Pre- to Postbiotics: The Beneficial Roles of Pediatric Dysbiosis Associated with Inflammatory Bowel Diseases
Abstract
:1. Introduction
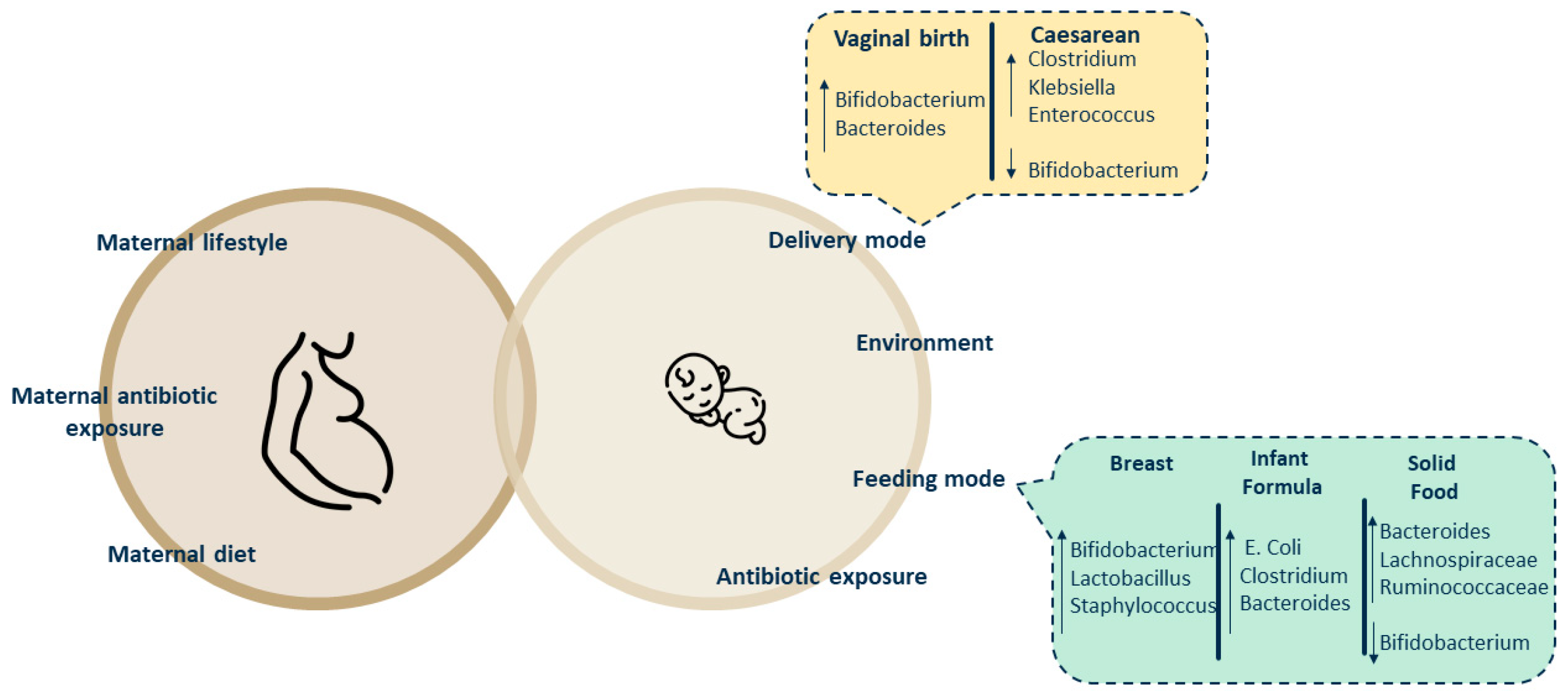
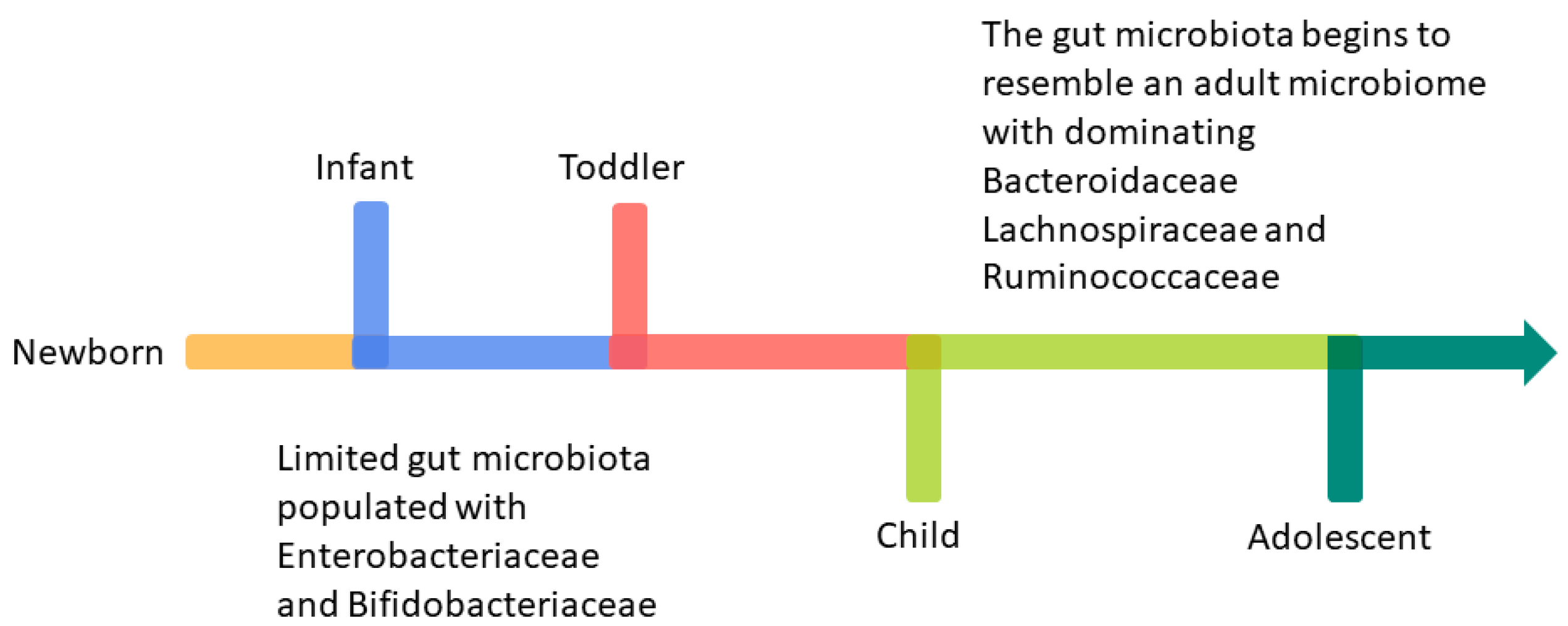
Development of Infant Intestinal Microbiota
2. Methods
3. Prebiotics, Probiotics, Synbiotics, and Paraprobiotics: Their Effects on IBD Intestinal Dysbiosis Affecting Children
3.1. Prebiotics
- Indigestible by the host’s enzyme.
- Fermented selectively by gastrointestinal microbiota.
3.2. Probiotics
3.3. Synbiotics
- Complementary, where the selection of the probiotic is based on beneficial impacts intended for the host, while the prebiotic is selected separately to enhance the levels of beneficial microbial components. The prebiotic can support the growth and activity of the probiotic but does so indirectly as part of its broader target spectrum.
- Synergistic, in which the probiotic is again chosen based on specific beneficial effects on the host, but the prebiotic is chosen to stimulate specifically the growth and activity of the selected probiotic. In this case, the prebiotic is selected to have a higher affinity for the probiotic and is chosen to enhance its survival and growth in the host. It can also increase levels of microbiota beneficial in the host, but the main target is the ingested probiotic.
