Role of Carbon, Nitrogen, Phosphate and Sulfur Metabolism in Secondary Metabolism Precursor Supply in Streptomyces spp.
Abstract
1. Actinobacteria and Their Primary Metabolism as Basis for Secondary Metabolism Precursor Supply
1.1. Carbon Metabolism as a Source of Precursors
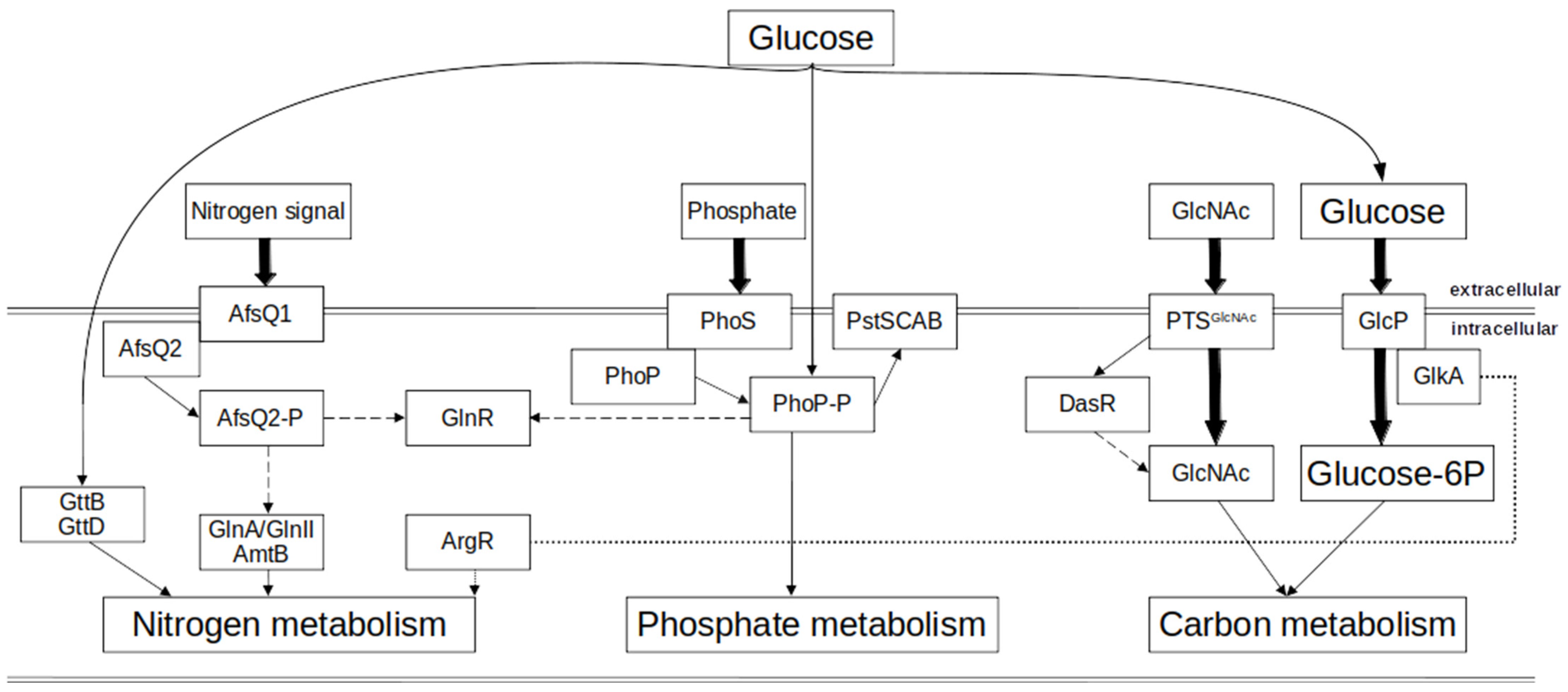
1.2. Primary Metabolism in Actinobacteria: Nitrogen Metabolism
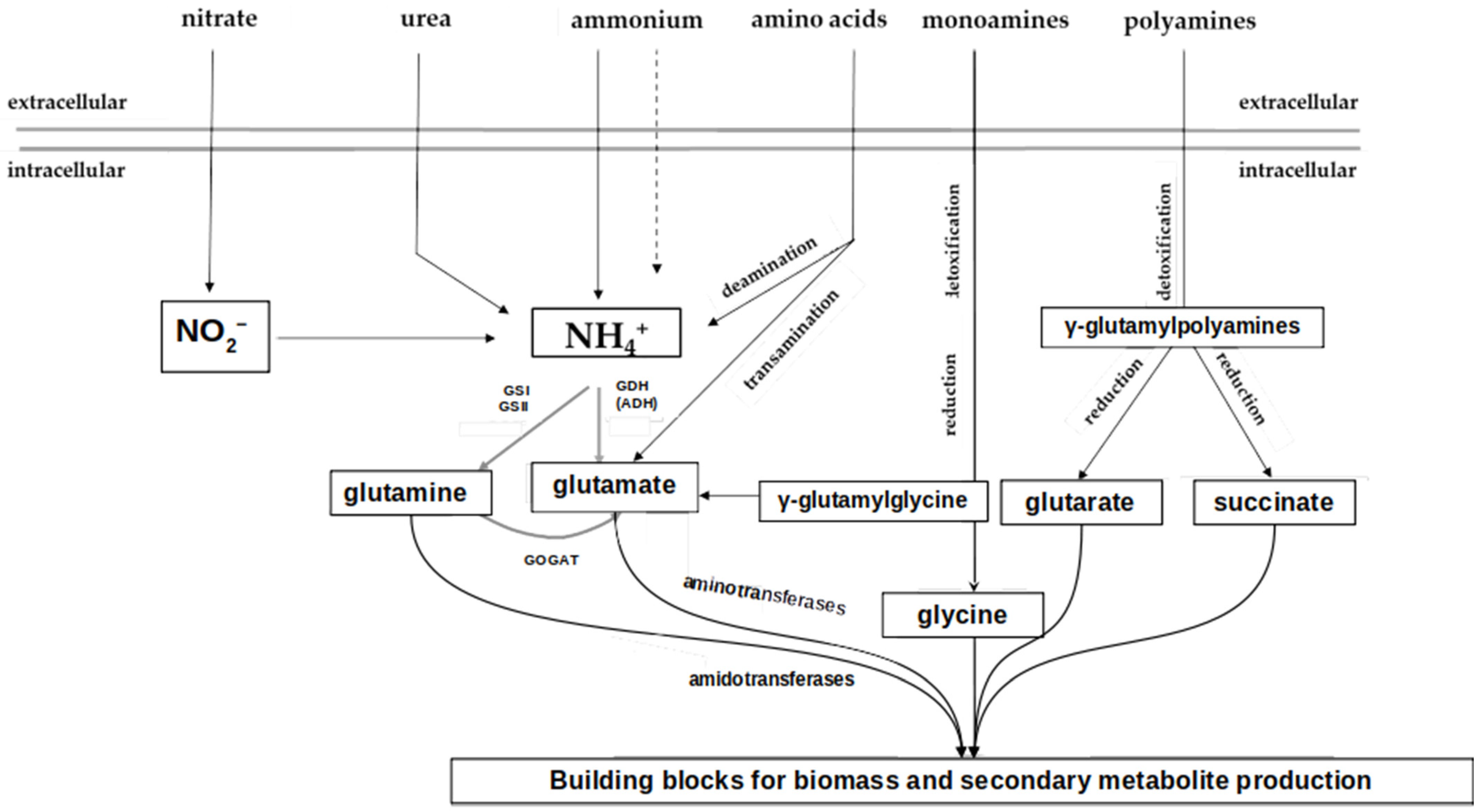
1.2.1. Biochemistry of Nitrogen Metabolism
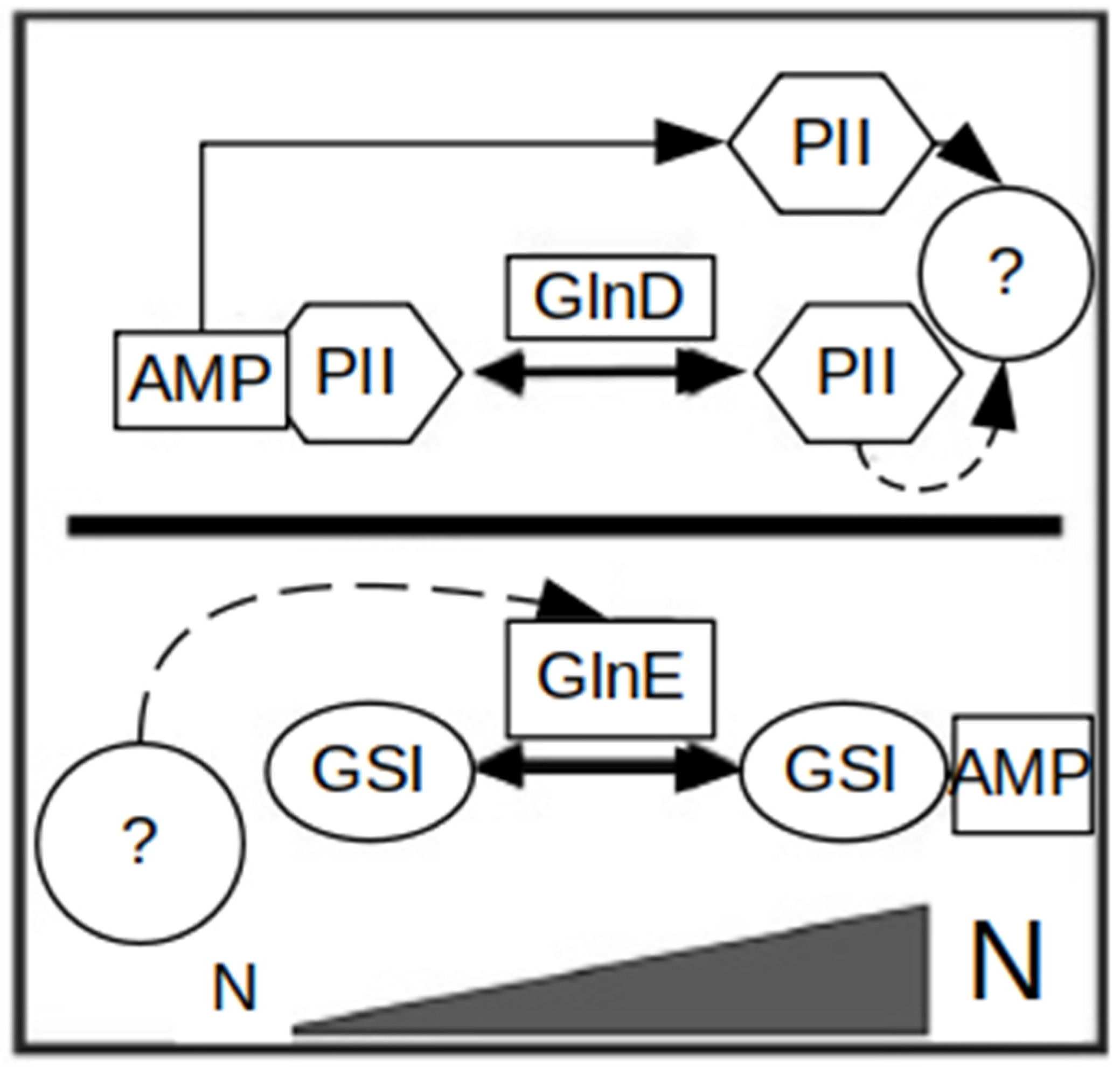
1.2.2. Regulation of Nitrogen Metabolism in Actinobacteria
1.3. Primary Metabolism in Actinobacteria: Phosphate Metabolism
1.3.1. Biochemistry of Phosphate Metabolism
1.3.2. Regulation of Phosphate Metabolism
1.4. Primary Metabolism in Actinobacteria: Sulfur Metabolism
2. Specialized Metabolic Pathways for Supply of Alternative Precursors for Secondary Metabolism
2.1. Alternative Metabolism in Actinobacteria—Polyamine Metabolism
2.2. Alternative Metabolism in Actinobacteria—Ethanolamine Metabolism
2.3. Exoenzymes and Proteases
3. Utilization of Precursor Compounds from Primary Metabolism in Secondary Metabolism
3.1. Secondary Metabolism in Actinobacteria
3.2. Supply of Key Precursors Carbon, Nitrogen, Phosphate and Sulfur for Secondary Metabolism
3.2.1. Supply of Carbon for Secondary Metabolism
3.2.2. Supply of Nitrogen for Secondary Metabolism
3.2.3. Supply of Phosphate for Secondary Metabolism
3.2.4. Supply of Sulfur for Secondary Metabolism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fatahi-Bafghi, M. Antibiotic resistance genes in the Actinobacteria phylum. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 1599–1624. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Lacey, H.J.; Rutledge, P.J. Recently Discovered Secondary Metabolites from Streptomyces Species. Molecules 2022, 27, 887. [Google Scholar] [CrossRef]
- Urem, M.; Świątek-Połatyńska, M.A.; Rigali, S.; van Wezel, G.P. Intertwining nutrient-sensory networks and the control of antibiotic production in Streptomyces. Mol. Microbiol. 2016, 102, 183–195. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. The Balance Metabolism Safety Net: Integration of Stress Signals by Interacting Transcriptional Factors in Streptomyces and Related Actinobacteria. Front. Microbiol. 2020, 10, 3120. [Google Scholar] [CrossRef]
- Hodgson, D.A. Primary metabolism and its control in streptomycetes: A most unusual group of bacteria. Adv. Microb. Physiol. 2000, 42, 47–238. [Google Scholar] [CrossRef]
- Borodina, I.; Krabben, P.; Nielsen, J. Genome-scale analysis of Streptomyces coelicolor A3(2) metabolism. Genome Res. 2005, 15, 820–828. [Google Scholar] [CrossRef]
- Krysenko, S.; Matthews, A.; Busche, T.; Bera, A.; Wohlleben, W. Poly- and Monoamine Metabolism in Streptomyces coelicolor: The New Role of Glutamine Synthetase-Like Enzymes in the Survival under Environmental Stress. Microb. Physiol. 2021, 31, 233–247. [Google Scholar] [CrossRef]
- Paulsen, I.T. Carbon metabolism and its regulation in Streptomyces and other high GC gram-positive bacteria. Res. Microbiol. 1996, 147, 535–541. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Rocha, D.; Ruiz-Villafán, B.; Guzmán-Trampe, S.; Maldonado-Carmona, N.; Vázquez-Hernández, M.; Zelarayán, A.; Rodríguez-Sanoja, R.; Sánchez, S. Carbon catabolite regulation in Streptomyces: New insights and lessons learned. World J. Microbiol. Biotechnol. 2017, 33, 162. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Jahreis, K.; Pimentel-Schmitt, E.F.; Brückner, R.; Titgemeyer, F. Ins and outs of glucose transport systems in eubacteria. FEMS Microbiol. Rev. 2008, 32, 891–907. [Google Scholar] [CrossRef]
