approaches in IBD
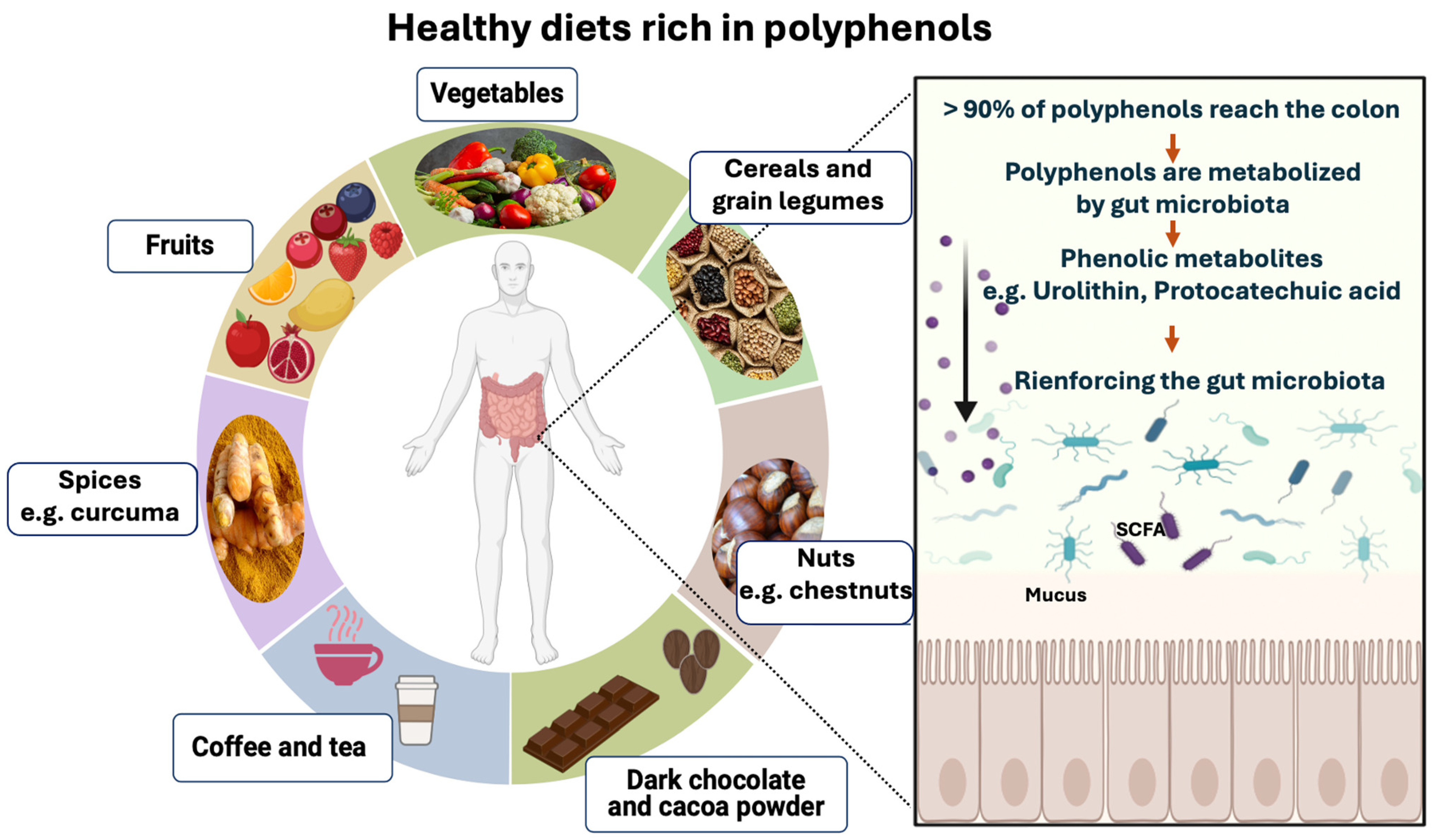
4. Polyphenol-Rich Foods
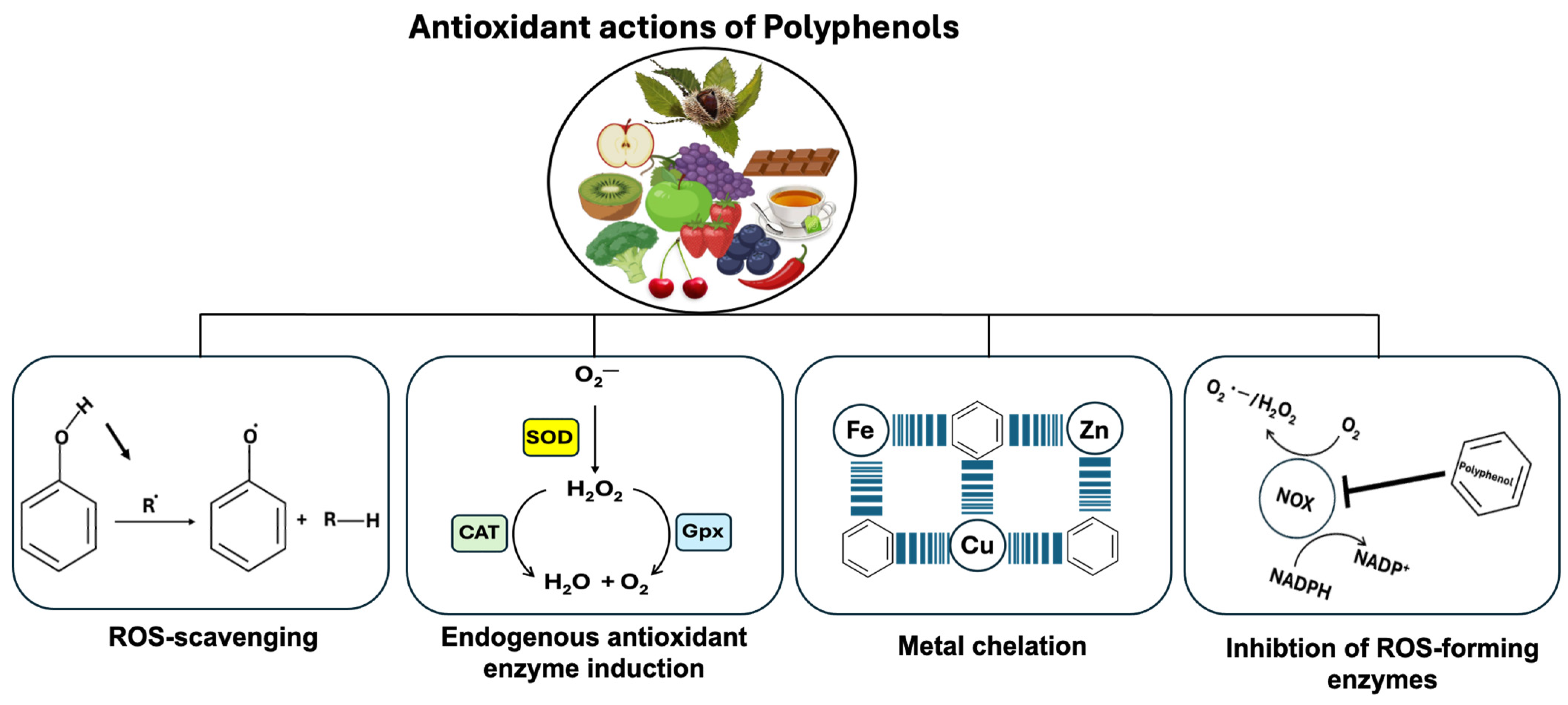
5. Polyphenol-Rich Foods and Their Role in Oxidative Stress Inhibition
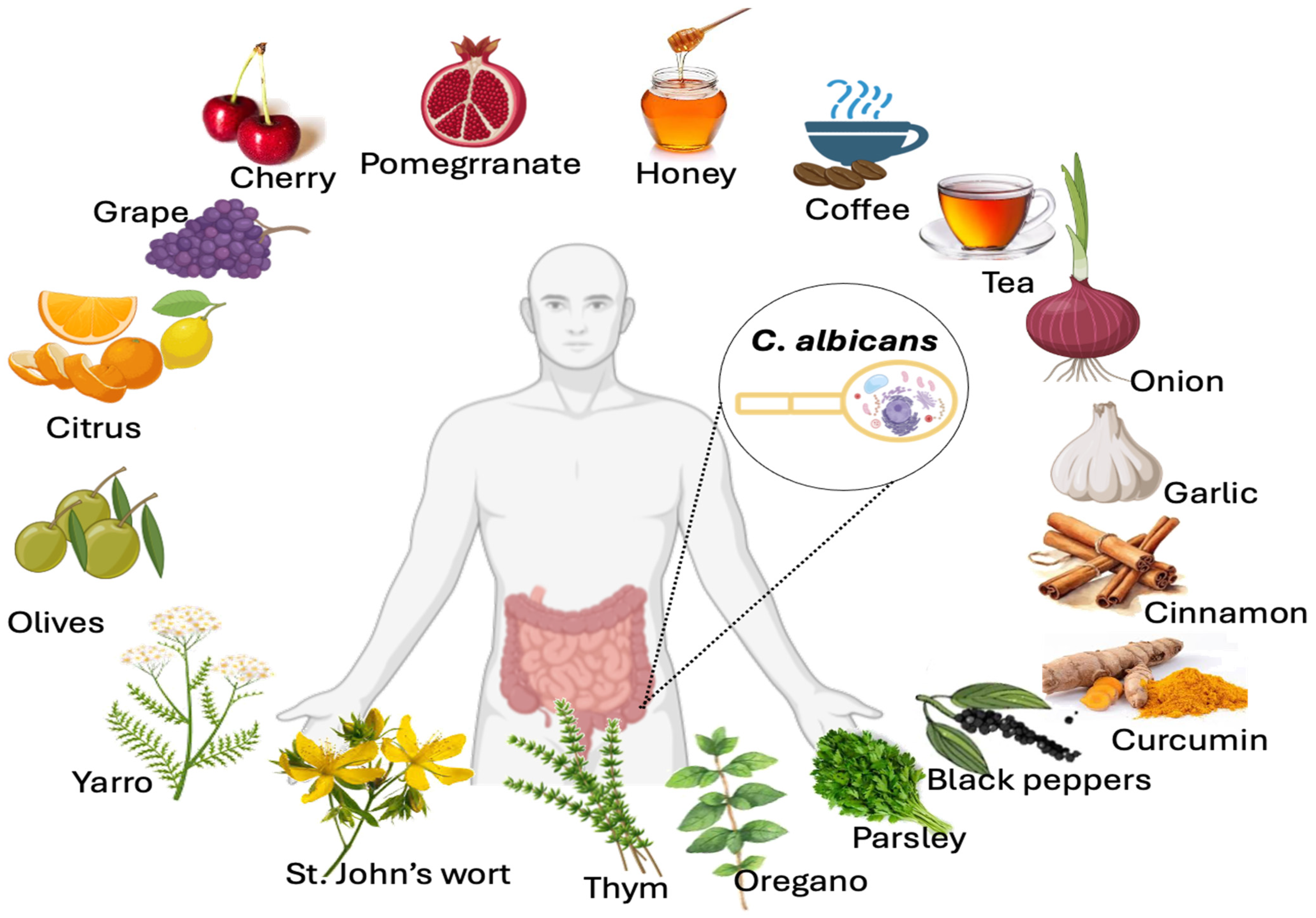
6. Impact of Polyphenol-Rich Foods on Opportunistic Yeast Candida albicans Growth Inhibition
7. Conclusions
Funding
Conflicts of Interest
References
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Mendall, M.; Karagiannis, D.; Karatzas, P.; Ipenburg, N.; Sebastian, S.; Rizzello, F.; Limdi, J.; Katsanos, K.; et al. European Crohn’s and Colitis Organisation Topical Review on IBD in the Elderly. J. Crohns Colitis 2017, 11, 263–273. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, A.; Korzenik, J. Environmental triggers for IBD. Curr. Gastroenterol. Rep. 2014, 16, 396. [Google Scholar] [CrossRef] [PubMed]
- Gabbani, T.; Deiana, S.; Marocchi, M.; Annese, V. Genetic risk variants as therapeutic targets for Crohn’s disease. Expert. Opin. Ther. Targets 2017, 21, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; Yuan, S.; Wang, X.; Theodoratou, E.; Li, X.; et al. Intake of Ultra-processed Foods Is Associated with an Increased Risk of Crohn’s Disease: A Cross-sectional and Prospective Analysis of 187 154 Participants in the UK Biobank. J. Crohns Colitis 2023, 17, 535–552. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z.; Xu, C.; Kan, S.; Chen, D. Disturbances of the gut microbiota and microbiota-derived metabolites in inflammatory bowel disease. Nutrients 2022, 14, 5140. [Google Scholar] [CrossRef]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014, 20, 1192. [Google Scholar] [CrossRef]
- Swidsinski, A.; Weber, J.; Loening-Baucke, V.; Hale, L.P.; Lochs, H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 2005, 43, 3380–3389. [Google Scholar] [CrossRef]
- Hansen, R.; Russell, R.K.; Reiff, C.; Louis, P.; McIntosh, F.; Berry, S.H.; Mukhopadhya, I.; Bisset, M.W.; Barclay, A.R.; Bishop, J. Microbiota of De-Novo Pediatric IBD: Increased Faecali bacterium Prausnitziiand Reduced Bacterial Diversity in Crohn’s But Not in Ulcerative Colitis. Am. J. Gastroenterol. 2012, 107, 1913–1922. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Barnich, N.; Denizot, J.; Darfeuille-Michaud, A.E. coli-mediated gut inflammation in genetically predisposed Crohn’s disease patients. Pathol. Biol. 2013, 61, e65–e69. [Google Scholar] [CrossRef]
- Guzik, T.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Herulf, M.; Ljung, T.; Hellström, P.; Weitzberg, E.; Lundberg, J. Increased luminal nitric oxide in inflammatory bowel disease as shown with a novel minimally invasive method. Scand. J. Gastroenterol. 1998, 33, 164–169. [Google Scholar] [CrossRef]
- Zhou, G.X.; Liu, Z.J. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017, 18, 495–503. [Google Scholar] [CrossRef]
- Neish, A.S. Mucosal immunity and the microbiome. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. S1), S28–S32. [Google Scholar] [CrossRef]