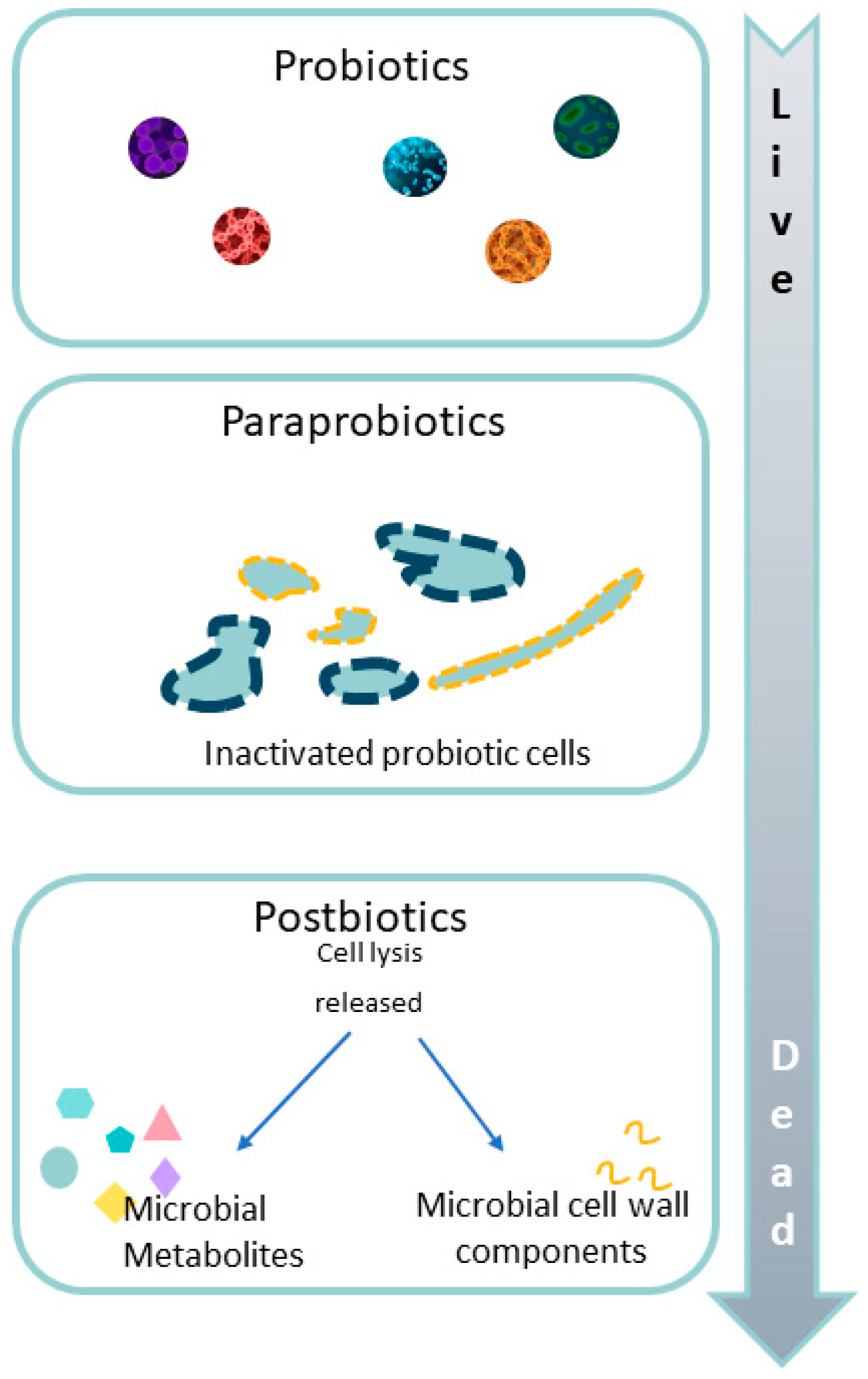
3.4. Paraprobiotics
3.5. Next-Generation Probiotics
4. Postbiotics, Metabiotics, Biogenics, or Simply Microbiota Metabolites: Beyond Probiotics and Prebiotics on Pediatric Intestinal Dysbiosis in IBD
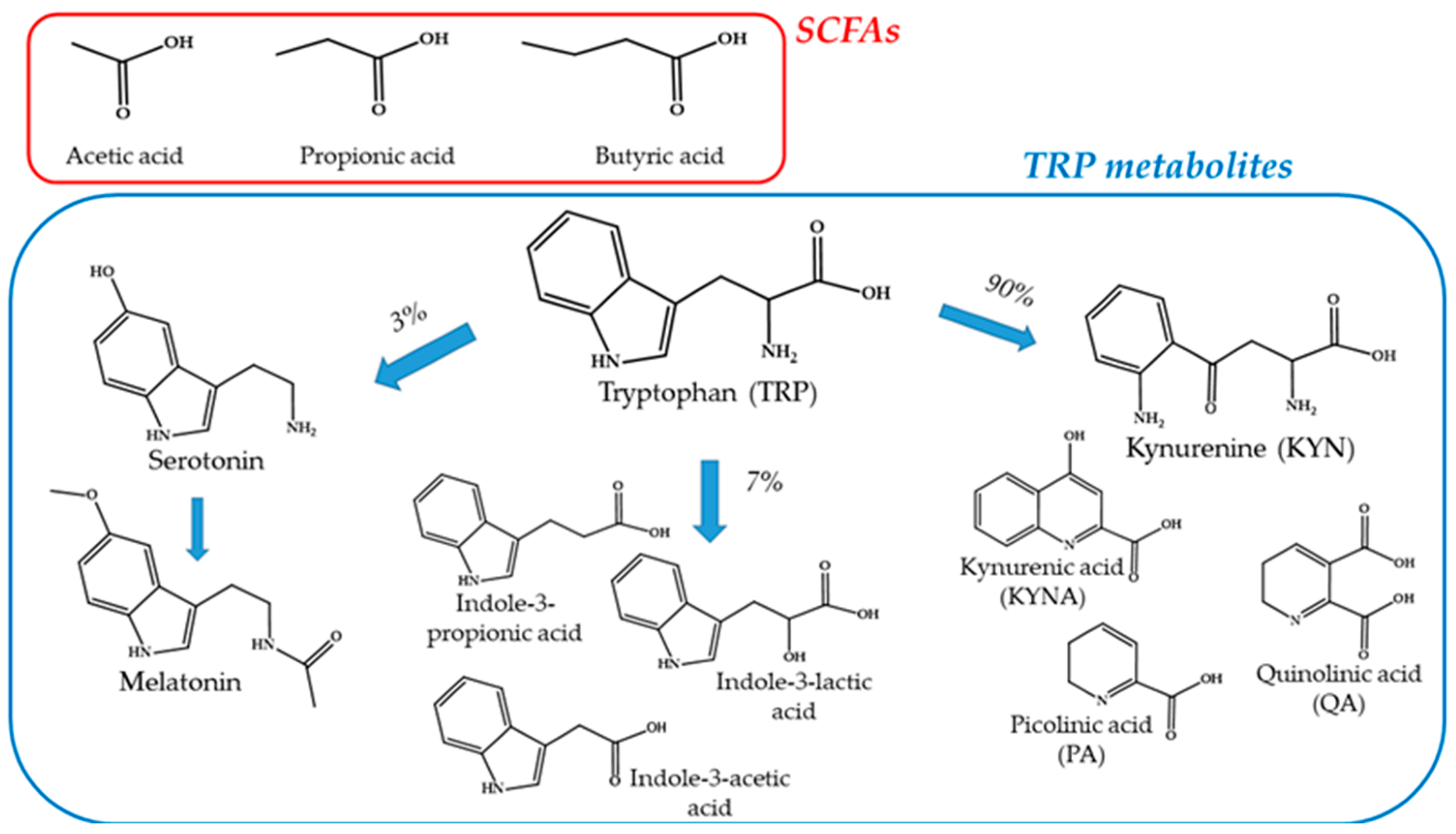
4.1. Short-Chain Fatty Acids (SCFAs)
4.2. Tryptophan Metabolites
5. Other Supplements
5.1. Polyunsaturated Fatty Acids
5.2. Vitamin D
5.3. Minerals: Zinc
6. Other Therapies for Pediatric IBD
6.1. Fecal Microbiota Transplantation (FMT)
6.2. Vaginal Seeding
7. Clinical Trials
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Landman, C.; Quévrain, E. Le microbiote intestinal: Description, rôle et implication physiopathologique. Rev. Med. Interne 2016, 37, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Selber-Hnatiw, S.; Rukundo, B.; Ahmadi, M.; Akoubi, H.; Al-Bizri, H.; Aliu, A.F.; Ambeaghen, T.U.; Avetisyan, L.; Bahar, I.; Baird, A.; et al. Human Gut Microbiota: Toward an Ecology of Disease. Front. Microbiol. 2017, 8, 1265. [Google Scholar] [CrossRef]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in inflammatory bowel disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef]
- Thapar, N.; Benninga, M.A.; Crowell, M.D.; Di Lorenzo, C.; Mack, I.; Nurko, S.; Saps, M.; Shulman, R.J.; Szajewska, H.; van Tilburg, M.A.L.; et al. Paediatric functional abdominal pain disorders. Nat. Rev. Dis. Primers 2020, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Sung, V.; D’Amico, F.; Cabana, M.D.; Chau, K.; Koren, G.; Savino, F.; Szajewska, H.; Deshpande, G.; Dupont, C.; Indrio, F.; et al. Lactobacillus reuteri to Treat Infant Colic: A Meta-analysis. Pediatrics 2018, 141, e20171811. