Diversity of Microbial Functional Genes Promotes Soil Nitrogen Mineralization in Boreal Forests
Abstract
:1. Introduction
2. Materials and Methods
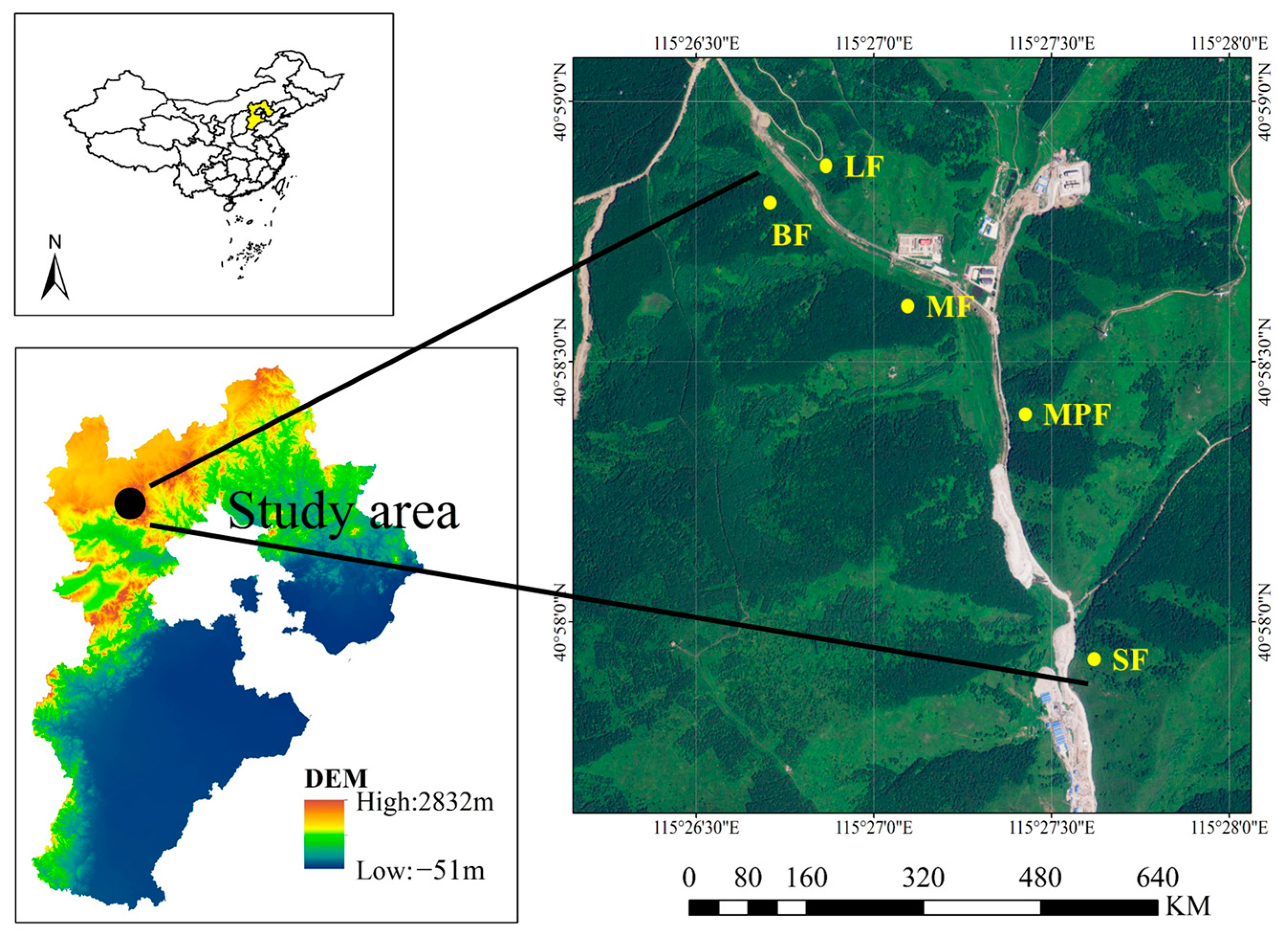
2.1. Study Site
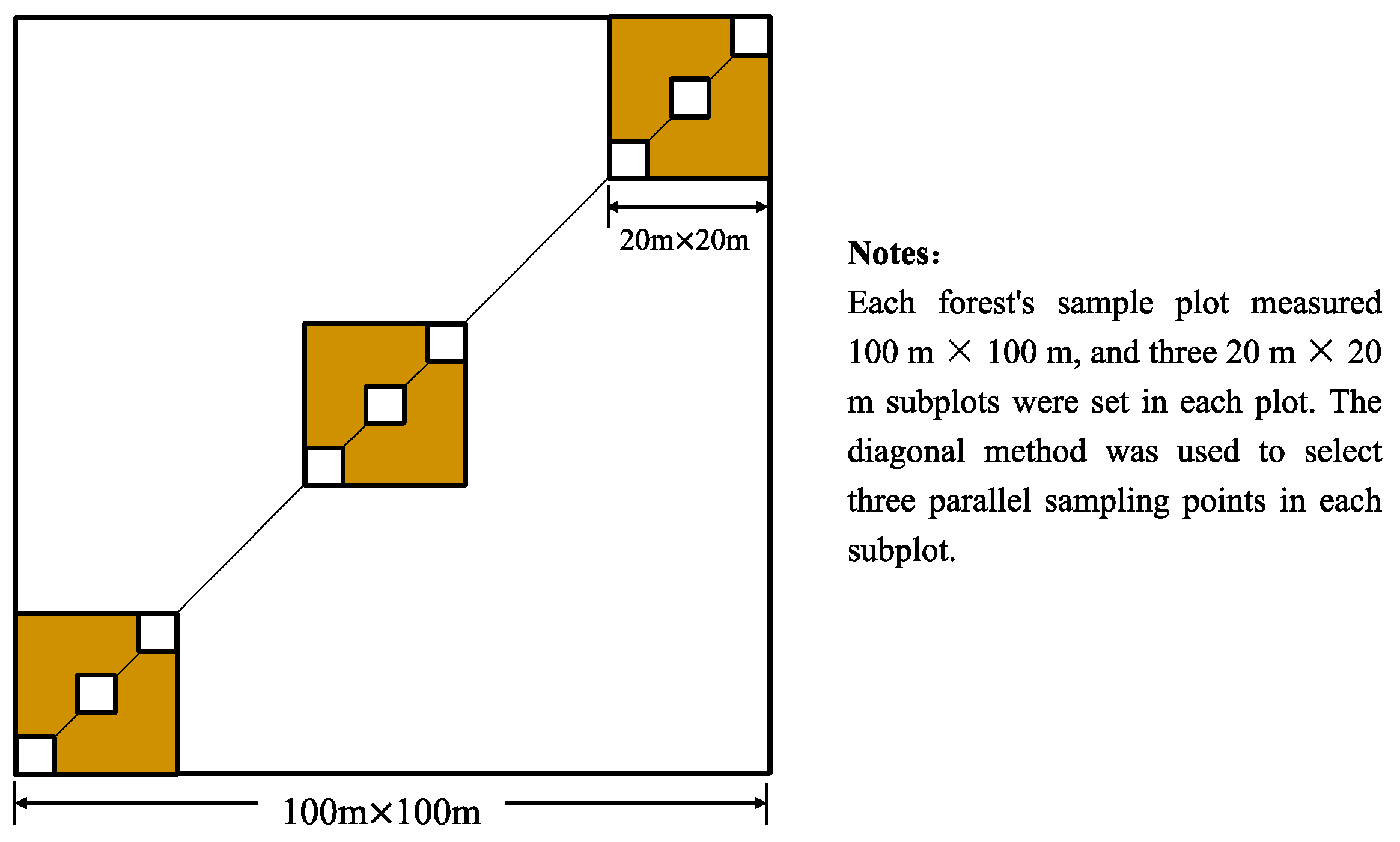
2.2. Experimental Design and Field Sampling
2.3. Soil Physicochemical Properties
2.4. Measurement of Soil Microbial Functional Genes Involved in N Mineralization
2.5. Statistical Analysis
3. Results
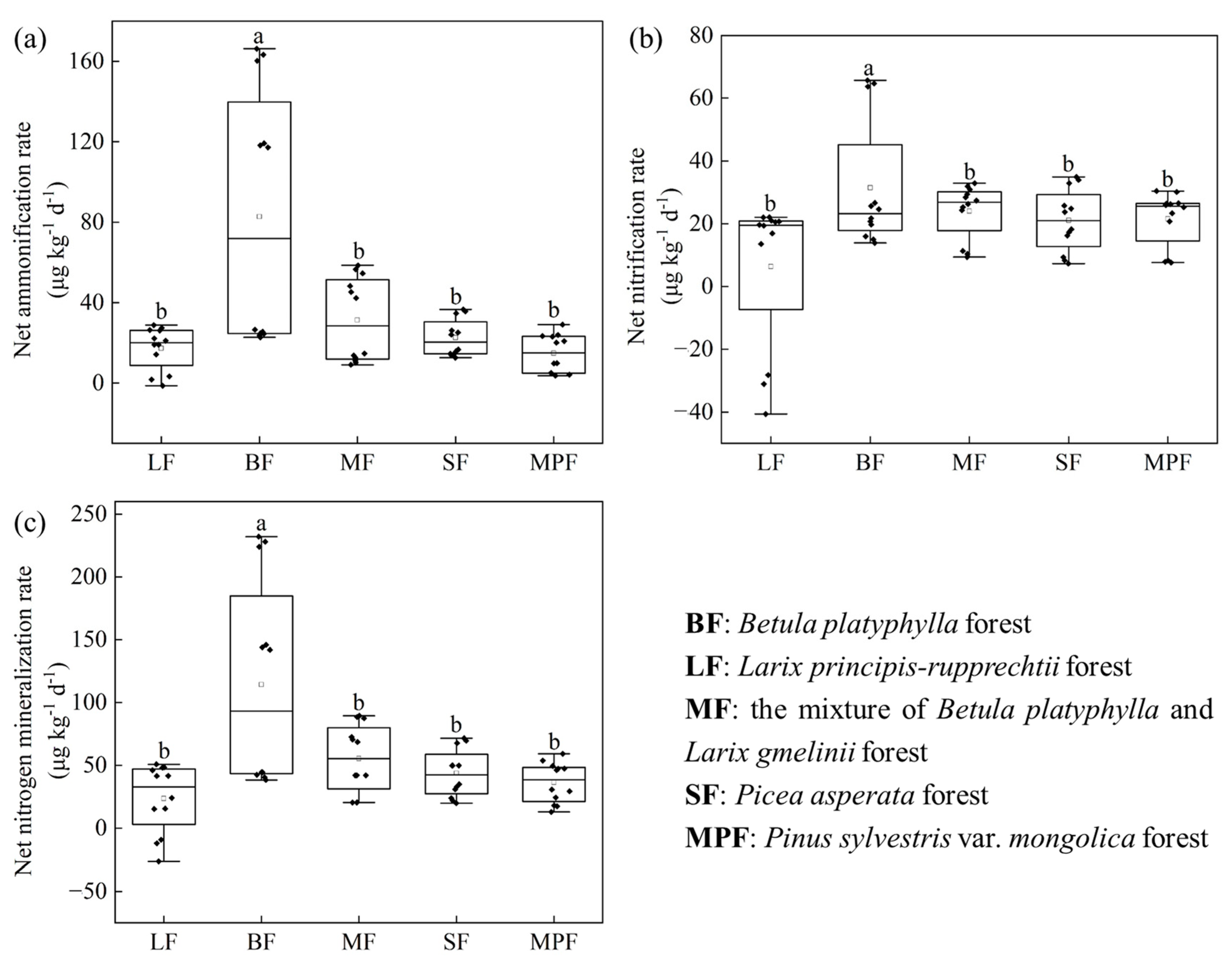
3.1. Soil Net N Mineralization
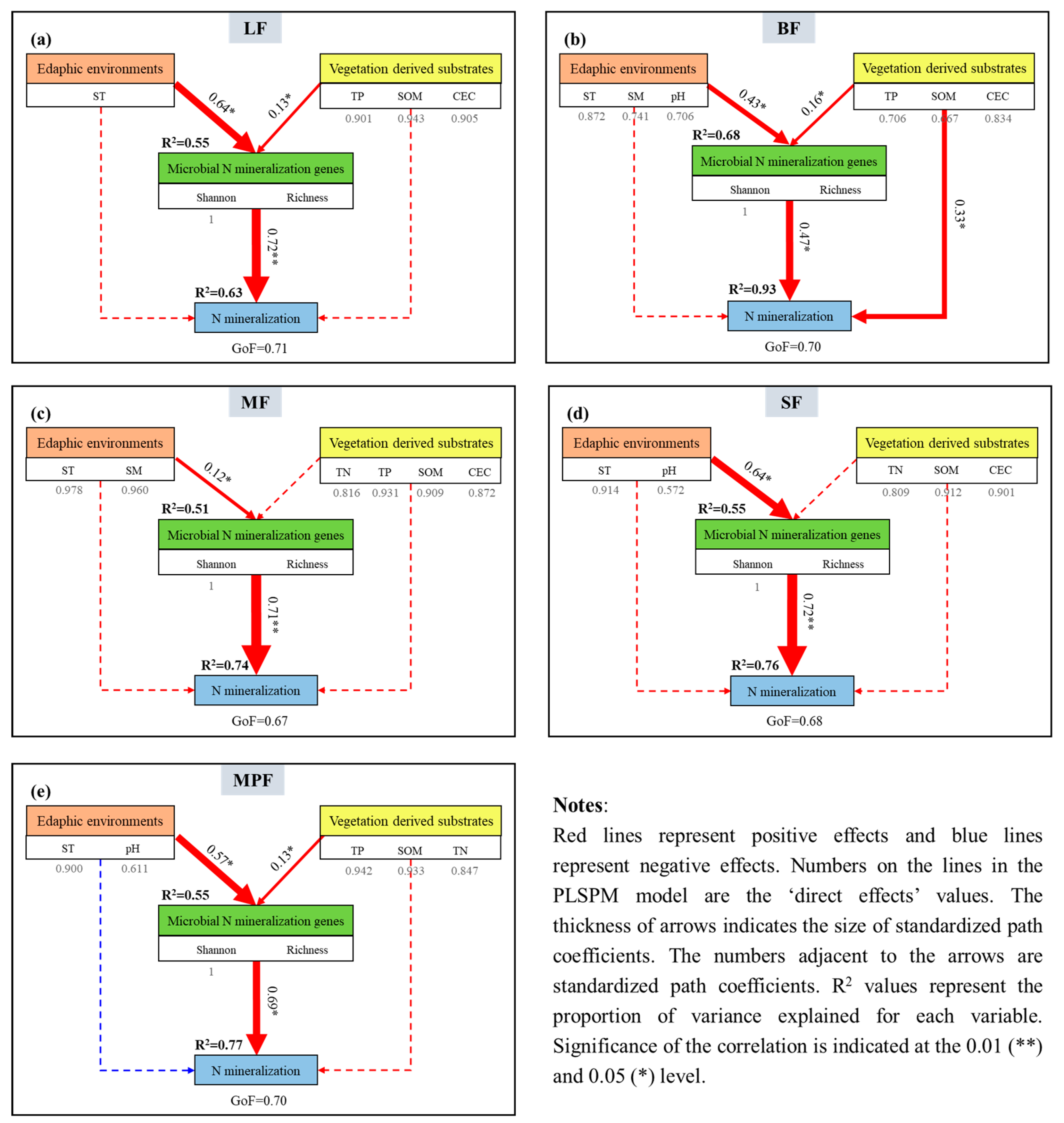
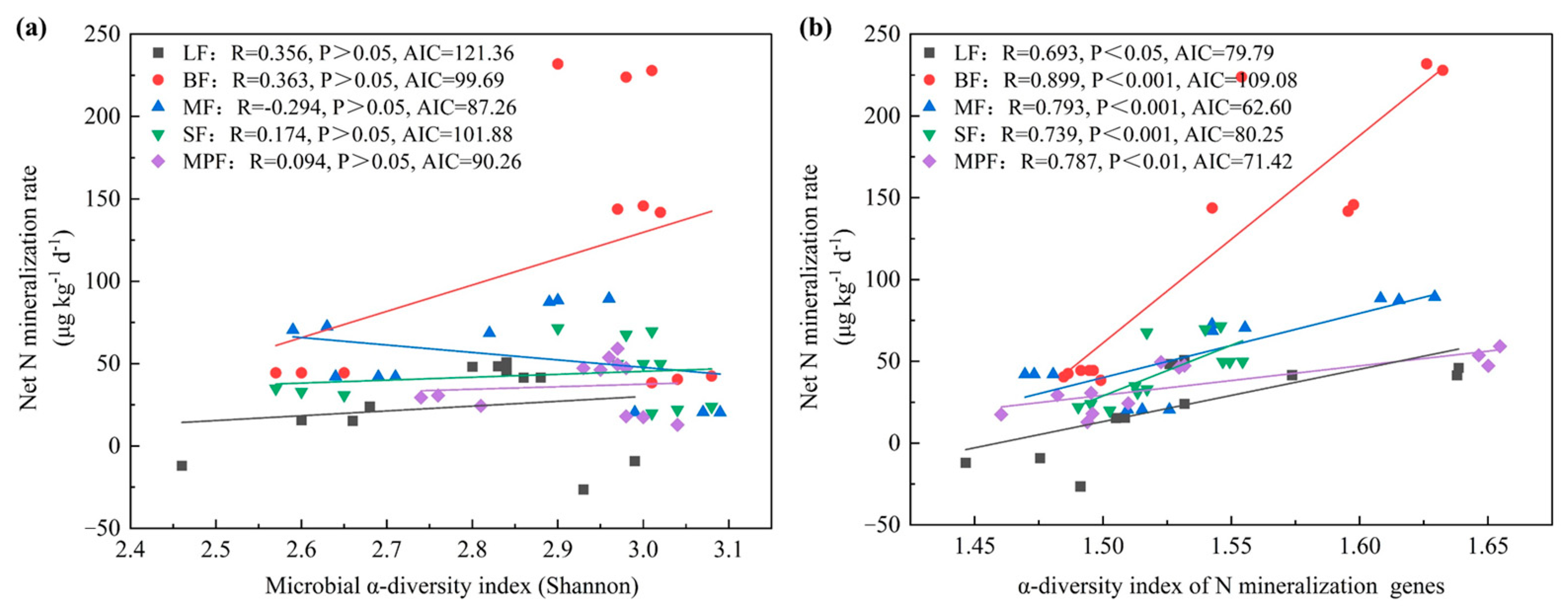
3.2. Relationships among Soil Factors, Microbial Community Diversity and Soil N Mineralization
3.3. Factors Driving Soil N Mineralization
4. Discussion
4.1. Influencing Factors of Soil N Mineralization
4.2. Determinants of Soil N Mineralization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar] [CrossRef]
- Mitchell, M.J. Nitrate dynamics of forested watersheds: Spatial and temporal patterns in North America, Europe and Japan. J. For. Res. 2011, 16, 333. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhao, W.; Hu, H.; Feng, X.; Müller, C.; Cai, Z. Soil gross nitrogen transformations along the Northeast China Transect (NECT) and their response to simulated rainfall events. Sci. Rep. 2016, 6, 22830. [Google Scholar] [CrossRef]
- Dannenmann, M.; Gasche, R.; Papen, H. Nitrogen turnover and N2O production in the forest floor of beech stands as influenced by forest management. J. Plant Nutr. Soil Sci. 2007, 170, 134–144. [Google Scholar] [CrossRef]
- Keuper, F.; Dorrepaal, E.; van Bodegom, P.M.; van Logtestijn, R.; Venhuizen, G.; van Hal, J.; Aerts, R. Experimentally increased nutrient availability at the permafrost thaw front selectively enhances biomass production of deep-rooting subarctic peatland species. Glob. Chang. Biol. 2017, 23, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; He, N.; Wen, X.; Gao, Y.; Li, S.; Niu, S.; Butterbach-Bahl, K.; Luo, Y.; Yu, G. A global synthesis of the rate and temperature sensitivity of soil nitrogen mineralization: Latitudinal patterns and mechanisms. Glob. Chang. Biol. 2017, 23, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Groffman, P.M. Nitrogen transformations. In Soil Microbiology, Ecology and Biochemistry, 5th ed.; Paul, E.A., Frey, S.