- Virolle, M.J.; Gagnat, J. Sequences involved in growth-phase-dependent expression and glucose repression of a Streptomyces alpha-amylase gene. Microbiology 1994, 140 Pt 5, 1059–1067. [Google Scholar] [CrossRef][Green Version]
- Vilches, C.; Méndez, C.; Hardisson, C.; Salas, J.A. Biosynthesis of oleandomycin by Streptomyces antibioticus: Influence of nutritional conditions and development of resistance. J. Gen. Microbiol. 1990, 136, 1447–1454. [Google Scholar] [CrossRef]
- van Wezel, G.P.; Mahr, K.; König, M.; Traag, B.A.; Pimentel-Schmitt, E.F.; Willimek, A.; Titgemeyer, F. GlcP constitutes the major glucose uptake system of Streptomyces coelicolor A3(2). Mol. Microbiol. 2005, 55, 624–636. [Google Scholar] [CrossRef]
- Lu, X.; Liu, X.; Chen, Z.; Li, J.; van Wezel, G.P.; Chen, W.; Wen, Y. The ROK-family regulator Rok7B7 directly controls carbon catabolite repression, antibiotic biosynthesis, and morphological development in Streptomyces avermitilis. Environ. Microbiol. 2020, 22, 5090–5108. [Google Scholar] [CrossRef]
- van Wezel, G.P.; König, M.; Mahr, K.; Nothaft, H.; Thomae, A.W.; Bibb, M.; Titgemeyer, F. A new piece of an old jigsaw: Glucose kinase is activated posttranslationally in a glucose transport-dependent manner in Streptomyces coelicolor A3(2). J. Mol. Microbiol. Biotechnol. 2007, 12, 67–74. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Ruiz-Villafán, B.; Tierrafría, V.H.; Rodríguez-Sanoja, R.; Sánchez, S. Carbon Catabolite Regulation of Secondary Metabolite Formation and Morphological Differentiation in Streptomyces coelicolor. Appl. Biochem. Biotechnol. 2016, 180, 1152–1166. [Google Scholar] [CrossRef]
- Gubbens, J.; Janus, M.M.; Florea, B.I.; Overkleeft, H.S.; van Wezel, G.P. Identification of glucose kinase-dependent and -independent pathways for carbon control of primary metabolism, development and antibiotic production in Streptomyces coelicolor by quantitative proteomics. Mol. Microbiol. 2012, 86, 1490–1507. [Google Scholar] [CrossRef]
- Sánchez, S.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Avalos, M.; Guzmán-Trampe, S.; et al. Carbon source regulation of antibiotic production. J. Antibiot. 2010, 63, 442–459. [Google Scholar] [CrossRef]
- Schniete, J.K.; Cruz-Morales, P.; Selem-Mojica, N.; Fernández-Martínez, L.T.; Hunter, I.S.; Barona-Gómez, F.; Hoskisson, P.A. Expanding Primary Metabolism Helps Generate the Metabolic Robustness To Facilitate Antibiotic Biosynthesis in Streptomyces. mBio 2018, 9, e02283-17. [Google Scholar] [CrossRef]
- Ordóñez-Robles, M.; Santos-Beneit, F.; Albillos, S.M.; Liras, P.; Martín, J.F.; Rodríguez-García, A. Streptomyces tsukubaensis as a new model for carbon repression: Transcriptomic response to tacrolimus repressing carbon sources. Appl. Microbiol. Biotechnol. 2017, 101, 8181–8195. [Google Scholar] [CrossRef]
- Santos-Beneit, F.; Rodríguez-García, A.; Sola-Landa, A.; Martín, J.F. Cross-talk between two global regulators in Streptomyces: PhoP and AfsR interact in the control of afsS, pstS and phoRP transcription. Mol. Microbiol. 2009, 72, 53–68. [Google Scholar] [CrossRef]
- Martín, J.F.; Ramos, A.; Liras, P. Regulation of Geldanamycin Biosynthesis by Cluster-Situated Transcription Factors and the Master Regulator PhoP. Antibiotics 2019, 8, 87. [Google Scholar] [CrossRef]