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, J.R.; Claud, E.C. Necrotizing Enterocolitis and the Preterm Infant Microbiome. Adv. Exp. Med. Biol. 2019, 1125, 25–36. [Google Scholar] [CrossRef]
- Zhang, S.; Qian, Y.; Li, Q.; Xu, X.; Li, X.; Wang, C.; Cai, H.; Zhu, J.; Yu, Y. Metabolic and Neural Mechanisms Underlying the Associations Between Gut Bacteroides and Cognition: A Large-Scale Functional Network Connectivity Study. Front. Neurosci. 2021, 15, 750704. [Google Scholar] [CrossRef]
- Saeed, N.K.; Al-Beltagi, M.; Bediwy, A.S.; El-Sawaf, Y.; Toema, O. Gut microbiota in various childhood disorders: Implication and indications. World J. Gastroenterol. 2022, 28, 1875–1901. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.L.; Hornig, M.; Parekh, T.; Lipkin, W.I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio 2012, 3, 10–1128. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
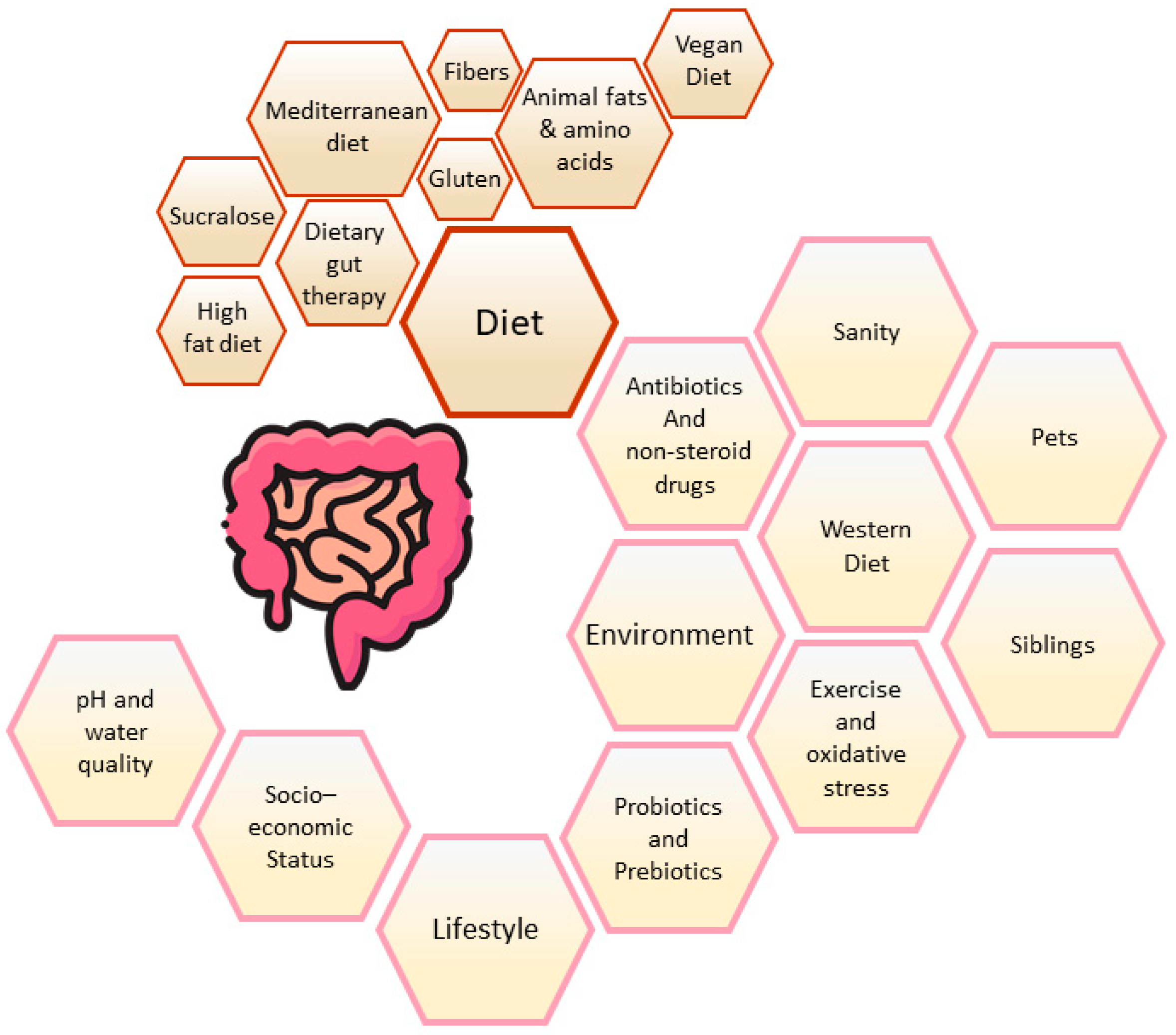
- Bajinka, O.; Tan, Y.; Abdelhalim, K.A.; Özdemir, G.; Qiu, X. Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Expr. 2020, 10, 130. [Google Scholar] [CrossRef]
