Abstract
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, involves chronic inflammatory disorders of the digestive tract. Oxidative stress, associated with increased reactive oxygen species generation, is a major risk factor for IBD pathogenesis. Industrialized lifestyles expose us to a variety of factors that contribute to deteriorating gut health, especially for IBD patients. Many alternative therapeutic strategies have been developed against oxidative stress along with conventional therapy to alleviate IBD pathogenesis. Polyphenol-rich foods have attracted growing interest from scientists due to their antioxidant properties. Polyphenols are natural compounds found in plants, fruits, vegetables, and nuts that exhibit antioxidant properties and protect the body from oxidative damage. This review presents an overview of polyphenol benefits and describes the different types of polyphenols. It also discusses polyphenols’ role in inhibiting oxidative stress and fungal growth prevention. Overall, this review highlights how a healthy and balanced diet and avoiding the industrialized lifestyles of our modern society can minimize oxidative stress damage and protect against pathogen infections. It also highlights how polyphenol-rich foods play an important role in protecting against oxidative stress and fungal growth.
1. Introduction
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis, two chronic inflammatory diseases of the gastrointestinal tract (GI) with extremely complex etiologies [1,2]. The etiology of IBD is not yet fully understood, but it is believed to involve a combination of genetic, environmental, and immune system factors [1,2]. Genetic predisposition, environmental factors, gut dysbiosis, and an overactive immune response are all thought to play a role in IBD development and progression [3,4]. IBD has increased significantly in the past few decades, affecting 3.1 million adults in the U.S. and 2.2 million in Europe, as well as an increase in Asian populations [5,6,7]. The rising incidence of IBD in industrialized countries suggests a possible link between the disease and modern lifestyle factors such as diet, stress, and exposure to environmental toxins. Additionally, changes in gut microbiota composition due to the Westernization of diets and reduced exposure to beneficial bacteria may also contribute to the development and progression of IBD [8,9,10].
In recent years, research has increasingly focused on the gut microbiota’s role in IBD [11,12]. The breakdown of host–microbial mutualism is probably what defines IBD development as a definitive change in the normal gut microbiota [13].
The gut microbiota has been consistently altered in IBD patients, particularly by Firmicute reduction. Bacteroides species may have been spatially reorganized in IBD patients, with Bacteroides fragilis occupying an increased proportion of the bacterial mass [14]. A shift in the balance between beneficial and harmful bacteria is observed in IBD pathogenesis, marked by a decline in Firmicutes and a spatial redistribution of Bacteroides species.
Among Firmicutes species, Faecalibacterium prausnitzii (F. prausnitzii) decreases significantly in patients with CD, particularly those with ileal CD, suggesting that F. prausnitzii is an anti-inflammatory bacterium. In addition, it is likely that its decrease in adult CD patients is a result of inflammation caused by the disease. A pediatric cohort has shown an increase of F. prausnitzii, suggesting a more dynamic role for the species [15].
In addition to the reduction in Firmicutes and the spatial reorganization of Bacteroides species, IBD is linked to increased members of the Proteobacteria phylum, which have been identified as key players. The Proteobacteria phylum is associated with the production of endotoxins, which are molecules that can stimulate an inflammatory response. This inflammation then triggers the other components of the immune system, leading to further inflammation and the development of IBD [16].
The increase in E. coli, a member of the Proteobacteria phylum, in patients with IBD, especially those with ileal CD, suggests its potential role in the development and progression of the disease. The immune response triggered by E. coli endotoxins in IBD contributes to chronic inflammation, tissue damage, and immune cell recruitment [17].
During mucosal inflammation, inflammatory cytokines activate NADPH oxidase (NOX) and inducible nitric oxide synthase (iNOS), leading to the release of superoxide from intestinal epithelial cells, neutrophils, and macrophages [18]. Excessive levels of reactive oxygen species (ROS) referring to oxidative stress can damage proteins in the cytoskeleton, contributing to inflammation and increasing the permeability of tight junctions in intestinal walls. This disruption of the intestinal epithelial barrier ultimately results in further mucosal inflammatory responses [19]. There is evidence that inflammation of the gut caused by oxidative stress is the precursor to the onset of IBD in humans [19].
Microvascular networks that surround epithelial cells can attract inflammatory mediators, leading to more tissue damage and an escalation of intestinal inflammation caused by circulating inflammatory mediators [20]. Various morphological lesions have been associated with intestinal inflammation, including the loss of goblet cells, decreased mucus production, ulceration, and hyperplasia of colonic crypt cells [21,22]. These morphological epithelial lesions contribute to reduced intestinal barrier function, allowing pathogenic bacteria and toxins into the circulation and causing systemic inflammation that leads to further tissue damage.
2. An Overview of Adverse Factors That Trigger Oxidative Stress, with a Special Focus on ROS Generation in IBD
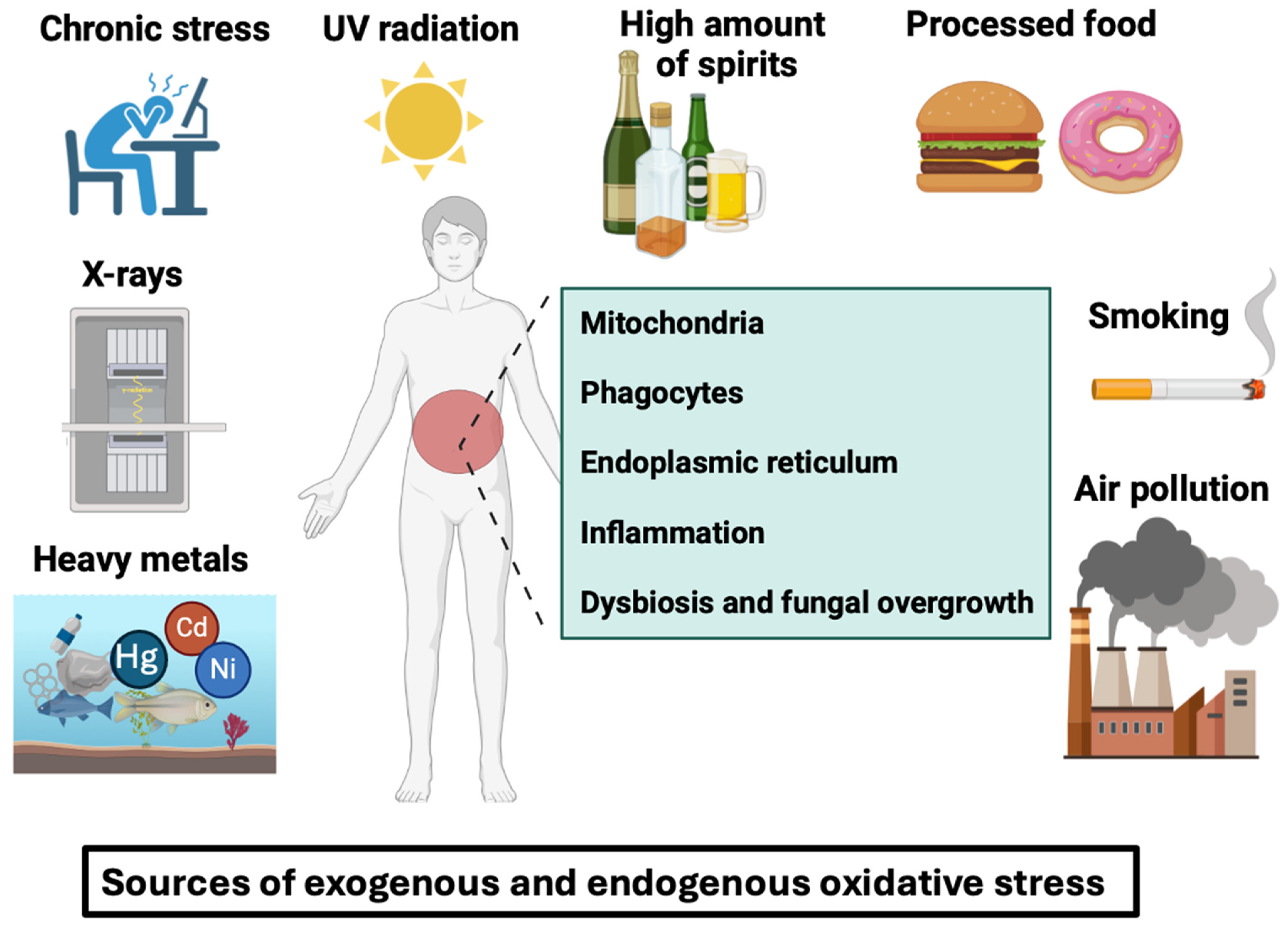
Our current industrialized lifestyle exposes us to a variety of exogenous unhealthy factors (smoking, processed food, hydrogenated oils, chronic stress, alcohol, air pollution, heavy metals, and ultraviolet light) and endogenous conditions (mitochondria and phagocyte NADPH oxidases) that can damage our cells and deteriorate our digestive tract, especially for IBD patients. These unhealthy factors can lead to chronic inflammation and oxidative stress, further exacerbating IBD pathogenesis and progression (Figure 1).

Figure 1.
Oxidative stress can be defined as an imbalance caused by excessive reactive oxygen species production by cells that exceeds the body’s capacity to neutralize them. Free radicals and peroxides damage intracellular structures such as proteins, lipids, and DNA and disrupt intrinsic cell mechanisms. These free radicals can be induced by exogenous factors such as smoking, air pollution, processed foods, chronic stress, UV radiation, X-rays, heavy metals (e.g., nickel, cobalt, and mercury) or by endogenous factors like intracellular mitochondria, phagocytes, endoplasmic reticulum, inflammation, gut dysbiosis, and fungal overgrowth in the gut.
Smoking is one of the most significant sources of ROS, which can have adverse effects on the GI tract [23]. The smoking substance list includes aldehydes, quinones, epoxides, nitric oxides, and several other compounds which are sources of ROS production [24]. The most common GI disorders include CD, reflux disease, cancers of the esophagus, and many more [24].
In terms of processed food, a clinical study showed that antioxidant enzyme activities such as catalase (CAT) and superoxide dismutase (SOD) are low in ultra-processed food consumers. However, ROS generation and myeloperoxidase activity are higher in these consumers [25]. Additionally, the study conducted by Narula et al. showed that individuals who consume ultra-processed foods (soft drinks, refined sweetened foods, salty snacks, and processed meats) are more likely to develop IBD [26]. The intake of white meat, red meat, dairy products, starches, fruit, vegetables, and legumes was not associated with incidents of IBD [26]. Of note, high consumption of red meat has been linked to a higher incidence of colon cancer [27]. The high iron content of red meat may catalyze ROS formation in the colon during digestion [27].