- Rom, O.; Avezov, K.; Aizenbud, D.; Reznick, A.Z. Cigarette smoking and inflammation revisited. Respir. Physiol. Neurobiol. 2013, 187, 5–10. [Google Scholar] [CrossRef]
- Baskara, I.; Kerbrat, S.; Dagouassat, M.; Nguyen, H.Q.; Guillot-Delost, M.; Surenaud, M.; Baillou, C.; Lemoine, F.M.; Morin, D.; Boczkowski, J.; et al. Cigarette smoking induces human CCR6+Th17 lymphocytes senescence and VEGF-A secretion. Sci. Rep. 2020, 10, 6488. [Google Scholar] [CrossRef]
- Quetglas-Llabres, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Mateos, D.; Ugarriza, L.; Gomez, C.; Tur, J.A.; Sureda, A. Oxidative stress and inflammatory biomarkers are related to high intake of ultra-processed food in old adults with metabolic syndrome. Antioxidants 2023, 12, 1532. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Mazúr, M.; Rapta, P.; Bilton, R.F. Oxygen free radical generating mechanisms in the colon: Do the semiquinones of vitamin K play a role in the aetiology of colon cancer? Biochim. Biophys. Acta (BBA)-General. Subj. 2001, 1527, 161–166. [Google Scholar] [CrossRef]
- Bryk, D.; Zapolska-Downar, D.; Malecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Trans fatty acids induce a proinflammatory response in endothelial cells through ROS-dependent nuclear factor-kappaB activation. J. Physiol. Pharmacol. 2011, 62, 229–238. [Google Scholar]
- Haag, F.; Janicova, A.; Xu, B.; Powerski, M.; Fachet, M.; Bundkirchen, K.; Neunaber, C.; Marzi, I.; Relja, B.; Sturm, R. Reduced phagocytosis, ROS production and enhanced apoptosis of leukocytes upon alcohol drinking in healthy volunteers. Eur. J. Trauma. Emerg. Surg. 2022, 48, 2689–2699. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol. Res. Health 2003, 27, 277–284. [Google Scholar]
- Bailey, S.M.; Cunningham, C.C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 2002, 32, 11–16. [Google Scholar] [CrossRef]
- Nordmann, R.; Ribière, C.; Rouach, H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic. Biol. Med. 1992, 12, 219–240. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Poznyak, A.V.; Nikiforov, N.G.; Sobenin, I.A.; Orekhov, A.N. The link between chronic stress and accelerated aging. Biomedicines 2020, 8, 198. [Google Scholar] [CrossRef]
- Herbet, M.; Korga, A.; Gawrońska-Grzywacz, M.; Izdebska, M.; Piątkowska-Chmiel, I.; Poleszak, E.; Wróbel, A.; Matysiak, W.; Jodłowska-Jędrych, B.; Dudka, J. Chronic variable stress is responsible for lipid and DNA oxidative disorders and activation of oxidative stress response genes in the brain of rats. Oxid. Med. Cell. Longev. 2017, 2017, 7313090. [Google Scholar] [CrossRef]
- Gardiner, P.; Sadikova, E.; Filippelli, A.C.; Mitchell, S.; White, L.F.; Saper, R.; Kaptchuk, T.J.; Jack, B.W.; Fredman, L. Stress Management and Relaxation Techniques use among underserved inpatients in an inner city hospital. Complement. Ther. Med. 2015, 23, 405–412. [Google Scholar] [CrossRef]