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 407–438. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 12733–12737. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Urakawa, R.; Ohte, N.; Shibata, H.; Isobe, K.; Tateno, R.; Oda, T.; Hishi, T.; Fukushima, K.; Inagaki, Y.; Hirai, K.; et al. Factors contributing to soil nitrogen mineralization and nitrification rates of forest soils in the Japanese archipelago. For. Ecol. Manag. 2016, 361, 382–396. [Google Scholar] [CrossRef]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil respiration and rates of soil carbon turnover differ among six common European tree species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Ste-Marie, C.; Paré, D. Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol. Biochem. 1999, 31, 1579–1589. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; O’Connell, A.M.; Carlyle, J.C.; Smethurst, P.J.; Khanna, P.K. Defining the relation between soil water content and net nitrogen mineralization. Eur. J. Soil Sci. 2003, 54, 39–48. [Google Scholar] [CrossRef]
- Xue, K.; Yuan, M.M.; Shi, Z.J.; Qin, Y.; Deng, Y.; Cheng, L.; Wu, L.; He, Z.; Van Nostrand, J.D.; Bracho, R.; et al. Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 2016, 6, 595–600. [Google Scholar] [CrossRef]
- Nelson, M.B.; Martiny, A.C.; Martiny, J.B.H. Global biogeography of microbial nitrogen-cycling traits in soil. Proc. Natl. Acad. Sci. USA 2016, 113, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Monteux, S.; Keuper, F.; Fontaine, S.; Gavazov, K.; Hallin, S.; Juhanson, J.; Krab, E.J.; Revaillot, S.; Verbruggen, E.; Walz, J.; et al. Carbon and nitrogen cycling in Yedoma permafrost controlled by microbial functional limitations. Nat. Geosci. 2020, 13, 794–798. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Hu, C.; Wu, L.; Duan, Y.; Zhang, W.; Aziz, T.; Cai, A.; Abrar, M.M.; Xu, M. Soil and microbial biomass stoichiometry regulate soil organic carbon and nitrogen mineralization in rice-wheat rotation subjected to long-term fertilization. J. Soils Sediments 2020, 20, 3103–3113. [Google Scholar] [CrossRef]