Trans fatty acids are found in partially hydrogenated oils, which are commonly used in processed foods such as fried and baked goods, margarine, and snack foods. These unhealthy fats have been linked to the presence of acrylamide in food [28]. These trans fatty acids increased phosphorylation of NF-κB and ROS generation in human aortic endothelial cells [28].
Alcohol, and especially distilled spirits, in high amounts can damage the mucosal layer of the GI tract [29]. Additionally, alcohol can disrupt the balance of beneficial gut bacteria, further compromising the health of the GI tract. ROS generation from ethanol can contribute to liver diseases caused by alcohol consumption [30,31,32]. Of note, a breakdown of alcohol in the liver by cytochrome reductase CYP2E1 forms acetaldehyde during excessive alcohol consumption. This enzyme CYP2E1 can transfer electrons to oxygen to form a superoxide (O2.−) radical or catalyze lipid peroxidation that results in ROS generation [30,31,32].
Chronic stress is another factor linked to increased oxidative stress levels in our modern society [33,34]. Many approaches to dealing with stress situations can help minimize oxidative stress in individuals with chronic stress. It is important for these individuals to know how to handle difficult and stressful situations by choosing the most effective stress-handling techniques for themselves. These techniques include analyzing and planning future tasks, socializing, meeting with friends, explaining the situation, and finding solutions to problems [35,36,37]. It is also worthwhile to focus on self-care activities such as engaging in hobbies and exercising meditation and relaxation techniques.
Air pollution is considered one of the world’s leading environmental health threats [38,39]. Various pollutants are emitted by industrial processes, including particulate matter (PM), nitrogen oxides (NO2), sulfur dioxide (SO2), and volatile organic compounds, which can have adverse effects on both the environment and health [38,39]. The study of Jin et al. showed that PM penetration into damaged skin leads to inflammation and adverse effects on the skin [40]. Experimental studies conducted on animals have shown that the adverse effects of ROS activity on rodent respiratory systems depend on particle size and emission sources [41].
In terms of heavy metals, some individuals are exposed to redox-inert elements such as cadmium and arsenic which are toxic at low concentrations [42]. These individuals living in contaminated areas are exposed to these elements through a variety of natural sources, such as air pollution and contaminated drinking water and food [42].
It is well known that human skin is susceptible to constant exposure to ultraviolet (UV) light [43]. Exposure to the sun stimulates melanin synthesis, which results in post-inflammatory hyperpigmentation of the skin [44]. Excessive sunlight exposure generates ROS from epidermis melanocytes, which adversely affects these cells. An excessive increase in ROS generation could disrupt homeostasis, leading to the malignant transformation of these cells [43,44].
Among the endogenous sources of cellular ROS generation, mitochondria and phagocyte NADPH oxidases are the two most important sources of cellular ROS generation [45]. ROS are produced in mitochondria by the oxidation of metabolic intermediates in the electron transport chain (ETC). However, this process is tightly regulated to prevent oxidative damage to cells [46]. Mitochondrial ROS are produced via ETC in the form of superoxide, with complex I being the most common source of ROS in mitochondria [46]. Phagocyte NADPH oxidase (e.g., NOX2) is responsible for the generation of large amounts of ROS in phagosomes, which function as a direct or indirect mechanism of killing ingested microbes [45,47].
In addition to mitochondrial and phagocyte NADPH oxidases, ROS can also be generated by inflammation and gut dysbiosis (Figure 1). A variety of enteric pathogens are able to cause inflammation by stimulating the production of proinflammatory cytokines, which are further responsible for oxidative stress production. The damage caused by oxidative stress is aggravated by leukocyte activation in chronic intestinal disorders [48]. These factors result in excessive ROS production, which exceeds the antioxidant defenses and perpetuates or worsens inflammation in the mucosa. ROS molecules generated by unstable types of oxygen, such as superoxide ions, hydrogen peroxides, and hydroxyl radicals, are the major pro-oxidant molecules involved in the oxidative reaction [49]. These highly reactive molecules contribute to oxidative damage in the mucosa, exacerbating inflammation in chronic intestinal disorders.
3. Overview of Oxidative Stress Inhibition Approaches in IBD
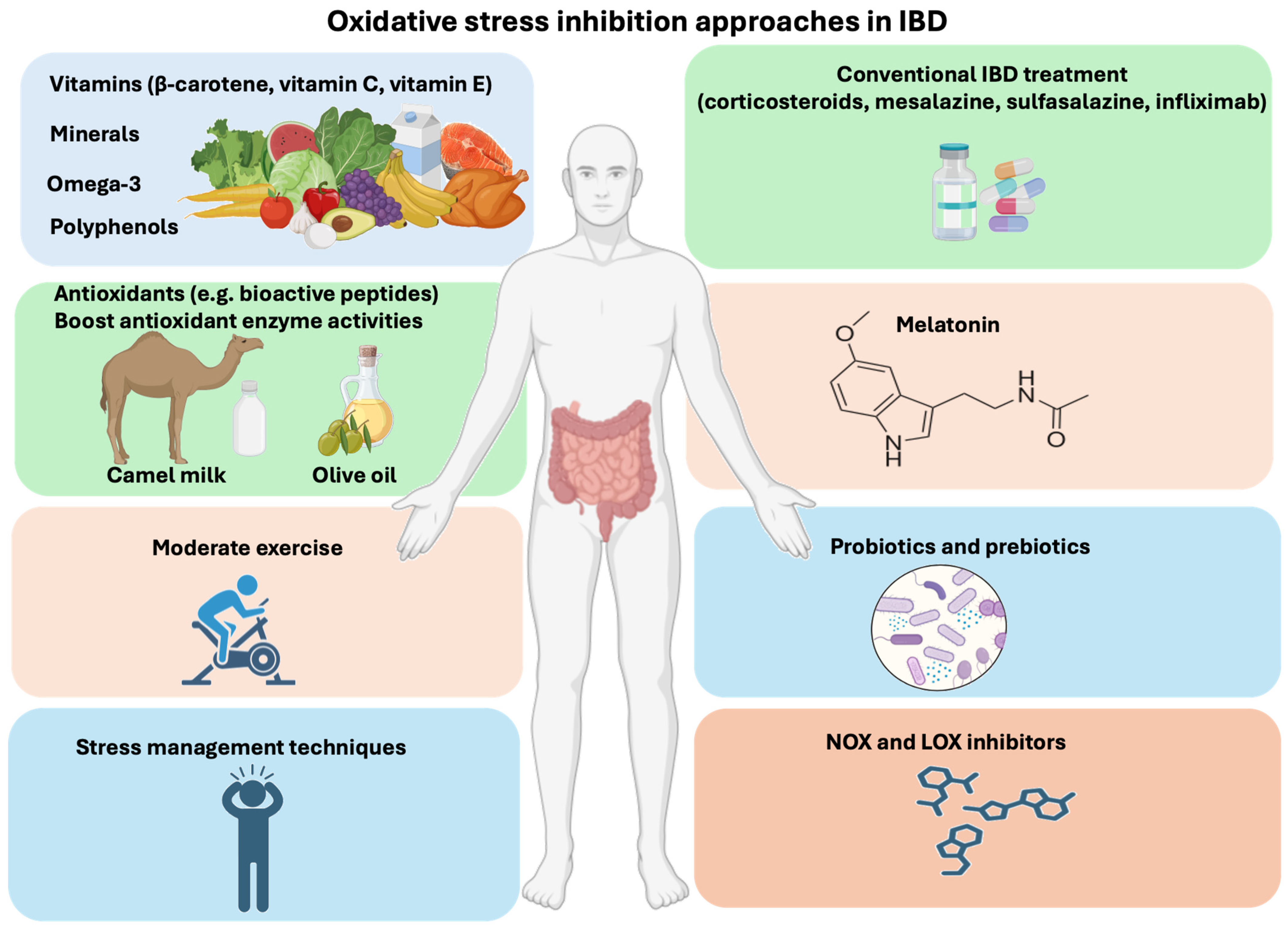
The conventional therapeutic approach to treating IBD consists of anti-inflammatory medicines such as corticosteroids, mesalazine, sulfasalazine, and infliximab for quick relief from IBD discomfort [50,51]. In parallel, many complementary therapeutic approaches, including some in clinical trials, are used to alleviate oxidative stress in IBD patients (Figure 2). One of these approaches, a new class of NOX inhibitors, shows potential anti-ROS properties. In addition to NOX inhibitors, LOXs (lipoxygenases) are a group of enzymes involved in the metabolism of arachidonic acid and other polyunsaturated fatty acids in cells [52]. Overactivation of LOXs generates ROS in cells. However, LOX inhibitors protect against inflammatory diseases and prevent ROS generation (Figure 2) [53].

Figure 2.
Oxidative stress inhibitors for IBD. Along with conventional therapy, many alternative therapeutic strategies have been developed to alleviate IBD pathogenesis. Various options for reducing oxidative stress in IBD include antioxidants such as melatonin or LOX and NOX inhibitors. In addition, lifestyle modifications by consuming foods rich in fruits and vegetables and medicinal plants rich in β-carotene (pro-vitamin A), vitamin C, vitamin E, minerals, omega-3 polyunsaturated fatty acids, and polyphenols and engaging in regular exercise in combination with foods abundant in probiotics and prebiotics, can reduce oxidative stress and inflammation. Some foods (e.g., olive oil) increase antioxidant enzyme activities (CAT, SOD, and GPx). In addition, camel milk contains lactoferrin and antioxidants (bioactive peptides and vitamin C), which protect against oxidative stress in IBD patients.
Melatonin, a hormone synthesized in the pineal gland that regulates the sleep–wake cycle, can also block oxidative stress through its ability to cross physiological barriers, such as the mitochondrial membrane [54]. Melatonin’s antioxidant activity plays a protective role in the early and advanced stages of several diseases that involve ROS metabolites, including IBD [54,55,56].
The consumption of a healthy diet, including fruits and vegetables as well as medicinal plants abundant in β-carotene (pro-vitamin A), vitamin C, vitamin E, minerals, omega-3 polyunsaturated fatty acids, and polyphenols can reduce oxidative stress [57,58]. It is worth noting that some foods (e.g., olive oil) increase the amount of antioxidant enzymes such as CAT, SOD, and glutathione peroxidase (GPx) that can also prevent ROS production in the body [59,60].
In addition to fruits, vegetables, and medicinal plants that offer a variety of benefits to patients with IBD, camel milk provides an abundant source of minerals, vitamins, insulin, lactoferrin, and antioxidants (e.g., bioactive peptides) that have potential as a nutritional supplement for IBD patients [61,62,63]. With its antioxidant and anti-inflammatory properties, and its low content of sugar (lactose), protein (casein), saturated fat, and cholesterol, camel milk makes a valuable option for replenishing and improving IBD patients’ immune responses [64].
Moderate exercise and stress management techniques improve quality of life, reduce chronic inflammation, and enhance the immune response [37,65]. The study of Agarwal et al. showed that exercise training significantly reduced ROS production in animals’ hearts, as well as induced upregulation of antioxidant enzymes, which promoted a low-redox environment [66].