- Milne, B.; Joachim, G.; Niedhardt, J. A stress management programme for inflammatory bowel disease patients. J. Adv. Nurs. 1986, 11, 561–567. [Google Scholar] [CrossRef]
- Miedziun, P.; Czabała, J.C. Stress management techniques. Arch. Psychiatry Psychother. 2015, 17, 23–31. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J. Introductory lecture: Atmospheric chemistry in the Anthropocene. Faraday Discuss. 2017, 200, 11–58. [Google Scholar] [CrossRef]
- Lelieveld, J. Clean air in the Anthropocene. Faraday Discuss. 2017, 200, 693–703. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018. [Google Scholar] [CrossRef]
- Cho, C.-C.; Hsieh, W.-Y.; Tsai, C.-H.; Chen, C.-Y.; Chang, H.-F.; Lin, C.-S. In vitro and in vivo experimental studies of PM2.5 on disease progression. Int. J. Environ. Res. Public. Health 2018, 15, 1380. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, Z.M.; Henneke, P.; Kolter, J. From flies to men: ROS and the NADPH oxidase in phagocytes. Front. Cell Dev. Biol. 2021, 9, 628991. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer. 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Allergy Clin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.K.; Kumar, R. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 2015, 30, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Ashique, S.; Mishra, N.; Garg, A.; Sibuh, B.Z.; Taneja, P.; Rai, G.; Djearamane, S.; Wong, L.S.; Al-Dayan, N.; Roychoudhury, S.; et al. Recent updates on correlation between reactive oxygen species and synbiotics for effective management of ulcerative colitis. Front. Nutr. 2023, 10, 1126579. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Abreu, M.T.; Cohen, R.; Tremaine, W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006, 130, 940–987. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, R.P. Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol. Rep. 2012, 64, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Probst, L.; Dächert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, A.; Triantafillidis, J. Melatonin: A potent antioxidant agent with anti-inflammatory and anti-apoptotic effects that might be useful in the treatment of IBD patients. Ann. Gastroenterol. 2009, 10–12. [Google Scholar]
- Li, J.-H.; Yu, J.-P.; Yu, H.-G.; Xu, X.-M.; Yu, L.-L.; Liu, J.; Luo, H.-S. Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediat. Inflamm. 2005, 2005, 185. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of melatonin on intestinal microbiota and oxidative stress in colitis mice. Biomed. Res. Int. 2018, 2018, 2607679. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Am. J. Med. Sci. 2013, 5, 126–139. [Google Scholar] [CrossRef]