- Liu, Y.; He, N.P.; Wen, X.F.; Yu, G.R.; Gao, Y.; Jia, Y.L. Patterns and regulating mechanisms of soil nitrogen mineralization and temperature sensitivity in Chinese terrestrial ecosystems. Agric. Ecosyst. Environ. 2016, 215, 40–46. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; de Neve, S. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. GCB Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Rousk, K.; Michelsen, A.; Rousk, J. Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatments. Glob. Chang. Biol. 2016, 22, 4150–4161. [Google Scholar] [CrossRef]
- Bagheri Novair, S.; Cheraghi, M.; Faramarzi, F.; Asgari Lajayer, B.; Senapathi, V.; Astatkie, T.; Price, G.W. Reviewing the role of biochar in paddy soils: An agricultural and environmental perspective. Ecotoxicol. Environ. Saf. 2023, 263, 115228. [Google Scholar] [CrossRef]
- Cheng, J.M.; Zhao, M.X.; Cong, J.; Qi, Q.; Xiao, Y.; Cong, W.; Deng, Y.; Zhou, J.Z.; Zhang, Y.G. Soil pH exerts stronger impacts than vegetation type and plant diversity on soil bacterial community composition in subtropical broad-leaved forests. Plant Soil 2020, 450, 273–286. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kou, D.; Chen, Y.; Xue, K.; Ernakovich, J.G.; Chen, L.; Yang, G.; Yang, Y. Altered microbial structure and function after thermokarst formation. Glob. Chang. Biol. 2021, 27, 823–835. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, Y.; Wang, S.; Xu, D.; Yu, H.; Wu, L.; Lin, Q.; Hu, Y.; Li, X.; He, Z.; et al. The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J. 2014, 8, 430–440. [Google Scholar] [CrossRef]
- Huang, Z.; Wan, X.; He, Z.; Yu, Z.; Wang, M.; Hu, Z.; Yang, Y. Soil microbial biomass, community composition and soil nitrogen cycling in relation to tree species in subtropical China. Soil Biol. Biochem. 2013, 62, 68–75. [Google Scholar] [CrossRef]
- Liu, S.; Da, L.; Cheng, R.; Wu, J.; Yang, H.; Shi, Z. Temporal variability in soil net nitrogen mineralization among forest regeneration patterns in eastern Tibetan Plateau. Ecol. Indic. 2021, 128, 107811. [Google Scholar] [CrossRef]