Probiotics, especially lactic acid bacteria, are living microorganisms involved in the balance of the microbiota in the gut [67,68]. There are a variety of foods rich in lactic acid bacteria, such as yogurt, kefir, sauerkraut, and kimchi [69,70,71]. Many beneficial biological effects are reported for probiotics belonging to the genera Lactobacillus and Bifidobacterium, particularly Lactococcus lactis, which has an antioxidative mechanism that involves scavenging oxidants, chelating metal ions, and preventing ROS formation [67,72].
Prebiotics are found in a variety of foods (fruits and vegetables) like fructo-oligosaccharides, which are non-digestible carbohydrates [73,74]. Prebiotics help the growth of beneficial bacteria in the gut, maintaining a balanced and healthy gut microbiota. The fermentation of prebiotics by Bifidobacteria has been linked to an improvement in oxidative parameters, which reduces free radicals. This effect can be explained by the production of hydrogen gas in the large intestine, which stimulates the growth of lactic acid bacteria [75,76].
4. Polyphenol-Rich Foods
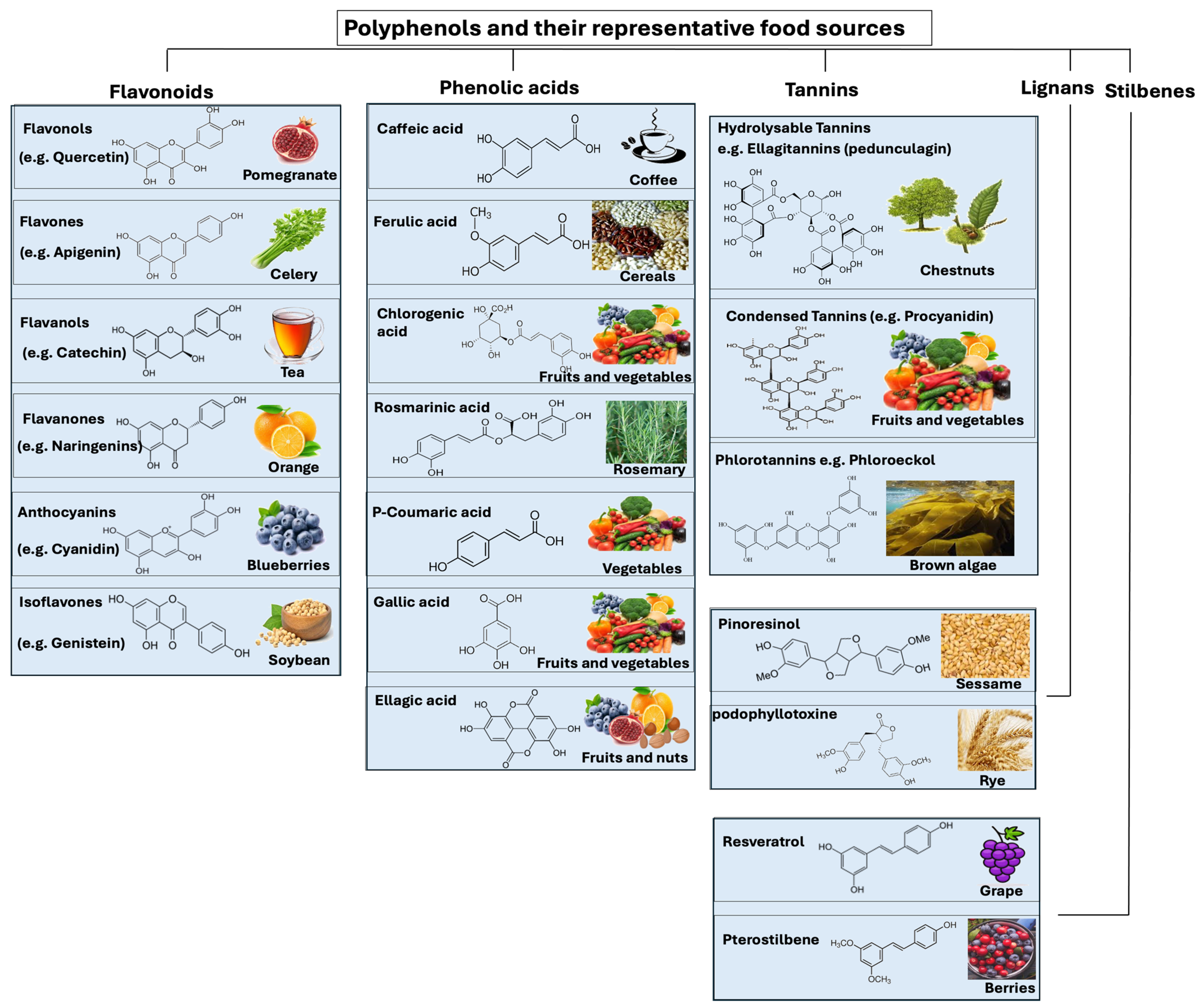
During the last decade, there has been a growing interest in polyphenol-rich foods and their potential role as antioxidants in preventing oxidative stress. Polyphenols are natural compounds found in plants, fruits, vegetables, and nuts (Figure 3). The antioxidant properties of these polyphenols include their ability to scavenge free radicals. Polyphenols protect plants from ultraviolet radiation and pathogens. Depending on how many phenol-rings the polyphenols contain, in addition to the structural elements that connect these rings together, polyphenols are classified into different groups such as flavonoids, phenolic acids, tannins, stilbenes, and lignans (Figure 3).

Figure 3.
Polyphenols and their representative food sources. Polyphenols are classified into different groups such as flavonoids, phenolic acids, tannins, stilbenes, and lignans.
Flavonoids are polyphenolic compounds found in plants, fruits, vegetables, and leaves. Several studies have demonstrated that diet-related polyphenols may protect against lifestyle-related diseases such as metabolic syndrome, atherosclerosis, coronary heart disease, and osteoporosis [77,78,79,80]. Two benzene rings are found in a flavonoid, which are linked to one another by a heterocyclic ring containing oxygen, which gives the flavonoid its structure [81]. In the case of flavonoids, the subclasses of flavonoid compounds include flavanols, flavanones, flavones, isoflavones, flavonols, and anthocyanidins [82]. Foods that contain flavonoids include fruits, vegetables, legumes, tea, dark chocolate, etc. Some foods have more flavonoids in specific parts, such as fruit peels. Flavonoid-containing foods can vary across countries based on culinary habits [83]. During digestion, flavonoids are metabolized through the intestine, and their metabolites are transported to the liver for further metabolic processing [81,84]. By entering the enterohepatic circulation through bile excretion, liver metabolites can be transported to target cells, metabolized by the microbiota into aglycones, or excreted through urine and feces [84]. Flavonoid metabolites that pass through the intestine but are not absorbed can be degraded by gut microbiota and reabsorbed [84]. In general, flavonoids have a low bioavailability, depending on their subclass [85]. Several approaches have been used to improve flavonoids’ bioavailability, including inhibition of enzymes that limit their availability, changes in the food matrix composition, and enhanced dissolution rates [86,87].
Phenolic acids are categorized into two groups belonging to benzoic acid and cinnamic acid hydroxy derivatives. They are bound in forms such as esters, amides, and glycosides. Lignin is one of the most common compounds containing hydroxybenzoic acids in their cell wall fractions. Gallic, syringic, p-hydroxybenzoic, and vanillic acids are well-known hydroxybenzoic acids [86]. The hydroxycinnamic acids (caffeic acid, ferulic acid, coumaric acid, chlorogenic acid, and isoferulic acid) are found in all parts of plants. They are most concentrated in ripe fruits and vegetables. Coffee contains high concentrations of chlorogenic acids, which are hydroxycinnamic acids obtained from the combination of caffeic and quinic acids. Considering phenolic acids’ role in diets, it has been shown that drinking coffee regularly reduces type 2 diabetes risk [88]. Additionally, the chlorogenic acid in coffee has prebiotic properties in vivo, which contribute to preventing obesity and lifestyle-related diseases [89,90]. A clinical study found a lower risk of advanced prostate cancer associated with higher intakes of caffeic acid and ferulic acid [91].
Tannins represent in plants a class of polyphenolic biomolecules with a high molecular weight (500 Da to 20 kDa) [92]. Tannins provide several benefits to plants. They act as a natural defense mechanism against herbivores, insects, and pathogens, deterring them from feeding on the plant. Tannins also play a role in regulating the plant’s growth and development, as well as helping to preserve the structural integrity of plant tissues [92,93]. The most common sources of tannins are bark, stems, roots, leaves, buds, seeds, as well as fruits and vegetables such as grapes, blackberries, strawberries, walnuts, cashew nuts, hazelnuts, mangoes, and tea. Tannins can be divided into three categories: hydrolyzable tannins, condensed tannins (also known as non-hydrolyzable tannins) and phlorotannins [94,95]. Hydrolyzable tannins are complex molecules that can be broken down by hydrolysis into smaller phenolic compounds [96]. A hydrolyzable tannin (e.g., chestnut wood extract) contains repeating structures of gallic (gallotannins) or ellagic (ellagitannins) acid with a sugar core [97]. One of the simplest hydrolyzable tannins is tannic acid, a mixture of digallic acid esters of glucose. Various applications of hydrolyzable tannins are possible, from human/animal nutrition to industrial processing [98]. As an example of its use in animal nutrition, it is well known that dietary supplementation with chestnut wood extract can improve broiler growth performance, nutrient digestibility, antioxidant status, immune response, and lipid metabolism [94,95].
Condensed tannins (e.g., mimosa, quebracho, pine, mangrove, and hemlock), also known as non-hydrolyzable tannins, are mainly composed of catechins and anthocyanidin aglycone scaffolds. Condensed tannins dominate the world market with more than 90% of the total commercial tannins, whereas hydrolyzable tannins are limited in nature [94,95]. Unlike hydrolyzable tannins, they cannot be broken down by hydrolysis. Plants use condensed tannins for protection against herbivores and pathogens, as they bind to proteins and prevent them from being digested [99,100]. Aside from contributing to the astringent taste of certain fruits and beverages, condensed tannins also possess antioxidant and anti-inflammatory properties [101].
In terms of phlorotannins, they are oligomers of phloroglucinol, a compound found in algae. Phlorotannins have the ability to bind heavy metals, such as cadmium and lead, thereby reducing environmental pollution [102,103].
Stilbenes are plant secondary metabolites derived from the phenylpropanoid pathway, associated with plant defense. These compounds can be found in several foods, including berries, grapes, and peanuts, and feature a distinctive structure consisting of two aromatic rings linked by an ethylene molecule. Resveratrol (cis and trans), found in high concentrations in the fresh skin of red grapes, is the main representative of stilbenes. Stilbenes’ health benefits include cardiovascular, anti-obesity, antidiabetic, chemopreventive, and neuroprotective effects [104,105].