- Okvenda, A.Z.; Yerizel, E. Olive oil increase catalase activity and gluthatione peroxidase level in hyperglycemic rats. Acta Biochim. Indones. 2023, 6, 137. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Monticolo, R.; D’Amico, A.; Stefanini, L.; Pagano, F.; Pastori, D.; Cangemi, R. Antioxidant activity from extra virgin olive oil via inhibition of hydrogen peroxide–mediated NADPH-oxidase 2 activation. Nutrition 2018, 55, 36–40. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Eid, A.H.; Omar, H.A.; Arafa, E.-S.A.; Maghrabi, I.A. Camel’s milk ameliorates TNBS-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem. Toxicol. 2014, 69, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, M.M.; Al-Ghariebeh, R.B.; Alhaija, A.A.A.; Al-Zoubi, H.; Al-Qaoud, K.M. Camel milk whey inhibits inflammatory colorectal cancer development via down regulation of pro-inflammatory cytokines in induced AOM/DSS mouse model. Emir. J. Food Agric. 2019, 31, 256–262. [Google Scholar] [CrossRef]
- Awais, M.A.; Arif, A.; Asif, R.; Hussain, M.I.; Niaz, A. A review on camel milk and its medicinal properties. Riphah J. Allied Health Sci. 2022, 1. [Google Scholar] [CrossRef]
- Arain, M.A.; Khaskheli, G.B.; Shah, A.H.; Marghazani, I.B.; Barham, G.S.; Shah, Q.A.; Khand, F.M.; Buzdar, J.A.; Soomro, F.; Fazlani, S.A. Nutritional significance and promising therapeutic/medicinal application of camel milk as a functional food in human and animals: A comprehensive review. Anim. Biotechnol. 2023, 34, 1988–2005. [Google Scholar] [CrossRef]
- Briones, A.M.; Touyz, R.M. Moderate exercise decreases inflammation and oxidative stress in hypertension: But what are the mechanisms? Hypertension 2009, 54, 1206–1208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, D.; Haque, M.; Sriramula, S.; Mariappan, N.; Pariaut, R.; Francis, J. Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension 2009, 54, 1393–1400. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Jawhara, S. Healthy diet and lifestyle improve the gut microbiota and help combat fungal infection. Microorganisms 2023, 11, 1556. [Google Scholar] [CrossRef]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as probiotic carrier food. Int. Dairy. J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W., III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir: A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, D.; Natesan, S.; Billamboz, M.; Jawhara, S. Role of bacteria-derived exopolysaccharides in inflammatory bowel disease with a special focus on cyanobacterial exopolysaccharides. Appl. Microbiol. 2024, 4, 250–274. [Google Scholar] [CrossRef]
- Wang, C.H.; Lai, P.; Chen, M.E.; Chen, H.L. Antioxidative capacity produced by Bifidobacterium-and Lactobacillus acidophilus-mediated fermentations of konjac glucomannan and glucomannan oligosaccharides. J. Sci. Food Agric. 2008, 88, 1294–1300. [Google Scholar] [CrossRef]
- Chen, H.-L.; Wang, C.-H.; Kuo, Y.-W.; Tsai, C.-H. Antioxidative and hepatoprotective effects of fructo-oligosaccharide in d-galactose-treated Balb/cJ mice. Br. J. Nutr. 2011, 105, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Calabro, V.; Prince, P.D.; Litterio, M.C.; Piotrkowski, B.; Vazquez-Prieto, M.A.; Miatello, R.M.; Oteiza, P.I.; Fraga, C.G. Flavonoids and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012, 1259, 87–94. [Google Scholar] [CrossRef]