- Sola-Landa, A.; Rodríguez-García, A.; Amin, R.; Wohlleben, W.; Martín, J.F. Competition between the GlnR and PhoP regulators for the glnA and amtB promoters in Streptomyces coelicolor. Nucleic Acids Res. 2013, 41, 1767–1782. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Maldonado-Carmona, N.; Ruiz-Villafán, B.; Koirala, N.; Rocha, D.; Sánchez, S. Interplay between carbon, nitrogen and phosphate utilization in the control of secondary metabolite production in Streptomyces. Antonie Van Leeuwenhoek 2018, 111, 761–781. [Google Scholar] [CrossRef]
- Sperber, A.M.; Herman, J.K. Metabolism Shapes the Cell. J. Bacteriol. 2017, 199, e00039-17. [Google Scholar] [CrossRef]
- Fischer, M.; Alderson, J.; van Keulen, G.; White, J.; Sawers, R.G. The obligate aerobe Streptomyces coelicolor A3(2) synthesizes three active respiratory nitrate reductases. Microbiology 2010, 156, 3166–3179. [Google Scholar] [CrossRef]
- Hopwood, D.A. Forty years of genetics with Streptomyces: From in vivo through in vitro to in silico. Microbiology 1999, 145, 2183–2202. [Google Scholar] [CrossRef]
- Kim Tiam, S.; Boubakri, H.; Bethencourt, L.; Abrouk, D.; Fournier, P.; Herrera-Belaroussi, A. Genomic Insights of Alnus-Infective Frankia Strains Reveal Unique Genetic Features and New Evidence on Their Host-Restricted Lifestyle. Genes 2023, 14, 530. [Google Scholar] [CrossRef]
- Shapiro, S.; Vining, L.C. Suppression of nitrate utilization by ammonium and its relationship to chloramphenicol production in Streptomyces venezuelae. Can. J. Microbiol. 1984, 30, 798–804. [Google Scholar] [CrossRef]
- Ives, P.R.; Bushell, M.E. Manipulation of the physiology of clavulanic acid production in Streptomyces clavuligerus. Microbiology 1997, 143 Pt 11, 3573–3579. [Google Scholar] [CrossRef]
- Fink, D.; Weissschuh, N.; Reuther, J.; Wohlleben, W.; Engels, A. Two transcriptional regulators GlnR and GlnRII are involved in regulation of nitrogen metabolism in Streptomyces coelicolor A3(2). Mol. Microbiol. 2002, 46, 331–347. [Google Scholar] [CrossRef]
- Kumada, Y.; Benson, D.R.; Hillemann, D.; Hosted, T.J.; Rochefort, D.A.; Thompson, C.J.; Wohlleben, W.; Tateno, Y. Evolution of the glutamine synthetase gene, one of the oldest existing and functioning genes. Proc. Natl. Acad. Sci. USA 1993, 90, 3009–3013. [Google Scholar] [CrossRef]
- Behrmann, I.; Hillemann, D.; Pühler, A.; Strauch, E.; Wohlleben, W. Overexpression of a Streptomyces viridochromogenes gene (glnII) encoding a glutamine synthetase similar to those of eucaryotes confers resistance against the antibiotic phosphinothricyl-alanyl-alanine. J. Bacteriol. 1990, 172, 5326–5334. [Google Scholar] [CrossRef]
- Hillemann, D.; Dammann, T.; Hillemann, A.; Wohlleben, W. Genetic and biochemical characterization of the two glutamine synthetases GSI and GSII of the phosphinothricyl-alanyl-alanine producer, Streptomyces viridochromogenes Tü494. J. Gen. Microbiol. 1993, 139, 1773–1783. [Google Scholar] [CrossRef][Green Version]
- Rexer, H.U.; Schäberle, T.; Wohlleben, W.; Engels, A. Investigation of the functional properties and regulation of three glutamine synthetase-like genes in Streptomyces coelicolor A3(2). Arch. Microbiol. 2006, 186, 447–458. [Google Scholar] [CrossRef]
- Krysenko, S.; Okoniewski, N.; Kulik, A.; Matthews, A.; Grimpo, J.; Wohlleben, W.; Bera, A. Gamma-Glutamylpolyamine Synthetase GlnA3 Is Involved in the First Step of Polyamine Degradation Pathway in Streptomyces coelicolor M145. Front. Microbiol. 2017, 8, 726. [Google Scholar] [CrossRef]