- Li, Z.; Tian, D.; Wang, B.; Wang, J.; Wang, S.; Chen, H.Y.H.; Xu, X.; Wang, C.; He, N.; Niu, S. Microbes drive global soil nitrogen mineralization and availability. Glob. Chang. Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef]
- Zhong, Z.; Makeschin, F. Differences of soil microbial biomass and nitrogen transformation under two forest types in central Germany. Plant Soil 2006, 283, 287–297. [Google Scholar] [CrossRef]
- Shu, D.; Guo, Y.; Zhang, B.; Zhang, C.; Van Nostrand, J.D.; Lin, Y.; Zhou, J.; Wei, G. Rare prokaryotic sub-communities dominate the complexity of ecological networks and soil multinutrient cycling during long-term secondary succession in China’s Loess Plateau. Sci. Total Environ. 2021, 774, 145737. [Google Scholar] [CrossRef] [PubMed]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef]
- LaRoche, J.; Breitbarth, E. Importance of the diazotrophs as a source of new nitrogen in the ocean. J. Sea Res. 2005, 53, 67–91. [Google Scholar] [CrossRef]
- Myrold, D.D.; Zeglin, L.H.; Jansson, J.K. The Potential of Metagenomic Approaches for Understanding Soil Microbial Processes. Soil Sci. Soc. Am. J. 2014, 78, 3–10. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wei, G. Biogeography and ecological diversity patterns of rare and abundant bacteria in oil-contaminated soils. Mol. Ecol. 2017, 26, 5305–5317. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xia, F.; Chang, S.X.; Brookes, P.C.; Dahlgren, R.A.; et al. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, K.; Denman, S.; Clark, D.R.; Forster, J.; Vanguelova, E.; Brown, N.; Whitby, C. Relationships between nitrogen cycling microbial community abundance and composition reveal the indirect effect of soil pH on oak decline. ISME J. 2021, 15, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, L.; Kushwaha, S.K.; Ahrén, D.; Hedlund, K. Agricultural land use determines functional genetic diversity of soil microbial communities. Soil Biol. Biochem. 2017, 115, 423–432. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Deng, Y.; Cong, J.; Lu, H.; Sun, X.; Yang, C.; Yuan, T.; Van Nostrand, J.D.; Li, D.; et al. Integrated metagenomics and network analysis of soil microbial community of the forest timberline. Sci. Rep. 2015, 5, 7994. [Google Scholar] [CrossRef]