- Chiba, M.; Nakane, K.; Komatsu, M. Westernized Diet is the Most Ubiquitous Environmental Factor in Inflammatory Bowel Disease. Perm. J. 2019, 23, 18–107. [Google Scholar] [CrossRef] [PubMed]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; van Schaik, F.D.M.; Tjønneland, A.; Olsen, A.; Dahm, C.C.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef]
- Sjögren, Y.M.; Jenmalm, M.C.; Böttcher, M.F.; Björkstén, B.; Sverremark-Ekström, E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clin. Exp. Allergy 2009, 39, 518–526. [Google Scholar] [CrossRef]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Sherriff, A.; Golding, J. Hygiene levels in a contemporary population cohort are associated with wheezing and atopic eczema in preschool infants. Arch. Dis. Child. 2002, 87, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Leontiadis, G.I.; Tse, F.; Yuan, Y.; Surette, M.; Moayyedi, P. Differences in Gut Microbiota in Patients With vs Without Inflammatory Bowel Diseases: A Systematic Review. Gastroenterology 2020, 158, 930–946.e1. [Google Scholar] [CrossRef] [PubMed]
- Kuenzig, M.E.; Fung, S.G.; Marderfeld, L.; Mak, J.W.Y.; Kaplan, G.G.; Ng, S.C.; Wilson, D.C.; Cameron, F.; Henderson, P.; Kotze, P.G.; et al. Twenty-first Century Trends in the Global Epidemiology of Pediatric-Onset Inflammatory Bowel Disease: Systematic Review. Gastroenterology 2022, 162, 1147–1159.e4. [Google Scholar] [CrossRef] [PubMed]