- Amiot, M.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hollman, P.; Katan, M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Basdra, E.; Papavassiliou, A.; Stefanadis, C. Flavonoids in atherosclerosis: An overview of their mechanisms of action. Curr. Med. Chem. 2013, 20, 2641–2660. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Wang, T.-y.; Li, Q.; Bi, K.-s. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of polyphenols and flavonoids in the era of precision medicine. Mol. Pharm. 2017, 14, 2861–2863. [Google Scholar] [CrossRef]
- Ravisankar, S.; Agah, S.; Kim, H.; Talcott, S.; Wu, C.; Awika, J. Combined cereal and pulse flavonoids show enhanced bioavailability by downregulating phase II metabolism and ABC membrane transporter function in Caco-2 model. Food Chem. 2019, 279, 88–97. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregório, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef]
- Huxley, R.; Lee, C.M.Y.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.L.; dePaula, J.; Silva, C.M.; Cruz, A.; Miguel, M.A.L.; Farah, A. Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.-J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants—Hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Robbins, C.; Hanley, T.; Hagerman, A.; Hjeljord, O.; Baker, D.; Schwartz, C.; Mautz, W. Role of tannins in defending plants against ruminants: Reduction in protein availability. Ecology 1987, 68, 98–107. [Google Scholar] [CrossRef]
- Tanner, G. Condensed tannins. Plant pigments and their manipulation. Ann. Plant Rev. 2004, 14, 150–184. [Google Scholar] [CrossRef]
- Porter, L.J. Structure and chemical properties of the condensed tannins. In Plant Polyphenols: Synthesis, Properties, Significance; Basic Life Sciences; Springer: Boston, MA, USA, 1992; pp. 245–258. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed. Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Turchetti, B.; Mulinacci, N.; Vincieri, F.F.; Buzzini, P. Analysis of condensed and hydrolysable tannins from commercial plant extracts. J. Pharm. Biomed. Anal. 2006, 41, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.P.; Clausen, T.P.; MacLean, S.F., Jr.; Redman, A.M.; Reichardt, P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Zhang, R.; Richardson, J.J.; Masters, A.F.; Maschmeyer, T. Removal of Pb2+ from water using sustainable brown seaweed phlorotannins. Langmuir 2022, 38, 8324–8333. [Google Scholar] [CrossRef] [PubMed]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- D Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita. 2007, 43, 348. [Google Scholar] [PubMed]