- Krysenko, S.; Matthews, A.; Okoniewski, N.; Kulik, A.; Girbas, M.G.; Tsypik, O.; Meyners, C.S.; Hausch, F.; Wohlleben, W.; Bera, A. Initial Metabolic Step of a Novel Ethanolamine Utilization Pathway and Its Regulation in Streptomyces coelicolor M145. mBio 2019, 10, e00326-19. [Google Scholar] [CrossRef]
- Harth, G.; Maslesa-Galić, S.; Tullius, M.V.; Horwitz, M.A. All four Mycobacterium tuberculosis glnA genes encode glutamine synthetase activities but only GlnA1 is abundantly expressed and essential for bacterial homeostasis. Mol. Microbiol. 2005, 58, 1157–1172. [Google Scholar] [CrossRef]
- Engel, P.C. Glutamate dehydrogenases: The why and how of coenzyme specificity. Neurochem. Res. 2014, 39, 426–432. [Google Scholar] [CrossRef]
- Merrick, M.J.; Edwards, R.A. Nitrogen control in bacteria. Microbiol. Rev. 1995, 59, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S. Impact of Nitrogen-Containing Compounds on Secondary Metabolism in Streptomyces spp.—A Source of Metabolic Engineering Strategies. SynBio 2023, 1, 204–225. [Google Scholar] [CrossRef]
- Rigali, S.; Titgemeyer, F.; Barends, S.; Mulder, S.; Thomae, A.W.; Hopwood, D.A.; van Wezel, G.P. Feast or famine: The global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep. 2008, 9, 670–675. [Google Scholar] [CrossRef]
- van Wezel, G.P.; McDowall, K.J. The regulation of the secondary metabolism of Streptomyces: New links and experimental advances. Nat. Prod. Rep. 2011, 28, 1311–1333. [Google Scholar] [CrossRef]
- Wohlleben, W.; Bera, A.; Mast, Y.; Stegmann, E. Regulation of Secondary Metabolites of Actinobacteria. In Biology and Biotechnology of Actinobacteria; Wink, J., Mohammadipanah, F., Hamedi, J., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; p. 395. [Google Scholar]
- Martin, J.F.; Demain, A.L. Control of antibiotic biosynthesis. Microbiol. Rev. 1980, 44, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P. Molecular Mechanisms of Phosphate Sensing, Transport and Signalling in Streptomyces and Related Actinobacteria. Int. J. Mol. Sci. 2021, 22, 1129. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Barreiro, C.; Santos-Beneit, F.; Sola-Landa, A.; Martín, J.F. Genome-wide transcriptomic and proteomic analysis of the primary response to phosphate limitation in Streptomyces coelicolor M145 and in a Delta phoP mutant. Proteomics 2007, 7, 2410–2429. [Google Scholar] [CrossRef]
- Allenby, N.E.; Laing, E.; Bucca, G.; Kierzek, A.M.; Smith, C.P. Diverse control of metabolism and other cellular processes in Streptomyces coelicolor by the PhoP transcription factor: Genome-wide identification of in vivo targets. Nucleic Acids Res. 2012, 40, 9543–9556. [Google Scholar] [CrossRef]
- Chouayekh, H.; Virolle, M.J. The polyphosphate kinase plays a negative role in the control of antibiotic production in Streptomyces lividans. Mol. Microbiol. 2002, 43, 919–930. [Google Scholar] [CrossRef]
- Ghorbel, S.; Smirnov, A.; Chouayekh, H.; Sperandio, B.; Esnault, C.; Kormanec, J.; Virolle, M.J. Regulation of ppk expression and in vivo function of Ppk in Streptomyces lividans TK24. J. Bacteriol. 2006, 188, 6269–6276. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Martín, J.F.; Santos-Beneit, F.; Rodríguez-García, A.; Sola-Landa, A.; Smith, M.C.; Ellingsen, T.E.; Nieselt, K.; Burroughs, N.J.; Wellington, E.M. Transcriptomic studies of phosphate control of primary and secondary metabolism in Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 2012, 95, 61–75. [Google Scholar] [CrossRef]
- Cheung, J.; Hendrickson, W.A. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 2010, 13, 116–123. [Google Scholar] [CrossRef]