- Li, Y.; Tremblay, J.; Bainard, L.D.; Cade-Menun, B.; Hamel, C. Long-term effects of nitrogen and phosphorus fertilization on soil microbial community structure and function under continuous wheat production. Environ. Microbiol. 2020, 22, 1066–1088. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, X.; Feng, Z.; Xiao, M.; Ge, T.; Li, Y.; Yao, H. Polyethylene microplastics alter the microbial functional gene abundances and increase nitrous oxide emissions from paddy soils. J. Hazard. Mater. 2022, 432, 128721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.; Cheng, X.; Zhang, Z.; Zhang, Q. Changes in soil properties rather than functional gene abundance control carbon and nitrogen mineralization rates during long-term natural revegetation. Plant Soil 2019, 443, 293–306. [Google Scholar] [CrossRef]
- Sponseller, R.A.; Gundale, M.J.; Futter, M.; Ring, E.; Nordin, A.; Näsholm, T.; Laudon, H. Nitrogen dynamics in managed boreal forests: Recent advances and future research directions. Ambio 2016, 45, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Man, X.; Duan, B.; Cai, T.; Ge, Z.; Li, X.; Vesala, T. Changes in soil bacterial communities and nitrogen mineralization with understory vegetation in boreal larch forests. Soil Biol. Biochem. 2022, 166, 108572. [Google Scholar] [CrossRef]
- Ribbons, R.R.; Levy-Booth, D.J.; Masse, J.; Grayston, S.J.; McDonald, M.A.; Vesterdal, L.; Prescott, C.E. Linking microbial communities, functional genes and nitrogen-cycling processes in forest floors under four tree species. Soil Biol. Biochem. 2016, 103, 181–191. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Yan, B.; Liu, G. The responses of soil nitrogen transformation to nitrogen addition are mainly related to the changes in functional gene relative abundance in artificial Pinus tabulaeformis forests. Sci. Total Environ. 2020, 723, 137679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, G.; Huang, T.; Feng, Y.; Tian, W.; Wu, X. Mixed Forest of Larix principis-rupprechtii and Betula platyphylla Modulating Soil Fauna Diversity and Improving Faunal Effect on Litter Decomposition. Forests 2022, 13, 703. [Google Scholar] [CrossRef]
- Raison, R.J.; Connell, M.J.; Khanna, P.K. Methodology for studying fluxes of soil mineral-N in situ. Soil Biol. Biochem. 1987, 19, 521–530. [Google Scholar] [CrossRef]