- Pérez-Chabela, M.L.; Hernández-Alcántara, A.M. Agroindustrial coproducts as sources of novel functional ingredients. In Food Processing for Increased Quality and Consumption; Elsevier: Amsterdam, The Netherlands, 2018; pp. 219–250. [Google Scholar]
- Zuiter, A. Proanthocyanidin: Chemistry and biology: From phenolic compounds to proanthocyanidins. In Module in Chemistry, Molecular Sciences and Chemical Engineering, 1st ed.; Reedijk, J., Ed.; Elsevier: Cambridge, MA, USA, 2014; Volume 1, pp. 1–29. [Google Scholar] [CrossRef]
- Almario, R.U.; Karakas, S.E. Lignan content of the flaxseed influences its biological effects in healthy men and women. J. Am. Coll. Nutr. 2013, 32, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and their derivatives from plants as antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The what and who of dietary lignans in human health: Special focus on prooxidant and antioxidant effects. Trends Food Sci. Technol. 2020, 106, 382–390. [Google Scholar] [CrossRef]
- Mridula, D.; Singh, K.; Barnwal, P. Development of omega-3 rich energy bar with flaxseed. J. Food Sci. Technol. 2013, 50, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Zhang, L.-y.; Wang, X.-l.; Sun, D.-x.; Liu, X.-x.; Hu, X.-y.; Kong, F. Regulation of zinc transporters by dietary flaxseed lignan in human breast cancer xenografts. Mol. Biol. Rep. 2008, 35, 595–600. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.; Chen, J.; Thompson, L.U. Flaxseed and its lignan precursor, secoisolariciresinol diglycoside, affect pregnancy outcome and reproductive development in rats. J. Nutr. 1998, 128, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Fu, H.; Sun, H.; Affoh, R.; Yu, H. The impact of polyphenol on general nutrient metabolism in the monogastric gastrointestinal tract. J. Food Qual. 2020, 2020, 5952834. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Baek, K.; Kim, J.-R.; Lee, J.-J.; Ryu, S.-H.; Chin, B.-R.; Baek, S.-H. Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp. Mol. Med. 2009, 41, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. [Google Scholar] [CrossRef]
- Jawhara, S. How gut bacterial dysbiosis can promote Candida albicans overgrowth during colonic inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef]
- Poulain, D.; Sendid, B.; Standaert-Vitse, A.; Fradin, C.; Jouault, T.; Jawhara, S.; Colombel, J.F. Yeasts: Neglected pathogens. Dig. Dis. 2009, 27 (Suppl. S1), 104–110. [Google Scholar] [CrossRef]
- Jawhara, S.; Habib, K.; Maggiotto, F.; Pignede, G.; Vandekerckove, P.; Maes, E.; Dubuquoy, L.; Fontaine, T.; Guerardel, Y.; Poulain, D. Modulation of intestinal inflammation by yeasts and cell wall extracts: Strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS ONE 2012, 7, e40648. [Google Scholar] [CrossRef]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Barbet, M.; Sendid, B.; Jawhara, S. A decrease in anaerobic bacteria promotes Candida glabrata overgrowth while beta-glucan treatment restores the gut microbiota and attenuates colitis. Gut Pathog. 2018, 10, 50. [Google Scholar] [CrossRef]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef]