- Martínez-Castro, M.; Barreiro, C.; Martín, J.F. Analysis and validation of the pho regulon in the tacrolimus-producer strain Streptomyces tsukubaensis: Differences with the model organism Streptomyces coelicolor. Appl. Microbiol. Biotechnol. 2018, 102, 7029–7045. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Curr. Opin. Microbiol. 2010, 13, 263–273. [Google Scholar] [CrossRef]
- Kertesz, M.A. Riding the sulfur cycle--metabolism of sulfonates and sulfate esters in gram-negative bacteria. FEMS Microbiol. Rev. 2000, 24, 135–175. [Google Scholar] [CrossRef]
- Bhave, D.P.; Muse, W.B., 3rd; Carroll, K.S. Drug targets in mycobacterial sulfur metabolism. Infect. Disord. Drug Targets 2007, 7, 140–158. [Google Scholar] [CrossRef]
- Lu, T.; Cao, Q.; Pang, X.; Xia, Y.; Xun, L.; Liu, H. Sulfane sulfur-activated actinorhodin production and sporulation is maintained by a natural gene circuit in Streptomyces coelicolor. Microb. Biotechnol. 2020, 13, 1917–1932. [Google Scholar] [CrossRef]
- Ferla, M.P.; Patrick, W.M. Bacterial methionine biosynthesis. Microbiology 2014, 160 Pt 8, 1571–1584. [Google Scholar] [CrossRef]
- Kulkarni, A.; Zeng, Y.; Zhou, W.; Van Lanen, S.; Zhang, W.; Chen, S. A Branch Point of Streptomyces Sulfur Amino Acid Metabolism Controls the Production of Albomycin. Appl. Environ. Microbiol. 2015, 82, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Ostash, B.O.; Stanchak, K.; Gren, T.; Fedorenko, V.O. Genetic analysis of sulfate assimilation gene cluster of Streptomyces coelicolor A3(2). Fakt. Eksp. Evol. Org. 2023, 32, 64–68. [Google Scholar] [CrossRef]
- Suzuki, H.; Kurihara, S. Polyamine Catabolism in Prokaryotes. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Kusano, T., Suzuki, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 47–59. [Google Scholar]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Burrell, M.; Hanfrey, C.C.; Kinch, L.N.; Elliott, K.A.; Michael, A.J. Evolution of a novel lysine decarboxylase in siderophore biosynthesis. Mol. Microbiol. 2012, 86, 485–499. [Google Scholar] [CrossRef]
- Young, C.C.; Chen, L.F. Polyamines in humic acid and their effect on radical growth of lettuce seedlings. Plant Soil 1997, 195, 143–149. [Google Scholar] [CrossRef]
- Krysenko, S.; Okoniewski, N.; Nentwich, M.; Matthews, A.; Bäuerle, M.; Zinser, A.; Busche, T.; Kulik, A.; Gursch, S.; Kemeny, A.; et al. A Second Gamma-Glutamylpolyamine Synthetase, GlnA2, Is Involved in Polyamine Catabolism in Streptomyces coelicolor. Int. J. Mol. Sci. 2022, 23, 3752. [Google Scholar] [CrossRef]
- Krysenko, S.; Wohlleben, W. Überlebenswichtig: Glutaminsynthetasehomologe Proteine in Streptomyceten. Biospektrum 2022, 28, 23–26. [Google Scholar] [CrossRef]
- Knaak, J.B.; Leung, H.W.; Stott, W.T.; Busch, J.; Bilsky, J. Toxicology of mono-, di-, and triethanolamine. Rev. Environ. Contam. Toxicol. 1997, 149, 1–86. [Google Scholar] [CrossRef]
- Nandedkar, A.K. Report on the utilization of ethanolamine-1-14C by Mycobacterium 607. Biochem. Med. 1974, 11, 67–70. [Google Scholar] [CrossRef]
- Chater, K.F.; Biró, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Siemieniewicz, K.W.; Kajla, M.K.; Schrempf, H. Elucidating the biosynthesis of chitin filaments and their configuration with specific proteins and electron microscopy. Macromol. Biosci. 2007, 7, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Chigwada, A.D.; Tekere, M. The plastic and microplastic waste menace and bacterial biodegradation for sustainable environmental clean-up a review. Environ. Res. 2023, 231 Pt 1, 116110. [Google Scholar] [CrossRef]