- Becker, H.; Uri, V.; Aosaar, J.; Varik, M.; Mander, Ü.; Soosaar, K.; Hansen, R.; Teemusk, A.; Morozov, G.; Kutti, S.; et al. The effects of clear-cut on net nitrogen mineralization and nitrogen losses in a grey alder stand. Ecol. Eng. 2015, 85, 237–246. [Google Scholar] [CrossRef]
- Wang, H.; Deng, N.; Wu, D.; Hu, S.; Kou, M. Long-term net transformation and quantitative molecular mechanisms of soil nitrogen during natural vegetation recovery of abandoned farmland on the Loess Plateau of China. Sci. Total Environ. 2017, 607, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Yu, P.; Wen, X.; Wang, W.; Jia, J.; Wang, X. Effects of cadmium addition on net nitrogen mineralization processes in the urban constructed wetland soils of a Chinese delta. Environ. Geochem. Health 2021, 43, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, X.; Song, Y. Effects of Different Land Use Patterns on Soil Organic Matter and Soil Cycle Process. IOP Conf. Ser. Earth Environ. Sci. 2021, 804, 022101. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 595–624. [Google Scholar]
- Liu, C.J.; Gong, X.W.; Dang, K.; Li, J.; Yang, P.; Gao, X.L.; Deng, X.P.; Feng, B.L. Linkages between nutrient ratio and the microbial community in rhizosphere soil following fertilizer management. Environ. Res. 2020, 184, 109261. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Soil and Agro-Chemical Analytical Methods; Agricultural Technical Press of China: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Bracho, R.; Natali, S.; Pegoraro, E.; Crummer, K.G.; Schädel, C.; Celis, G.; Hale, L.; Wu, L.; Yin, H.; Tiedje, J.M.; et al. Temperature sensitivity of organic matter decomposition of permafrost-region soils during laboratory incubations. Soil Biol. Biochem. 2016, 97, 1–14. [Google Scholar] [CrossRef]
- Paula, F.S.; Rodrigues, J.L.M.; Zhou, J.Z.; Wu, L.Y.; Mueller, R.C.; Mirza, B.S.; Bohannan, B.J.M.; Nusslein, K.; Deng, Y.; Tiedje, J.M.; et al. Land use change alters functional gene diversity, composition and abundance in Amazon forest soil microbial communities. Mol. Ecol. 2014, 23, 2988–2999. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, F.; Rong, X.; Fan, Z. Plant-soil properties associated with Nitrogen mineralization: Effect of conversion of natural secondary forests to Larch plantations in a headwater catchment in Northeast China. Forests 2018, 9, 386. [Google Scholar] [CrossRef]