- Standaert-Vitse, A.; Jouault, T.; Vandewalle, P.; Mille, C.; Seddik, M.; Sendid, B.; Mallet, J.M.; Colombel, J.F.; Poulain, D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology 2006, 130, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. Editorial of Special Issue “Human Pathogenic Fungi: Host-Pathogen Interactions and Virulence”. Microorganisms 2023, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.G. Naringin-generated ROS promotes mitochondria-mediated apoptosis in Candida albicans. IUBMB Life 2021, 73, 953–967. [Google Scholar] [CrossRef]
- Arismunandar, R.N.; Nosartika, I.; Purnomo, B.N.R.; Antari, A.L. The Effectivity of Parsley (Petroselinum crispum) Extract on The Growth Inhibition of Candida albicans. J. Biomed. Transl. Res. 2021, 7, 123–128. [Google Scholar] [CrossRef]
- Candiracci, M.; Citterio, B.; Piatti, E. Antifungal activity of the honey flavonoid extract against Candida albicans. Food Chem. 2012, 131, 493–499. [Google Scholar] [CrossRef]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef]
- Han, H.-K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert. Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef]
- Feng, Y.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Technol. 2020, 57, 4671–4687. [Google Scholar] [CrossRef]
- Priya, A.; Pandian, S.K. Piperine impedes biofilm formation and hyphal morphogenesis of Candida albicans. Front. Microbiol. 2020, 11, 528306. [Google Scholar] [CrossRef]
- Mateos-Martin, M.L.; Fuguet, E.; Quero, C.; Perez-Jimenez, J.; Torres, J.L. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1327–1336. [Google Scholar] [CrossRef]
- Atai, Z.; Ansari, M.; Mousavi, A.; Mirzaei, A. In-vitro study of antifungal effects of selected herbal extracts on standard and wild strains of Candida albicans. J. Iran. Dent. Assoc. 2007, 19, 91–97. [Google Scholar]
- Salman, S.; Öz, G.; Felek, R.; Haznedar, A.; Turna, T.; Özdemir, F. Effects of fermentation time on phenolic composition, antioxidant and antimicrobial activities of green, oolong, and black teas. Food Biosci. 2022, 49, 101884. [Google Scholar] [CrossRef]
- Sitheeque, M.; Panagoda, G.; Yau, J.; Amarakoon, A.; Udagama, U.; Samaranayake, L. Antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. Chemotherapy 2009, 55, 189–196. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant extracts rich in polyphenols as potent modulators in the growth of probiotic and pathogenic intestinal microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, A.; Shokri, H.; Katiraee, F. Anti-adherence and anti-fungal abilities of thymol and carvacrol against Candida species isolated from patients with oral candidiasis in comparison with fluconazole and voriconazole. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e65005. [Google Scholar] [CrossRef]