- Kotb, E.; Alabdalall, A.H.; Alghamdi, A.I.; Ababutain, I.M.; Aldakeel, S.A.; Al-Zuwaid, S.K.; Algarudi, B.M.; Algarudi, S.M.; Ahmed, A.A.; Albarrag, A.M. Screening for chitin degrading bacteria in the environment of Saudi Arabia and characterization of the most potent chitinase from Streptomyces variabilis Am1. Sci. Rep. 2023, 13, 11723. [Google Scholar] [CrossRef]
- Tsujibo, H.; Ohtsuki, T.; Iio, T.; Yamazaki, I.; Miyamoto, K.; Sugiyama, M.; Inamori, Y. Cloning and sequence analysis of genes encoding xylanases and acetyl xylan esterase from Streptomyces thermoviolaceus OPC-520. Appl. Environ. Microbiol. 1997, 63, 661–664. [Google Scholar] [CrossRef]
- King, R.R.; Calhoun, L.A. The thaxtomin phytotoxins: Sources, synthesis, biosynthesis, biotransformation and biological activity. Phytochemistry 2009, 70, 833–841. [Google Scholar] [CrossRef]
- Xu, W.; Gao, W.; Bu, Q.; Li, Y. Degradation Mechanism of AAA+ Proteases and Regulation of Streptomyces Metabolism. Biomolecules 2022, 12, 1848. [Google Scholar] [CrossRef]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef]
- Xie, F.; Chao, Y.; Yang, X.; Yang, J.; Xue, Z.; Luo, Y.; Qian, S. Purification and characterization of four keratinases produced by Streptomyces sp. strain 16 in native human foot skin medium. Bioresour. Technol. 2010, 101, 344–350. [Google Scholar] [CrossRef]
- Demain, A.L.; Fang, A. The natural functions of secondary metabolites. Adv. Biochem. Eng./Biotechnol. 2000, 69, 1–39. [Google Scholar] [CrossRef]
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988. [Google Scholar] [CrossRef]
- Barona-Gómez, F.; Wong, U.; Giannakopulos, A.E.; Derrick, P.J.; Challis, G.L. Identification of a cluster of genes that directs desferrioxamine biosynthesis in Streptomyces coelicolor M145. J. Am. Chem. Soc. 2004, 126, 16282–16283. [Google Scholar] [CrossRef]
- Steiger, S.; Perez-Fons, L.; Cutting, S.M.; Fraser, P.D.; Sandmann, G. Annotation and functional assignment of the genes for the C30 carotenoid pathways from the genomes of two bacteria: Bacillus indicus and Bacillus firmus. Microbiology 2015, 161 Pt 1, 194–202. [Google Scholar] [CrossRef]
- Madhusudhan, D.N.; Mazhari, B.B.; Dastager, S.G.; Agsar, D. Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitanus DMZ-3. BioMed Res. Int. 2014, 2014, 306895. [Google Scholar] [CrossRef]
- Moody, S.C.; Zhao, B.; Lei, L.; Nelson, D.R.; Mullins, J.G.; Waterman, M.R.; Kelly, S.L.; Lamb, D.C. Investigating conservation of the albaflavenone biosynthetic pathway and CYP170 bifunctionality in streptomycetes. FEBS J. 2012, 279, 1640–1649. [Google Scholar] [CrossRef]
- Bursy, J.; Kuhlmann, A.U.; Pittelkow, M.; Hartmann, H.; Jebbar, M.; Pierik, A.J.; Bremer, E. Synthesis and uptake of the compatible solutes ectoine and 5-hydroxyectoine by Streptomyces coelicolor A3(2) in response to salt and heat stresses. Appl. Environ. Microbiol. 2008, 74, 7286–7296. [Google Scholar] [CrossRef]
- Poralla, K.; Muth, G.; Härtner, T. Hopanoids are formed during transition from substrate to aerial hyphae in Streptomyces coelicolor A3(2). FEMS Microbiol. Lett. 2000, 189, 93–95. [Google Scholar] [CrossRef]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial–fungal interaction triggers bio- synthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef]
- Alam, K.; Mazumder, A.; Sikdar, S.; Zhao, Y.M.; Hao, J.; Song, C.; Wang, Y.; Sarkar, R.; Islam, S.; Zhang, Y.; et al. Streptomyces: The biofactory of secondary metabolites. Front. Microbiol. 2022, 13, 968053. [Google Scholar] [CrossRef]