- Burns, D.A.; Murdoch, P.S. Effects of a clearcut on the net rates of nitrification and N mineralization in a northern hardwood forest, Catskill Mountains, New York, USA. Biogeochemistry 2005, 72, 123–146. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, Y.; Niu, L.; Xu, X.; Tian, Y. Interactive effects of soil temperature and moisture on soil N mineralization in a Stipa krylovii grassland in Inner Mongolia, China. J. Arid Land 2014, 6, 571–580. [Google Scholar] [CrossRef]
- Mylliemngap, W.; Nath, D.; Barik, S.K. Changes in vegetation and nitrogen mineralization during recovery of a montane subtropical broadleaved forest in North-eastern India following anthropogenic disturbance. Ecol. Res. 2016, 31, 21–38. [Google Scholar] [CrossRef]
- Carmosini, N.; Devito, K.J.; Prepas, E.E. Net nitrogen mineralization and nitrification in trembling aspen forest soils on the Boreal Plain. Can. J. For. Res. 2003, 33, 2262–2268. [Google Scholar] [CrossRef]
- Elrys, A.S.; Wang, J.; Metwally, M.A.S.; Cheng, Y.; Zhang, J.-B.; Cai, Z.C.; Chang, S.X.; Müller, C. Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob. Chang. Biol. 2021, 27, 6512–6524. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, H.; Gao, K.; Fang, Y.; Wang, K.; Zhu, T.; Zhu, J.; Cheng, Y.; Li, D. Plant species diversity enhances soil gross nitrogen transformations in a subtropical forest, southwest China. J. Appl. Ecol. 2023, 60, 1364–1375. [Google Scholar] [CrossRef]
- Elrys, A.S.; Chen, Z.; Wang, J.; Uwiragiye, Y.; Helmy, A.M.; Desoky, E.S.M.; Cheng, Y.; Zhang, J.B.; Cai, Z.C.; Müller, C. Global patterns of soil gross immobilization of ammonium and nitrate in terrestrial ecosystems. Glob. Chang. Biol. 2022, 28, 4472–4488. [Google Scholar] [CrossRef]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Kirby, R. Actinomycetes and lignin degradation. Adv. Appl. Microbiol. 2005, 58, 125–168. [Google Scholar] [CrossRef]