Lignans are found in the seeds of oleaginous plants (such as flax seeds, sesame seeds, linseeds, and sunflower seeds). However, fibrous plants like rye, whole wheat, vegetables, and fruits also contain lignans, although in smaller amounts. Deglycosylation and demethylation of dietary lignans by the gut microbiota produce human lignan agents such as enterodiol and enterolactone. In recent years, many studies have pointed to the potential therapeutic properties of lignans and their derivatives in cancer chemotherapy and neurodegenerative diseases [105,106]. In terms of health benefit, lignans regulate cholesterol, prevent microbial infections, protect against cancer, improve athletic performance, and reduce inflammation [107,108,109,110,111]. Of note, flaxseed lignans (matairesinol, lariciresinol, and pinoresinol), which are mammalian estrogen precursors, are converted by anaerobic gut bacteria into enterolignans, enterodiol, and enterolactone [112]. As lignan metabolites bind to estrogen receptors, they affect estrogen function, reducing their circulation in the bloodstream and their biological activity, thereby reducing breast cancer risk [113,114]. Of note, excessive flaxseed consumption during pregnancy and breastfeeding can potentially lead to hormonal imbalances due to the high levels of phytoestrogens present in flaxseeds [115].
5. Polyphenol-Rich Foods and Their Role in Oxidative Stress Inhibition
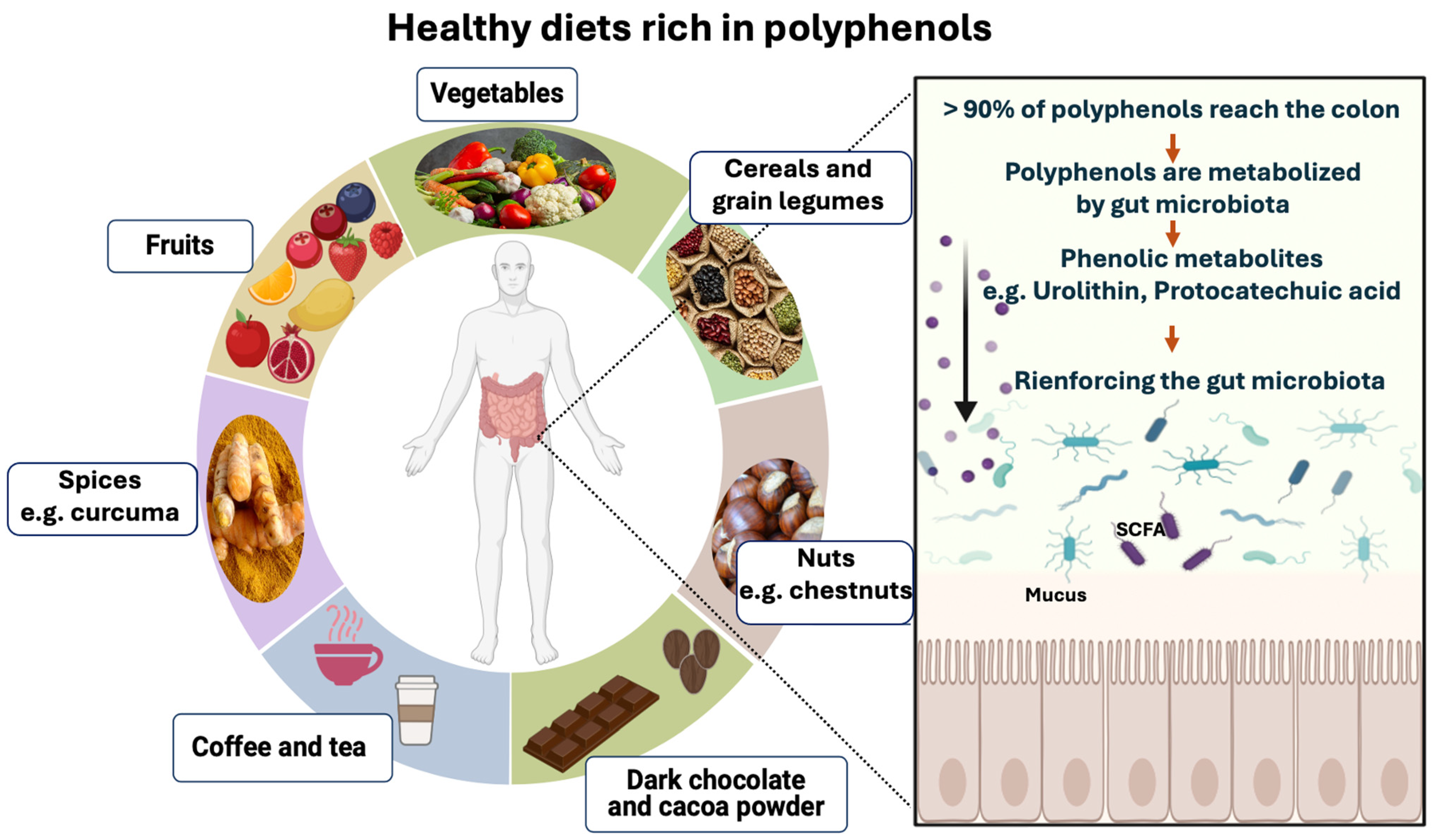
Several factors influence polyphenol-rich food absorption in the small intestine, including structural complexity and polymerization [116]. It is estimated that the small intestine absorbs less than 5–10% of ingested polyphenols (Figure 4). Leftover polyphenols (90–95%) can accumulate in the large intestine [116]. Then, the gut microbiota breaks down polyphenol glycosides to form aglycones through the opening of heterocycle rings. This catabolic process reduces polyphenol’s complex structure into low-molecular-weight phenolic metabolites that can be assimilated by gut cells [117,118]. Dietary phenolic substances, along with their aromatic metabolites, have the potential to enhance the gut microbiota community composition through their prebiotic effects (Figure 4) [117,118].

Figure 4.
An overview of polyphenol-rich foods and their potential effects on human health in association with gut microbiota. Polyphenols are compounds found in a wide variety of foods, including vegetables (e.g., artichoke heart, parsley, broccoli, celery, onion, garlic, lettuce, leek, zucchini, green bell pepper, tomato, cauliflower, etc.), fruits (e.g., blueberries, strawberries, raspberries, blackberries, cranberries, grapes, cherries, apricots, apples, pomegranates, oranges, grapefruits, etc.), cereals (e.g., wheat, rice, corn, rye, oat, etc.), grain legumes (e.g., beans, chickpeas, lentils, etc.), nuts (e.g., chestnuts, hazelnuts, pecan nuts, almonds, etc.), tea, coffee, dark chocolate, cocoa powder, and spices (e.g., curcuma or turmeric). For IBD patients in remission, it is important to reintroduce gradually restricted foods and drinks (vegetables, fruits, cereals, grain legumes, etc.) that contribute to a balanced diet in line with the “Eatwell Guide”. Polyphenols are mostly complex structures. Approximately 5–10% of food polyphenols are absorbed by the small intestine, while the majority (90–95%) reach the colon. They are then metabolized by the gut microbiota into absorbable simple phenolic compounds (e.g., urolithin, protocatechuic acid, etc.). Dietary phenolic compounds and their aromatic metabolites reinforce the gut microbiota through their prebiotic properties.
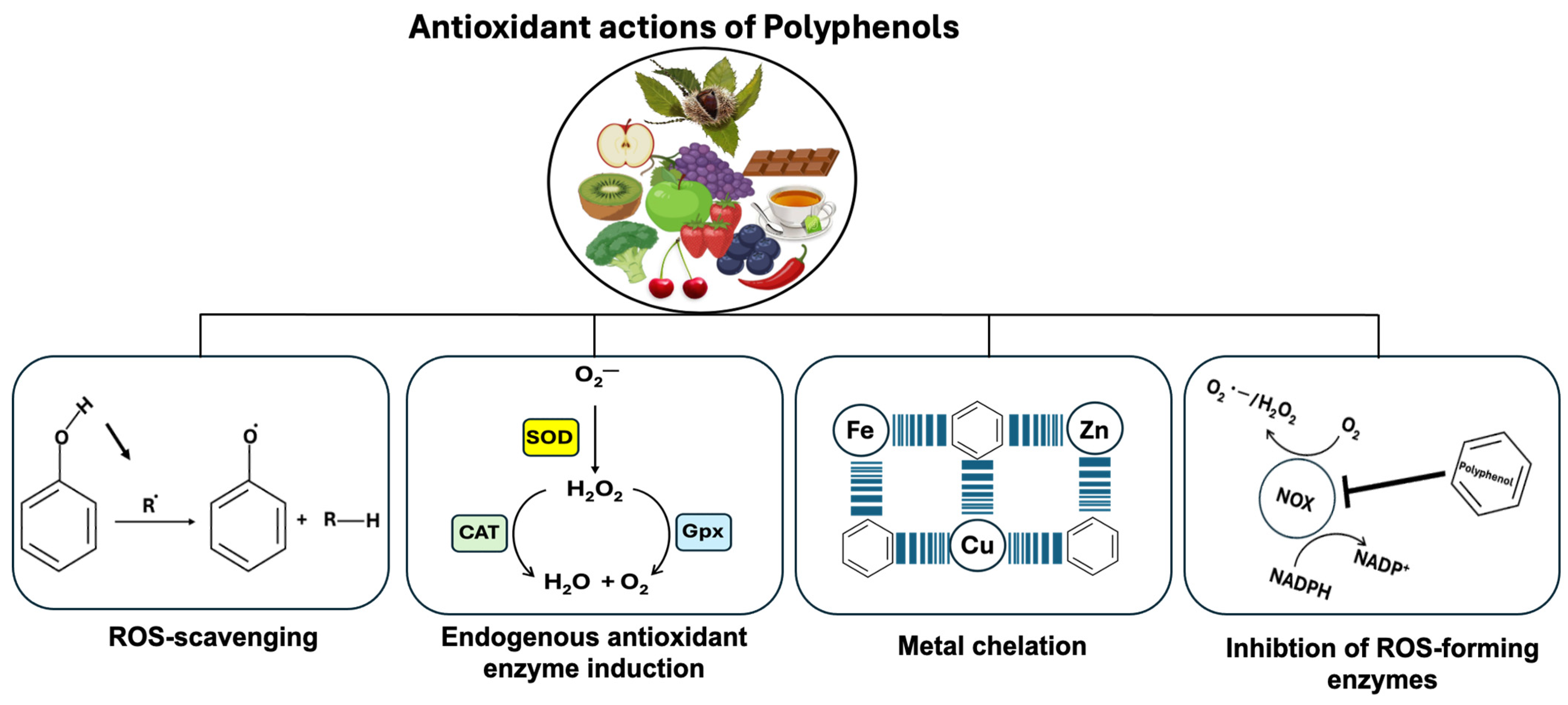
Polyphenols’ antioxidant properties are mediated by four major mechanisms (Figure 5). The first polyphenol action involves removing ROS directly, whereas the second increases endogenous antioxidant-synthesizing enzymes (Figure 5). As a third action, polyphenols prevent ROS formation by directly acting as metal ion chelators against transition metal ions such as copper (Cu), zinc (Zn), and iron (Fe) that play a critical role in the progression of different diseases including Alzheimer’s disease. For the fourth action, the polyphenol inhibits several enzymes involved in ROS formation (iNOS, NOX, and LOX). For example, NOX enzymes produce superoxide anion radicals (O2.−), while resveratrol treatment of macrophages inhibits LPS-induced Nox1 expression and ROS production [119].