- Baltz, R.H. Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588. [Google Scholar] [CrossRef]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Schall, C.; Stehle, T.; Breitmeyer, C.; Krysenko, S.; Mitulski, A.; Wohlleben, W. Optimization of the precursor supply for an enhanced FK506 production in Streptomyces tsukubaensis. Front. Bioeng. Biotechnol. 2022, 10, 1067467. [Google Scholar] [CrossRef] [PubMed]
- Metsä-Ketelä, M.; Salo, V.; Halo, L.; Hautala, A.; Hakala, J.; Mäntsälä, P.; Ylihonko, K. An efficient approach for screening minimal PKS genes from Streptomyces. FEMS Microbiol. Lett. 1999, 180, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Fraústo Da Silva, J.J. Evolution was chemically constrained. J. Theor. Biol. 2003, 220, 323–343. [Google Scholar] [CrossRef]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Strauch, E.; Takano, E.; Baylis, H.A.; Bibb, M.J. The stringent response in Streptomyces coelicolor A3(2). Mol. Microbiol. 1991, 5, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.U.; Lee, C.K.; Hwang, Y.I.; Kinosita, H.; Nihira, T. Gamma-butyrolactone autoregulators and receptor proteins in non- Streptomyces actinomycetes producing commercially important secondary metabolites. Arch. Microbiol. 2003, 180, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Augustijn, H.E.; Roseboom, A.M.; Medema, M.H.; van Wezel, G.P. Harnessing regulatory networks in Actinobacteria for natural product discovery. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae011. [Google Scholar] [CrossRef]
- Barbuto Ferraiuolo, S.; Cammarota, M.; Schiraldi, C.; Restaino, O.F. Streptomycetes as platform for biotechnological production processes of drugs. Appl. Microbiol. Biotechnol. 2021, 105, 551–568. [Google Scholar] [CrossRef]
- Boldt, J.; Lukoševičiūtė, L.; Fu, C.; Steglich, M.; Bunk, B.; Junker, V.; Gollasch, A.; Trunkwalter, B.; Mohr, K.I.; Beckstette, M.; et al. Bursts in biosynthetic gene cluster transcription are accompanied by surges of natural compound production in the myxobacterium Sorangium sp. Microb. Biotechnol. 2023, 16, 1054–1068. [Google Scholar] [CrossRef]
- Keasling, J.; Garcia Martin, H.; Lee, T.S.; Mukhopadhyay, A.; Singer, S.W.; Sundstrom, E. Microbial production of advanced biofuels. Nat. Rev. Microbiol. 2021, 19, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, R.R.; Schneider, T.; Sahl, H.-G.; Wiedemann, I. Insights into in vivo activities of lantibiotics from gallidermin and epidermin mode-of-action studies. Antimicrob. Agents Chemother. 2006, 50, 1449–1457. [Google Scholar] [CrossRef]
- Rauter, A.P. Recent Trends in Carbohydrate Chemistry. Volume 1: Synthesis, Structure, and Function of Carbohydrates; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-817467-8. [Google Scholar] [CrossRef]
- Mast, Y.; Weber, T.; Gölz, M.; Ort-Winklbauer, R.; Gondran, A.; Wohlleben, W.; Schinko, E. Characterization of the ‘pristinamycin supercluster’ of Streptomyces pristinaespiralis. Microb. Biotechnol. 2011, 4, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Nolden, S.; Gebhardt, P.; Heinzelmann, E.; Lange, C.; Puk, O.; Welzel, K.; Wohlleben, W.; Schwartz, D. Sequencing and analysis of the biosynthetic gene cluster of the lipopeptide antibiotic Friulimicin in Actinoplanes friuliensis. Antimicrob. Agents Chemother. 