- Braga, P.C.; Culici, M.; Alfieri, M.; Dal Sasso, M. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int. J. Antimicrob. Agents 2008, 31, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Del Bo, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef]
- Calheiros, D.; Dias, M.I.; Calhelha, R.C.; Barros, L.; Ferreira, I.; Fernandes, C.; Goncalves, T. Antifungal activity of spent coffee ground extracts. Microorganisms 2023, 11, 242. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef]
- Lemar, K.M.; Turner, M.P.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A. Inhibition of Candida adhesion to buccal epithelial cells by an aqueous extract of Allium sativum (garlic). J. Appl. Bacteriol. 1990, 68, 163–169. [Google Scholar] [CrossRef]
- Doddanna, S.J.; Patel, S.; Sundarrao, M.A.; Veerabhadrappa, R.S. Antimicrobial activity of plant extracts on Candida albicans: An in vitro study. Indian J. Dent. Res. 2013, 24, 401–405. [Google Scholar] [CrossRef]
- Almasaudi, S.; AlBureikan, M.O. Antimicrobial activity of onion juice (Allium cepa), honey, and onion-honey mixture on some sensitive and multi-resistant microorganisms. Life Sci. J. 2012, 9, 775–780. [Google Scholar]
- Ben Lagha, A.; LeBel, G.; Grenier, D. Tart cherry (Prunus cerasus L.) fractions inhibit biofilm formation and adherence properties of oral pathogens and enhance oral epithelial barrier function. Phytother. Res. 2020, 34, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Seu, Y.B.; Lee, D.G. Candicidal action of resveratrol isolated from grapes on human pathogenic yeast C. albicans. J. Microbiol. Biotechnol. 2007, 17, 1324–1329. [Google Scholar]
- Parera, L.; Lawa, Y.; More, E. The effect of ethanol extracts of red pomegranate peel (Punica granatum L.) on activities Candida albicans. Environ. Conserv. 2019, 25, 81–86. [Google Scholar]
- Prasad, D.; Kunnaiah, R. Punica granatum: A review on its potential role in treating periodontal disease. J. Indian Soc. Periodontol. 2014, 18, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Zoric, N.; Kopjar, N.; Bobnjaric, I.; Horvat, I.; Tomic, S.; Kosalec, I. Antifungal activity of oleuropein against Candida albicans-the in vitro study. Molecules 2016, 21, 1631. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jawhara, S. How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health? Microorganisms 2024, 12, 1570. https://doi.org/10.3390/microorganisms12081570
Jawhara S. How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health? Microorganisms. 2024; 12(8):1570. https://doi.org/10.3390/microorganisms12081570
Chicago/Turabian StyleJawhara, Samir. 2024. "How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health?" Microorganisms 12, no. 8: 1570. https://doi.org/10.3390/microorganisms12081570
APA StyleJawhara, S. (2024). How Do Polyphenol-Rich Foods Prevent Oxidative Stress and Maintain Gut Health? Microorganisms, 12(8), 1570. https://doi.org/10.3390/microorganisms12081570