- Brockway, M. The role of antibiotic exposure and the effects of breastmilk and human milk feeding on the developing infant gut microbiome. Front. Public Health 2024, 12, 1408246. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Järvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J. Allergy Clin. Immunol. 2022, 150, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Rubini, M.; Riboni, S.; Morelli, L.; Bessi, E.; Retetangos, C. Mode of delivery affects the bacterial community in the newborn gut. Early Hum. Dev. 2010, 86 (Suppl. S1), 13–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, Y.; Wu, J.-L. Influence of mode of delivery on infant gut microbiota composition: A pilot study. J. Obstet. Gynaecol. 2024, 44, 2368829. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.M.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 2015, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Kuhle, S.; Tong, O.S.; Woolcott, C.G. Association between caesarean section and childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, E.H.; Wingren, C.J.; Vincente, R.P.; Merlo, J.; Agardh, D. Perinatal risk factors increase the risk of being affected by both type 1 diabetes and coeliac disease. Acta Paediatr. 2015, 104, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Black, M.; Bhattacharya, S.; Philip, S.; Norman, J.E.; McLernon, D.J. Planned Cesarean Delivery at Term and Adverse Outcomes in Childhood Health. JAMA 2015, 314, 2271–2279. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Rushing, J. Cesarean versus vaginal delivery: Long-term infant outcomes and the hygiene hypothesis. Clin. Perinatol. 2011, 38, 321–331. [Google Scholar] [CrossRef]