Figure 5.
Polyphenols‘ antioxidant properties are mediated by four major mechanisms. Polyphenols can remove ROS directly, while a second action can be attributed to their ability to increase endogenous antioxidant-synthesizing enzymes. Catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD) are the most important antioxidant enzymes in cells. SOD and CAT are enzymes that catalyze the breakdown of superoxide and hydrogen peroxide, while GPx is an enzyme that catalyzes the reduction of hydrogen peroxide. As a third action, polyphenols prevent ROS formation by directly acting as metal ion chelators against transition metal ions such as copper (Cu), zinc (Zn), and iron (Fe) that play a critical role in the progression of different diseases including Alzheimer’s disease. For the fourth action, the polyphenol inhibits several enzymes involved in ROS formation (iNOS, NOX, and LOX). For example, the superoxide anion radical (O2.−) is produced by members of the NADPH oxidase (NOX) enzyme family, while resveratrol treatment inhibits Nox1 expression and ROS production in macrophages.
Whenever the amount of ROS produced by cells increases, the cellular defense mechanism against oxidative stress is activated to clear the cells of ROS [120]. In terms of endogenous antioxidant enzyme activity, several enzymes are involved in the cellular antioxidant defense system, including CAT, SOD, GPx, peroxiredoxins (Prxs), and NADPH ubiquinone oxidoreductase (NQO1) [121]. As well as antioxidant enzymes, there are non-enzymatic compounds that reduce ROS, such as glutathione (GSH), ascorbic acid (vitamin C), and tocopherol (vitamin E). During oxidative stress, SODs convert O2.− to H2O2 which is then eliminated by Prxs, GPx, and CAT. In this case, H2O2 is converted into H2O and O2 by CAT to protect cells against oxidative stress [122].
6. Impact of Polyphenol-Rich Foods on Opportunistic Yeast Candida albicans Growth Inhibition
In addition to polyphenols’ properties in preventing ROS generation, maintaining intestinal homeostasis, and boosting the biodiversity and health of the microbiota, they have also been shown to contribute to the elimination of pathogens, such as Candida albicans (Figure 6). This yeast is an opportunistic fungus that lives in humans’ vaginal and intestinal tracts [123,124,125]. This microorganism is the most common cause of yeast infections in humans. C. albicans infections can range from superficial to systemic and life-threatening [125]. Different factors may facilitate C. albicans overgrowth and its migration from the gut to the vital organs. These factors include changes in the gut microbiota, epithelial barrier ruptures, and immune system dysfunction [124]. Another important aspect is the fact that C. albicans overgrowth is associated with inflammatory diseases [126,127,128]. Evidence from experiments and clinical studies suggests a link between C. albicans and CD [129,130]. Of note, unhealthy diets high in fats and sugars, particularly processed foods consumed in Western countries, affect the gut microbiota, contributing to intestinal inflammation and fungal overgrowth [68,131]. The antifungal properties of polyphenol-rich foods have attracted considerable attention in recent years, especially those belonging to the flavonoid class (flavanols and flavonols) [132,133,134]. These polyphenols inhibit virulence factors such as biofilm formation, C. albicans filamentation, modulation of the fungal cell wall, and reduction of fungal adhesion to host cells [132,133,134].

Figure 6.
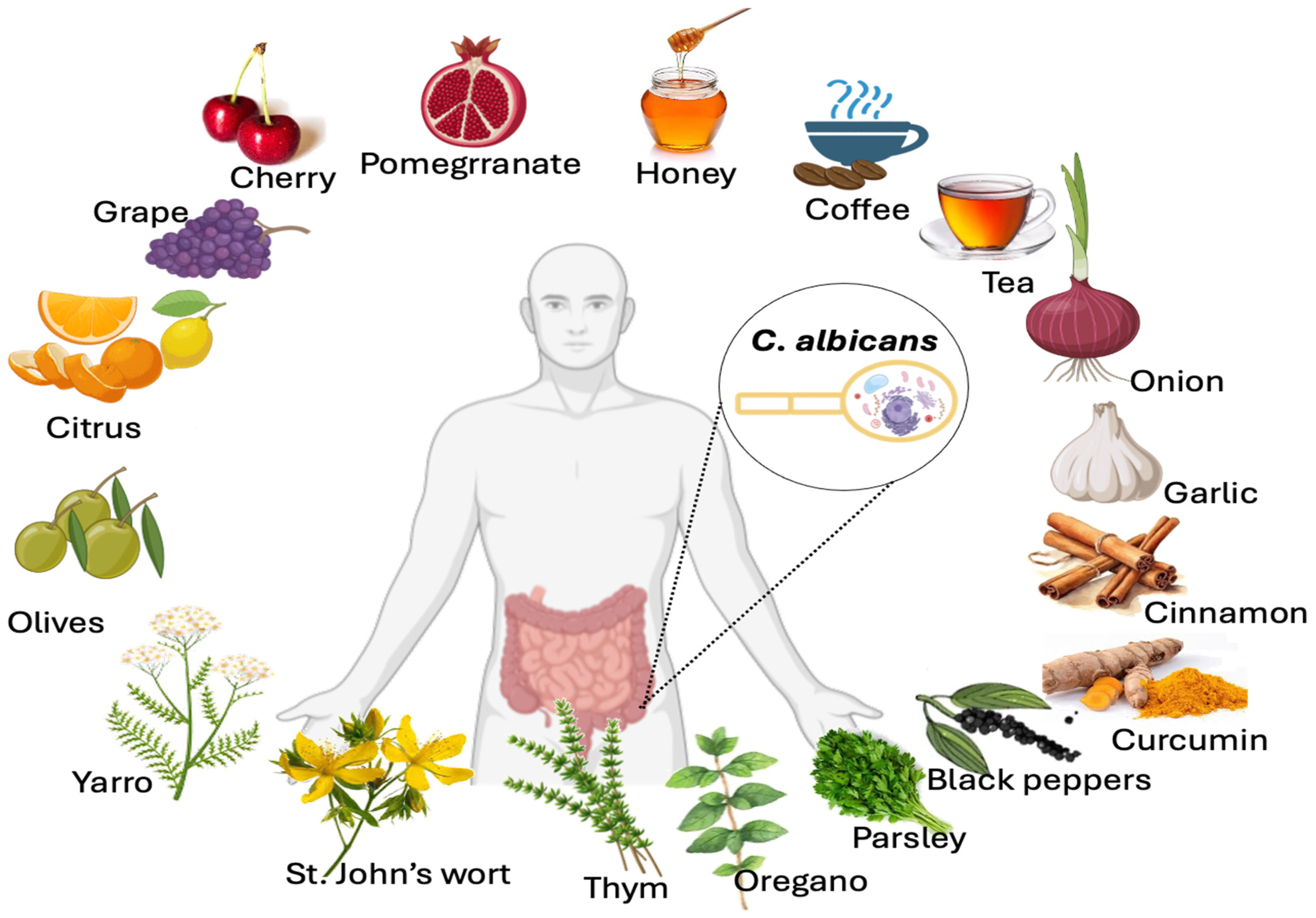
Effect of some representative polyphenol-rich foods on C. albicans growth inhibition. Food and spices rich in polyphenols that inhibit C. albicans growth include curcumin (curcuminoids), black pepper (p-hydroxybenzoic acid), cinnamon (proanthocyanidins), oolong tea or green tea (e.g., catechins), yarrow, St. John’s wort, oregano, thyme (carvacrol and thymol), coffee (phenolic acids), parsley (flavonoids), garlic (phenolic acids and flavonoids), onions (anthocyanins and flavonols), honey (flavonoids), cherries (procyanidins and quercetin), grapes (resveratrol), citrus fruits (naringin), pomegranates (caffeic acid, gallic acid, and epigallocatechin), and olives (hydroxytyrosol).
Food and spices rich in polyphenols that inhibit C. albicans growth include curcumin (curcuminoids), black pepper (p-hydroxybenzoic acid), cinnamon (proanthocyanidins), oolong tea or green tea (e.g., catechins), yarrow, St. John’s wort, winter savory, or willow gentian, thyme (carvacrol and thymol), coffee (phenolic acids), parsley (flavonoids), garlic (phenolic acids and flavonoids), onions (anthocyanins and flavonols), honey (flavonoids), cherries (procyanidins and quercetin), grapes (resveratrol), citrus fruits (naringin), pomegranates (caffeic acid, gallic acid, and epigallocatechin), and olives (hydroxytyrosol).
Curcumin, curcuminoids are one of the main compounds found in Curcuma longa rhizome (turmeric). Gut microbiota is directly regulated by curcumin, and curcumin itself is bio-transformed into active metabolites by gut microbiota [135]. In terms of curcumin’s role in fungal growth inhibition, the study of Shahzad et al. showed that curcumin inhibits the expression of hwp1 and als3 that affect C. albicans biofilm formation [136].
Black pepper contains piperine, a bioavailability enhancer, which increases curcumin’s bioavailability by 2000% [137]. One of the most prominent phenolic acids in black pepper is p-hydroxybenzoic acid [138]. Of note, piperine demonstrated concentration-dependent antibiofilm activity of C. albicans [139].
Cinnamon, commercial cinnamon contains the polyphenol proanthocyanidins, which may contribute to the health benefits [140]. A study conducted by Atai et al. found that Absinthium artemisia, eucalyptus, onion, cinnamon, curcumin, sage, mint, and Calendula officinalis all showed antifungal activity against C. albicans strains. Cinnamon was more potent and effective than onion, mint, C. officinalis, and sage. In contrast, curcumin, A. artemisia, and eucalyptus showed similar antifungal effects [141].
Tea, the antioxidant activity of black tea is lower than that of oolong teas or green teas as it contains the lowest level of polyphenols (catechins) [142]. Additionally, green tea was shown to have the highest antimicrobial properties, particularly against C. albicans compared to other types of tea [142]. It has been shown that catechins and flavins in black tea are both anti-Candida agents and inhibit all Candida species tested with the lowest MIC of 6.25 µg/mL observed for C. albicans (Figure 6) [143]. These catechins and flavins in black tea cause cell wall damage to C. albicans [143].