2007, 51, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Gatto, G.J.; Boyne, M.T.; Kelleher, N.L.; and Walsh, C.T. Biosynthesis of pipecolic acid by RapL, a lysine cyclodeaminase encoded in the rapamycin gene cluster. J. Am. Chem. Soc. 2006, 128, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.A.; Cavallieri, A.P.; Araujo, M.L. Enhancing effect of lysine combined with other compounds on cephamycin C production in Streptomyces clavuligerus. BMC Microbiol. 2013, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Edenhart, S.; Denneler, M.; Spohn, M.; Doskocil, E.; Kavšček, M.; Amon, T.; Kosec, G.; Smole, J.; Bardl, B.; Biermann, M.; et al. Metabolic engineering of Amycolatopsis japonicum for optimized production of [S,S]-EDDS, a biodegradable chelator. Metab. Eng. 2020, 60, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Esnault, C.; Prigent, M.; Holland, I.B.; Virolle, M.J. Phosphate Homeostasis in Conditions of Phosphate Proficiency and Limitation in the Wild Type and the phoP Mutant of Streptomyces lividans. PLoS ONE 2015, 10, e0126221. [Google Scholar] [CrossRef] [PubMed]
- Wentzel, A.; Sletta, H.; Stream Consortium; Ellingsen, T.E.; Bruheim, P. Intracellular Metabolite Pool Changes in Response to Nutrient Depletion Induced Metabolic Switching in Streptomyces coelicolor. Metabolites 2012, 2, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Peng, Q.; Li, S.; Deng, Z.; Gao, J. The intriguing biology and chemistry of fosfomycin: The only marketed phosphonate antibiotic. RSC Adv. 2019, 9, 42204–42218. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.; Berger, S.; Heinzelmann, E.; Muschko, K.; Welzel, K.; Wohlleben, W. Biosynthetic gene cluster of the herbicide phosphinothricin tripeptide from Streptomyces viridochromogenes Tü494. Appl. Environ. Microbiol. 2004, 70, 7093–7102. [Google Scholar] [CrossRef]
- Chakravarty, I.; Kundu, S. Improved production of Daptomycin in an airlift bioreactor by morphologically modified and immobilized cells of Streptomyces roseosporus. AMB Express 2016, 6, 101. [Google Scholar] [CrossRef]
- Sahl, H.G.; Jack, R.W.; Bierbaum, G. Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur. J. Biochem. 1995, 230, 827–853. [Google Scholar] [CrossRef] [PubMed]
- Kaysser, L.; Eitel, K.; Tanino, T.; Siebenberg, S.; Matsuda, A.; Ichikawa, S.; Gust, B. A new arylsulfate sulfotransferase involved in liponucleoside antibiotic biosynthesis in streptomycetes. J. Biol. Chem. 2010, 285, 12684–12694. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.L.; Fahey, R.C. Mycothiol biochemistry. Arch. Microbiol. 2002, 178, 388–394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysenko, S.; Wohlleben, W. Role of Carbon, Nitrogen, Phosphate and Sulfur Metabolism in Secondary Metabolism Precursor Supply in Streptomyces spp. Microorganisms 2024, 12, 1571. https://doi.org/10.3390/microorganisms12081571
Krysenko S, Wohlleben W. Role of Carbon, Nitrogen, Phosphate and Sulfur Metabolism in Secondary Metabolism Precursor Supply in Streptomyces spp. Microorganisms. 2024; 12(8):1571. https://doi.org/10.3390/microorganisms12081571
Chicago/Turabian StyleKrysenko, Sergii, and Wolfgang Wohlleben. 2024. "Role of Carbon, Nitrogen, Phosphate and Sulfur Metabolism in Secondary Metabolism Precursor Supply in Streptomyces spp." Microorganisms 12, no. 8: 1571. https://doi.org/10.3390/microorganisms12081571
APA StyleKrysenko, S., & Wohlleben, W. (2024). Role of Carbon, Nitrogen, Phosphate and Sulfur Metabolism in Secondary Metabolism Precursor Supply in Streptomyces spp. Microorganisms, 12(8), 1571. https://doi.org/10.3390/microorganisms12081571