- Chen, Q.L.; Ding, J.; Li, C.Y.; Yan, Z.Z.; He, J.Z.; Hu, H.W. Microbial functional attributes, rather than taxonomic attributes, drive top soil respiration, nitrification and denitrification processes. Sci. Total Environ. 2020, 734, 139479. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Imamura, S.; Tatsumi, C.; Taniguchi, T.; Tateno, R. Microbial functions and soil nitrogen mineralisation processes in the soil of a cool temperate forest in northern Japan. Biogeochemistry 2021, 155, 359–379. [Google Scholar] [CrossRef]
- Liang, Y.T.; Xiao, X.; Nuccio, E.E.; Yuan, M.T.; Zhang, N.; Xue, K.; Cohan, F.M.; Zhou, J.Z.; Sun, B. Differentiation strategies of soil rare and abundant microbial taxa in response to changing climatic regimes. Environ. Microbiol. 2020, 22, 1327–1340. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Trivedi, C.; Hu, H.W.; Anderson, I.C.; Jeffries, T.C.; Zhou, J.Z.; Singh, B.K. Microbial regulation of the soil carbon cycle: Evidence from gene-enzyme relationships. ISME J. 2016, 10, 2593–2604. [Google Scholar] [CrossRef]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; Garcia-Gomez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef]
- Wieder, W.R.; Allison, S.D.; Davidson, E.A.; Georgiou, K.; Hararuk, O.; He, Y.J.; Hopkins, F.; Luo, Y.Q.; Smith, M.J.; Sulman, B.; et al. Explicitly representing soil microbial processes in Earth system models. Glob. Biogeochem. Cycles 2015, 29, 1782–1800. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, H.; Wang, Z.; Tian, Y.; Tian, W.; Liu, Z. Diversity of Microbial Functional Genes Promotes Soil Nitrogen Mineralization in Boreal Forests. Microorganisms 2024, 12, 1577. https://doi.org/10.3390/microorganisms12081577
Zhang X, Zhang H, Wang Z, Tian Y, Tian W, Liu Z. Diversity of Microbial Functional Genes Promotes Soil Nitrogen Mineralization in Boreal Forests. Microorganisms. 2024; 12(8):1577. https://doi.org/10.3390/microorganisms12081577
Chicago/Turabian StyleZhang, Xiumin, Huayong Zhang, Zhongyu Wang, Yonglan Tian, Wang Tian, and Zhao Liu. 2024. "Diversity of Microbial Functional Genes Promotes Soil Nitrogen Mineralization in Boreal Forests" Microorganisms 12, no. 8: 1577. https://doi.org/10.3390/microorganisms12081577