Many medicinal plants, traditionally used to treat digestive problems, have been shown to suppress pathogen growth, including C. albicans, and to promote the growth of probiotic bacteria, such as yarrow, St. John’s wort, winter savory, or willow gentian extracts [144]. Additionally, carvacrol and thymol from thyme and oregano both exhibit strong fungicidal properties against all Candida isolates through inhibition of ergosterol biosynthesis as well as disruption of the integrity of the membrane of C. albicans [145]. Thymol is not only effective in inhibiting C. albicans adhesion to host cells, but it has also been shown to be effective in preventing fungal infections in host cells [146,147].
Coffee, having the remarkable contents of phenolic acids, represents one of the most consumed beverages and is also a major contributor to dietary antioxidant intake [148]. A significant reduction in the content of ergosterol, chitin, and β-glucan in Candida species was observed following treatment with spent coffee grounds, suggesting that these extracts target the synthesis of membranes and cell walls of Candida species [149].
Parsley, due to its high polyphenol content, especially of flavonoids, demonstrates antibacterial properties [133]. A study conducted by Arismunandar et al. showed that parsley (Petroselinum crispum) extract inhibits C. albicans growth [133].
Garlic, many phytomolecules found in garlic are biologically active, including organosulfur compounds, phenolic acids, allyl thiosulfinates, flavonoids, and vitamins [150]. Through its antioxidant activities (phenolic acids and flavonoids), garlic protects against ROS generation in the body. Additionally, allicin, allyl cysteine, allyl disulfide, and alliin are the four main compounds constituting garlic compounds that scavenge free radicals. Inhibition of succinate dehydrogenase is one mechanism by which allicin inhibits fungi growth [151]. Garlic has been reported to affect the lipid composition of the outer surface of C. albicans [152]. Furthermore, garlic extract inhibits C. albicans growth by forming pits on its surface [151].
Onions, anthocyanins and flavonols are the main flavonoid classes found in onions. Some onion varieties are red due to anthocyanins. Onions have yellow and brown skin due to flavonols such as quercetin. The research of Doddanna et al. showed that onion leaves and bulb extracts inhibit C. albicans growth [153].
In comparison with onion or honey alone, onion juice extracted from red Egyptian onion reduced the growth of C. albicans, while the honey–onion mixture was significantly more effective than onion juice alone [154]. Honey polyphenols, specifically flavonoids, have been found to possess natural antifungal properties that effectively combat C. albicans by protecting against the yeast-to-hyphal transition that occurs in C. albicans [134].
In addition to plants and herbs, fruits also contain polyphenols that play an antifungal role in preventing C. albicans by acting as an antifungal [155,156,157].
Cherry (Prunus cerasus L.) is an excellent source of procyanidins and quercetin that attenuate C. albicans adherence to the oral cavity epithelium [155].
Resveratrol derived from grapes displays potent fungicidal activity by significantly increasing intracellular trehalose content in the cells. This trehalose accumulation was induced by stress responses to resveratrol action, and C. albicans showed an arrest in their cell-cycle processes at S phase [156].
Naringin, a flavonoid found in citrus fruits, exhibits potent antifungal properties due to its ability to disrupt mitochondrial function in C. albicans. This disruption ultimately leads to mitochondrial dysfunction and triggers apoptosis in fungal cells [132].
Pomegranate (Punica granatum L.) juice has high polyphenol content, including caffeic acid, gallic acid, and epigallocatechin [158]. The pomegranate peel contains ellagitannin, flavonoids, triterpenes, and phenols which have been shown to have an antibacterial effect. Pomegranate peel inhibits fungal growth and C. albicans biofilm formation [157].
Polyphenol-rich Mediterranean fruits include olives (Olea europaea). Hydroxytyrosol is the main phenolic component in olives that contributes to their health benefits [159]. Hydroxytyrosol acts as a radical scavenger and induces apoptosis in cancer cells. Additionally, hydroxytyrosol exhibits antimicrobial properties [160]. The study conducted by Zoric et al. showed that hydroxytyrosol inhibits the transition of unicellular C. albicans yeast into filamentous forms and induces changes in the hydrophobicity of cell surfaces, factors affecting C. albicans adhesion to cell hosts [161].
7. Conclusions
In summary, the production of ROS is increased during inflammation by immune cells, such as monocytes and neutrophils, which causes further tissue damage. Oxidative stress, associated with increased ROS generation, is a major risk factor for IBD pathogenesis and progression. Additionally, a decrease in antioxidant enzyme expression (CAT, GPx, and SOD) in the colonic mucosa, submucosa, and serosa has been observed in IBD patients.
Currently, industrialized lifestyles expose us to a variety of exogenous factors (smoking, processed food, hydrogenated oils, alcohol, chronic stress, air pollution, heavy metals, and UV light) and endogenous conditions (mitochondria and phagocyte NADPH oxidases) that deteriorate the digestive tract, especially in IBD patients. These unhealthy factors can trigger chronic inflammation and oxidative stress, which further exacerbate IBD pathogenesis and progression.
Many alternative therapeutic strategies have been developed against oxidative stress along with conventional therapy to alleviate IBD pathogenesis. Some alternative therapeutic strategies that reduce oxidative stress in IBD include antioxidants, such as LOX, NOX inhibitors and melatonin. Furthermore, healthy lifestyle changes, such as adopting a healthy diet rich in fruits and vegetables as well as medicinal plants abundant in β-carotene (pro-vitamin A), vitamin C, vitamin E, minerals, omega-3 polyunsaturated fatty acids, and polyphenols. Additionally, regular exercise combined with food rich in probiotics and prebiotics can reduce oxidative stress and inflammation. Of note, the natural presence of antioxidants (bioactive peptides) and lactoferrin in some foods (e.g., camel milk) provides a potential opportunity for protection against oxidative stress in IBD patients.
Polyphenol-rich foods have attracted growing interest from scientists due to their antioxidant properties. Polyphenols are natural compounds found in plants, fruits, vegetables, and nuts. These polyphenol-rich foods exhibit antioxidant properties and protect the body from oxidative damage caused by free radicals. Polyphenols, particularly those belonging to the flavonoid class (flavanols and flavonols), have been shown to possess antifungal properties, particularly against C. albicans. Polyphenol-rich foods that inhibit C. albicans growth include curcumin, black pepper, cinnamon, oolong tea or green tea (e.g., catechins), yarrow, St. John’s wort, winter savory, or willow gentian, thyme (carvacrol and thymol), coffee, parsley, garlic, onions (anthocyanins and flavonols), honey (flavonoids), cherries, grapes (resveratrol), citrus fruits (naringin), pomegranates (caffeic acid, gallic acid, and epigallocatechin), and olives (hydroxytyrosol). These polyphenols inhibit virulence factors such as biofilm formation, C. albicans filamentation, modulation of the fungal cell wall, and reduce fungal adhesion to cell host.
Overall, a healthy and balanced diet abundant in polyphenols along with regular physical activity and practicing stress management techniques and avoiding the industrialized lifestyles of our modern society (the factors listed above) can minimize oxidative stress damage and prevent infectious diseases. Various factors influence polyphenol absorption in the gut, including polyphenol type, dietary components, gut microbiota composition, and individual gut health differences. Identification of these factors may enable us to develop strategies for improving polyphenol gut absorption and maximizing their health benefits.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Dubuquoy, L.; Rousseaux, C.; Thuru, X.; Peyrin-Biroulet, L.; Romano, O.; Chavatte, P.; Chamaillard, M.; Desreumaux, P. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut 2006, 55, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Mendall, M.; Karagiannis, D.; Karatzas, P.; Ipenburg, N.; Sebastian, S.; Rizzello, F.; Limdi, J.; Katsanos, K.; et al. European Crohn’s and Colitis Organisation Topical Review on IBD in the Elderly. J. Crohns Colitis 2017, 11, 263–273. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, A.; Korzenik, J. Environmental triggers for IBD. Curr. Gastroenterol. Rep. 2014, 16, 396. [Google Scholar] [CrossRef] [PubMed]
- Gabbani, T.; Deiana, S.; Marocchi, M.; Annese, V. Genetic risk variants as therapeutic targets for Crohn’s disease. Expert. Opin. Ther. Targets 2017, 21, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; Yuan, S.; Wang, X.; Theodoratou, E.; Li, X.; et al. Intake of Ultra-processed Foods Is Associated with an Increased Risk of Crohn’s Disease: A Cross-sectional and Prospective Analysis of 187 154 Participants in the UK Biobank. J. Crohns Colitis 2023, 17, 535–552. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z.; Xu, C.; Kan, S.; Chen, D. Disturbances of the gut microbiota and microbiota-derived metabolites in inflammatory bowel disease. Nutrients 2022, 14, 5140. [Google Scholar] [CrossRef]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014, 20, 1192. [Google Scholar] [CrossRef]
- Swidsinski, A.; Weber, J.; Loening-Baucke, V.; Hale, L.P.; Lochs, H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 2005, 43, 3380–3389. [Google Scholar] [CrossRef]
- Hansen, R.; Russell, R.K.; Reiff, C.; Louis, P.; McIntosh, F.; Berry, S.H.; Mukhopadhya, I.; Bisset, M.W.; Barclay, A.R.; Bishop, J. Microbiota of De-Novo Pediatric IBD: Increased Faecali bacterium Prausnitziiand Reduced Bacterial Diversity in Crohn’s But Not in Ulcerative Colitis. Am. J. Gastroenterol. 2012, 107, 1913–1922. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Barnich, N.; Denizot, J.; Darfeuille-Michaud, A.E. coli-mediated gut inflammation in genetically predisposed Crohn’s disease patients. Pathol. Biol. 2013, 61, e65–e69. [Google Scholar] [CrossRef]
- Guzik, T.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Herulf, M.; Ljung, T.; Hellström, P.; Weitzberg, E.; Lundberg, J. Increased luminal nitric oxide in inflammatory bowel disease as shown with a novel minimally invasive method. Scand. J. Gastroenterol. 1998, 33, 164–169. [Google Scholar] [CrossRef]
- Zhou, G.X.; Liu, Z.J. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017, 18, 495–503. [Google Scholar] [CrossRef]
- Neish, A.S. Mucosal immunity and the microbiome. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. S1), S28–S32. [Google Scholar] [CrossRef]
- Rom, O.; Avezov, K.; Aizenbud, D.; Reznick, A.Z. Cigarette smoking and inflammation revisited. Respir. Physiol. Neurobiol. 2013, 187, 5–10. [Google Scholar] [CrossRef]
- Baskara, I.; Kerbrat, S.; Dagouassat, M.; Nguyen, H.Q.; Guillot-Delost, M.; Surenaud, M.; Baillou, C.; Lemoine, F.M.; Morin, D.; Boczkowski, J.; et al. Cigarette smoking induces human CCR6+Th17 lymphocytes senescence and VEGF-A secretion. Sci. Rep. 2020, 10, 6488. [Google Scholar] [CrossRef]
- Quetglas-Llabres, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Mateos, D.; Ugarriza, L.; Gomez, C.; Tur, J.A.; Sureda, A. Oxidative stress and inflammatory biomarkers are related to high intake of ultra-processed food in old adults with metabolic syndrome. Antioxidants 2023, 12, 1532. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Mazúr, M.; Rapta, P.; Bilton, R.F. Oxygen free radical generating mechanisms in the colon: Do the semiquinones of vitamin K play a role in the aetiology of colon cancer? Biochim. Biophys. Acta (BBA)-General. Subj. 2001, 1527, 161–166. [Google Scholar] [CrossRef]
- Bryk, D.; Zapolska-Downar, D.; Malecki, M.; Hajdukiewicz, K.; Sitkiewicz, D. Trans fatty acids induce a proinflammatory response in endothelial cells through ROS-dependent nuclear factor-kappaB activation. J. Physiol. Pharmacol. 2011, 62, 229–238. [Google Scholar]
- Haag, F.; Janicova, A.; Xu, B.; Powerski, M.; Fachet, M.; Bundkirchen, K.; Neunaber, C.; Marzi, I.; Relja, B.; Sturm, R. Reduced phagocytosis, ROS production and enhanced apoptosis of leukocytes upon alcohol drinking in healthy volunteers. Eur. J. Trauma. Emerg. Surg. 2022, 48, 2689–2699. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol. Res. Health 2003, 27, 277–284. [Google Scholar]
- Bailey, S.M.; Cunningham, C.C. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic. Biol. Med. 2002, 32, 11–16. [Google Scholar] [CrossRef]
- Nordmann, R.; Ribière, C.; Rouach, H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic. Biol. Med. 1992, 12, 219–240. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Poznyak, A.V.; Nikiforov, N.G.; Sobenin, I.A.; Orekhov, A.N. The link between chronic stress and accelerated aging. Biomedicines 2020, 8, 198. [Google Scholar] [CrossRef]
- Herbet, M.; Korga, A.; Gawrońska-Grzywacz, M.; Izdebska, M.; Piątkowska-Chmiel, I.; Poleszak, E.; Wróbel, A.; Matysiak, W.; Jodłowska-Jędrych, B.; Dudka, J. Chronic variable stress is responsible for lipid and DNA oxidative disorders and activation of oxidative stress response genes in the brain of rats. Oxid. Med. Cell. Longev. 2017, 2017, 7313090. [Google Scholar] [CrossRef]
- Gardiner, P.; Sadikova, E.; Filippelli, A.C.; Mitchell, S.; White, L.F.; Saper, R.; Kaptchuk, T.J.; Jack, B.W.; Fredman, L. Stress Management and Relaxation Techniques use among underserved inpatients in an inner city hospital. Complement. Ther. Med. 2015, 23, 405–412. [Google Scholar] [CrossRef]
- Milne, B.; Joachim, G.; Niedhardt, J. A stress management programme for inflammatory bowel disease patients. J. Adv. Nurs. 1986, 11, 561–567. [Google Scholar] [CrossRef]
- Miedziun, P.; Czabała, J.C. Stress management techniques. Arch. Psychiatry Psychother. 2015, 17, 23–31. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J. Introductory lecture: Atmospheric chemistry in the Anthropocene. Faraday Discuss. 2017, 200, 11–58. [Google Scholar] [CrossRef]
- Lelieveld, J. Clean air in the Anthropocene. Faraday Discuss. 2017, 200, 693–703. [Google Scholar] [CrossRef]
- Jin, S.P.; Li, Z.; Choi, E.K.; Lee, S.; Kim, Y.K.; Seo, E.Y.; Chung, J.H.; Cho, S. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018. [Google Scholar] [CrossRef]
- Cho, C.-C.; Hsieh, W.-Y.; Tsai, C.-H.; Chen, C.-Y.; Chang, H.-F.; Lin, C.-S. In vitro and in vivo experimental studies of PM2.5 on disease progression. Int. J. Environ. Res. Public. Health 2018, 15, 1380. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, Z.M.; Henneke, P.; Kolter, J. From flies to men: ROS and the NADPH oxidase in phagocytes. Front. Cell Dev. Biol. 2021, 9, 628991. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer. 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Allergy Clin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.K.; Kumar, R. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 2015, 30, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Ashique, S.; Mishra, N.; Garg, A.; Sibuh, B.Z.; Taneja, P.; Rai, G.; Djearamane, S.; Wong, L.S.; Al-Dayan, N.; Roychoudhury, S.; et al. Recent updates on correlation between reactive oxygen species and synbiotics for effective management of ulcerative colitis. Front. Nutr. 2023, 10, 1126579. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Abreu, M.T.; Cohen, R.; Tremaine, W. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006, 130, 940–987. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, R.P. Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol. Rep. 2012, 64, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Probst, L.; Dächert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, A.; Triantafillidis, J. Melatonin: A potent antioxidant agent with anti-inflammatory and anti-apoptotic effects that might be useful in the treatment of IBD patients. Ann. Gastroenterol. 2009, 10–12. [Google Scholar]
- Li, J.-H.; Yu, J.-P.; Yu, H.-G.; Xu, X.-M.; Yu, L.-L.; Liu, J.; Luo, H.-S. Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediat. Inflamm. 2005, 2005, 185. [Google Scholar] [CrossRef]
- Zhu, D.; Ma, Y.; Ding, S.; Jiang, H.; Fang, J. Effects of melatonin on intestinal microbiota and oxidative stress in colitis mice. Biomed. Res. Int. 2018, 2018, 2607679. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and oxidative stress: Friend or foe? Oxid. Med. Cell Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Diet Antioxidant Capacity: Relationships to Oxidative Stress and Health. Am. J. Med. Sci. 2013, 5, 126–139. [Google Scholar] [CrossRef]
- Okvenda, A.Z.; Yerizel, E. Olive oil increase catalase activity and gluthatione peroxidase level in hyperglycemic rats. Acta Biochim. Indones. 2023, 6, 137. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Monticolo, R.; D’Amico, A.; Stefanini, L.; Pagano, F.; Pastori, D.; Cangemi, R. Antioxidant activity from extra virgin olive oil via inhibition of hydrogen peroxide–mediated NADPH-oxidase 2 activation. Nutrition 2018, 55, 36–40. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Eid, A.H.; Omar, H.A.; Arafa, E.-S.A.; Maghrabi, I.A. Camel’s milk ameliorates TNBS-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem. Toxicol. 2014, 69, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, M.M.; Al-Ghariebeh, R.B.; Alhaija, A.A.A.; Al-Zoubi, H.; Al-Qaoud, K.M. Camel milk whey inhibits inflammatory colorectal cancer development via down regulation of pro-inflammatory cytokines in induced AOM/DSS mouse model. Emir. J. Food Agric. 2019, 31, 256–262. [Google Scholar] [CrossRef]
- Awais, M.A.; Arif, A.; Asif, R.; Hussain, M.I.; Niaz, A. A review on camel milk and its medicinal properties. Riphah J. Allied Health Sci. 2022, 1. [Google Scholar] [CrossRef]
- Arain, M.A.; Khaskheli, G.B.; Shah, A.H.; Marghazani, I.B.; Barham, G.S.; Shah, Q.A.; Khand, F.M.; Buzdar, J.A.; Soomro, F.; Fazlani, S.A. Nutritional significance and promising therapeutic/medicinal application of camel milk as a functional food in human and animals: A comprehensive review. Anim. Biotechnol. 2023, 34, 1988–2005. [Google Scholar] [CrossRef]
- Briones, A.M.; Touyz, R.M. Moderate exercise decreases inflammation and oxidative stress in hypertension: But what are the mechanisms? Hypertension 2009, 54, 1206–1208. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Haque, M.; Sriramula, S.; Mariappan, N.; Pariaut, R.; Francis, J. Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension 2009, 54, 1393–1400. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Jawhara, S. Healthy diet and lifestyle improve the gut microbiota and help combat fungal infection. Microorganisms 2023, 11, 1556. [Google Scholar] [CrossRef]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as probiotic carrier food. Int. Dairy. J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Park, K.-Y.; Jeong, J.-K.; Lee, Y.-E.; Daily, J.W., III. Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir: A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, D.; Natesan, S.; Billamboz, M.; Jawhara, S. Role of bacteria-derived exopolysaccharides in inflammatory bowel disease with a special focus on cyanobacterial exopolysaccharides. Appl. Microbiol. 2024, 4, 250–274. [Google Scholar] [CrossRef]
- Wang, C.H.; Lai, P.; Chen, M.E.; Chen, H.L. Antioxidative capacity produced by Bifidobacterium-and Lactobacillus acidophilus-mediated fermentations of konjac glucomannan and glucomannan oligosaccharides. J. Sci. Food Agric. 2008, 88, 1294–1300. [Google Scholar] [CrossRef]
- Chen, H.-L.; Wang, C.-H.; Kuo, Y.-W.; Tsai, C.-H. Antioxidative and hepatoprotective effects of fructo-oligosaccharide in d-galactose-treated Balb/cJ mice. Br. J. Nutr. 2011, 105, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Calabro, V.; Prince, P.D.; Litterio, M.C.; Piotrkowski, B.; Vazquez-Prieto, M.A.; Miatello, R.M.; Oteiza, P.I.; Fraga, C.G. Flavonoids and metabolic syndrome. Ann. N. Y. Acad. Sci. 2012, 1259, 87–94. [Google Scholar] [CrossRef]
- Amiot, M.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hollman, P.; Katan, M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tousoulis, D.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Basdra, E.; Papavassiliou, A.; Stefanadis, C. Flavonoids in atherosclerosis: An overview of their mechanisms of action. Curr. Med. Chem. 2013, 20, 2641–2660. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Wang, T.-y.; Li, Q.; Bi, K.-s. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of polyphenols and flavonoids in the era of precision medicine. Mol. Pharm. 2017, 14, 2861–2863. [Google Scholar] [CrossRef]
- Ravisankar, S.; Agah, S.; Kim, H.; Talcott, S.; Wu, C.; Awika, J. Combined cereal and pulse flavonoids show enhanced bioavailability by downregulating phase II metabolism and ABC membrane transporter function in Caco-2 model. Food Chem. 2019, 279, 88–97. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregório, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef]
- Huxley, R.; Lee, C.M.Y.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.L.; dePaula, J.; Silva, C.M.; Cruz, A.; Miguel, M.A.L.; Farah, A. Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.-J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.I.; Campisi, D.; Di Mauro, M.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary Consumption of Phenolic Acids and Prostate Cancer: A Case-Control Study in Sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants—Hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Robbins, C.; Hanley, T.; Hagerman, A.; Hjeljord, O.; Baker, D.; Schwartz, C.; Mautz, W. Role of tannins in defending plants against ruminants: Reduction in protein availability. Ecology 1987, 68, 98–107. [Google Scholar] [CrossRef]
- Tanner, G. Condensed tannins. Plant pigments and their manipulation. Ann. Plant Rev. 2004, 14, 150–184. [Google Scholar] [CrossRef]
- Porter, L.J. Structure and chemical properties of the condensed tannins. In Plant Polyphenols: Synthesis, Properties, Significance; Basic Life Sciences; Springer: Boston, MA, USA, 1992; pp. 245–258. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed. Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Turchetti, B.; Mulinacci, N.; Vincieri, F.F.; Buzzini, P. Analysis of condensed and hydrolysable tannins from commercial plant extracts. J. Pharm. Biomed. Anal. 2006, 41, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.P.; Clausen, T.P.; MacLean, S.F., Jr.; Redman, A.M.; Reichardt, P.B. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 1997, 78, 1696–1712. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini-Rev. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Zhang, R.; Richardson, J.J.; Masters, A.F.; Maschmeyer, T. Removal of Pb2+ from water using sustainable brown seaweed phlorotannins. Langmuir 2022, 38, 8324–8333. [Google Scholar] [CrossRef] [PubMed]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- D Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita. 2007, 43, 348. [Google Scholar] [PubMed]
- Pérez-Chabela, M.L.; Hernández-Alcántara, A.M. Agroindustrial coproducts as sources of novel functional ingredients. In Food Processing for Increased Quality and Consumption; Elsevier: Amsterdam, The Netherlands, 2018; pp. 219–250. [Google Scholar]
- Zuiter, A. Proanthocyanidin: Chemistry and biology: From phenolic compounds to proanthocyanidins. In Module in Chemistry, Molecular Sciences and Chemical Engineering, 1st ed.; Reedijk, J., Ed.; Elsevier: Cambridge, MA, USA, 2014; Volume 1, pp. 1–29. [Google Scholar] [CrossRef]
- Almario, R.U.; Karakas, S.E. Lignan content of the flaxseed influences its biological effects in healthy men and women. J. Am. Coll. Nutr. 2013, 32, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and their derivatives from plants as antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The what and who of dietary lignans in human health: Special focus on prooxidant and antioxidant effects. Trends Food Sci. Technol. 2020, 106, 382–390. [Google Scholar] [CrossRef]
- Mridula, D.; Singh, K.; Barnwal, P. Development of omega-3 rich energy bar with flaxseed. J. Food Sci. Technol. 2013, 50, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and gut microbiota: An interplay revealing potential health implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Zhang, L.-y.; Wang, X.-l.; Sun, D.-x.; Liu, X.-x.; Hu, X.-y.; Kong, F. Regulation of zinc transporters by dietary flaxseed lignan in human breast cancer xenografts. Mol. Biol. Rep. 2008, 35, 595–600. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A potential functional food source. J. Food Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.; Chen, J.; Thompson, L.U. Flaxseed and its lignan precursor, secoisolariciresinol diglycoside, affect pregnancy outcome and reproductive development in rats. J. Nutr. 1998, 128, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Fu, H.; Sun, H.; Affoh, R.; Yu, H. The impact of polyphenol on general nutrient metabolism in the monogastric gastrointestinal tract. J. Food Qual. 2020, 2020, 5952834. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Baek, K.; Kim, J.-R.; Lee, J.-J.; Ryu, S.-H.; Chin, B.-R.; Baek, S.-H. Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp. Mol. Med. 2009, 41, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Jawhara, S. How Fungal Glycans Modulate Platelet Activation via Toll-Like Receptors Contributing to the Escape of Candida albicans from the Immune Response. Antibiotics 2020, 9, 385. [Google Scholar] [CrossRef]
- Jawhara, S. How gut bacterial dysbiosis can promote Candida albicans overgrowth during colonic inflammation. Microorganisms 2022, 10, 1014. [Google Scholar] [CrossRef]
- Poulain, D.; Sendid, B.; Standaert-Vitse, A.; Fradin, C.; Jouault, T.; Jawhara, S.; Colombel, J.F. Yeasts: Neglected pathogens. Dig. Dis. 2009, 27 (Suppl. S1), 104–110. [Google Scholar] [CrossRef]
- Jawhara, S.; Habib, K.; Maggiotto, F.; Pignede, G.; Vandekerckove, P.; Maes, E.; Dubuquoy, L.; Fontaine, T.; Guerardel, Y.; Poulain, D. Modulation of intestinal inflammation by yeasts and cell wall extracts: Strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS ONE 2012, 7, e40648. [Google Scholar] [CrossRef]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Barbet, M.; Sendid, B.; Jawhara, S. A decrease in anaerobic bacteria promotes Candida glabrata overgrowth while beta-glucan treatment restores the gut microbiota and attenuates colitis. Gut Pathog. 2018, 10, 50. [Google Scholar] [CrossRef]
- Jawhara, S.; Poulain, D. Saccharomyces boulardii decreases inflammation and intestinal colonization by Candida albicans in a mouse model of chemically-induced colitis. Med. Mycol. 2007, 45, 691–700. [Google Scholar] [CrossRef]
- Standaert-Vitse, A.; Jouault, T.; Vandewalle, P.; Mille, C.; Seddik, M.; Sendid, B.; Mallet, J.M.; Colombel, J.F.; Poulain, D. Candida albicans is an immunogen for anti-Saccharomyces cerevisiae antibody markers of Crohn’s disease. Gastroenterology 2006, 130, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S. Editorial of Special Issue “Human Pathogenic Fungi: Host-Pathogen Interactions and Virulence”. Microorganisms 2023, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.G. Naringin-generated ROS promotes mitochondria-mediated apoptosis in Candida albicans. IUBMB Life 2021, 73, 953–967. [Google Scholar] [CrossRef]
- Arismunandar, R.N.; Nosartika, I.; Purnomo, B.N.R.; Antari, A.L. The Effectivity of Parsley (Petroselinum crispum) Extract on The Growth Inhibition of Candida albicans. J. Biomed. Transl. Res. 2021, 7, 123–128. [Google Scholar] [CrossRef]
- Candiracci, M.; Citterio, B.; Piatti, E. Antifungal activity of the honey flavonoid extract against Candida albicans. Food Chem. 2012, 131, 493–499. [Google Scholar] [CrossRef]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef]
- Han, H.-K. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert. Opin. Drug Metab. Toxicol. 2011, 7, 721–729. [Google Scholar] [CrossRef]
- Feng, Y.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Technol. 2020, 57, 4671–4687. [Google Scholar] [CrossRef]
- Priya, A.; Pandian, S.K. Piperine impedes biofilm formation and hyphal morphogenesis of Candida albicans. Front. Microbiol. 2020, 11, 528306. [Google Scholar] [CrossRef]
- Mateos-Martin, M.L.; Fuguet, E.; Quero, C.; Perez-Jimenez, J.; Torres, J.L. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1327–1336. [Google Scholar] [CrossRef]
- Atai, Z.; Ansari, M.; Mousavi, A.; Mirzaei, A. In-vitro study of antifungal effects of selected herbal extracts on standard and wild strains of Candida albicans. J. Iran. Dent. Assoc. 2007, 19, 91–97. [Google Scholar]
- Salman, S.; Öz, G.; Felek, R.; Haznedar, A.; Turna, T.; Özdemir, F. Effects of fermentation time on phenolic composition, antioxidant and antimicrobial activities of green, oolong, and black teas. Food Biosci. 2022, 49, 101884. [Google Scholar] [CrossRef]
- Sitheeque, M.; Panagoda, G.; Yau, J.; Amarakoon, A.; Udagama, U.; Samaranayake, L. Antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. Chemotherapy 2009, 55, 189–196. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant extracts rich in polyphenols as potent modulators in the growth of probiotic and pathogenic intestinal microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, A.; Shokri, H.; Katiraee, F. Anti-adherence and anti-fungal abilities of thymol and carvacrol against Candida species isolated from patients with oral candidiasis in comparison with fluconazole and voriconazole. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e65005. [Google Scholar] [CrossRef]
- Braga, P.C.; Culici, M.; Alfieri, M.; Dal Sasso, M. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int. J. Antimicrob. Agents 2008, 31, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Del Bo, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee Consumption and Oxidative Stress: A Review of Human Intervention Studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef]
- Calheiros, D.; Dias, M.I.; Calhelha, R.C.; Barros, L.; Ferreira, I.; Fernandes, C.; Goncalves, T. Antifungal activity of spent coffee ground extracts. Microorganisms 2023, 11, 242. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Cheng, S.; Li, P.; Du, J.; Chang, Y.; Meng, H. Evaluation of garlic cultivars for polyphenolic content and antioxidant properties. PLoS ONE 2013, 8, e79730. [Google Scholar] [CrossRef]
- Lemar, K.M.; Turner, M.P.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A. Inhibition of Candida adhesion to buccal epithelial cells by an aqueous extract of Allium sativum (garlic). J. Appl. Bacteriol. 1990, 68, 163–169. [Google Scholar] [CrossRef]
- Doddanna, S.J.; Patel, S.; Sundarrao, M.A.; Veerabhadrappa, R.S. Antimicrobial activity of plant extracts on Candida albicans: An in vitro study. Indian J. Dent. Res. 2013, 24, 401–405. [Google Scholar] [CrossRef]
- Almasaudi, S.; AlBureikan, M.O. Antimicrobial activity of onion juice (Allium cepa), honey, and onion-honey mixture on some sensitive and multi-resistant microorganisms. Life Sci. J. 2012, 9, 775–780. [Google Scholar]
- Ben Lagha, A.; LeBel, G.; Grenier, D. Tart cherry (Prunus cerasus L.) fractions inhibit biofilm formation and adherence properties of oral pathogens and enhance oral epithelial barrier function. Phytother. Res. 2020, 34, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Seu, Y.B.; Lee, D.G. Candicidal action of resveratrol isolated from grapes on human pathogenic yeast C. albicans. J. Microbiol. Biotechnol. 2007, 17, 1324–1329. [Google Scholar]
- Parera, L.; Lawa, Y.; More, E. The effect of ethanol extracts of red pomegranate peel (Punica granatum L.) on activities Candida albicans. Environ. Conserv. 2019, 25, 81–86. [Google Scholar]
- Prasad, D.; Kunnaiah, R. Punica granatum: A review on its potential role in treating periodontal disease. J. Indian Soc. Periodontol. 2014, 18, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Zoric, N.; Kopjar, N.; Bobnjaric, I.; Horvat, I.; Tomic, S.; Kosalec, I. Antifungal activity of oleuropein against Candida albicans-the in vitro study. Molecules 2016, 21, 1631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).