- Laursen, M.F.; Bahl, M.I.; Michaelsen, K.F.; Licht, T.R. First Foods and Gut Microbes. Front. Microbiol. 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.C.; Totten, S.M.; Huang, J.O.; Nagshbandi, S.; Kirmiz, N.; Garrido, D.A.; Lewis, Z.T.; Wu, L.D.; Smilowitz, J.T.; German, J.B.; et al. Identification of Oligosaccharides in Feces of Breast-fed Infants and Their Correlation with the Gut Microbial Community. Mol. Cell. Proteom. 2016, 15, 2987–3002. [Google Scholar] [CrossRef] [PubMed]
- Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019, 17, e05780. [Google Scholar] [CrossRef] [PubMed]
- Schiess, S.; Grote, V.; Scaglioni, S.; Luque, V.; Martin, F.; Stolarczyk, A.; Vecchi, F.; Koletzko, B. Introduction of complementary feeding in 5 European countries. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Przyrembel, H. Timing of introduction of complementary food: Short- and long-term health consequences. Ann. Nutr. Metab. 2012, 60 (Suppl. S2), 8–20. [Google Scholar] [CrossRef]
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 77, 21–34. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O. Potential role of the intestinal microbiota in programming health and disease. Nutr. Rev. 2015, 73 (Suppl. S1), 32–40. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, S.; Mehta, V.; Ali El Enshasy, H.; Thevarajoo, S.; El Adawi, H.; Zeini, I.; Pham, K.; Varzakas, T.; Abomoelak, B. Efficacy of Probiotics-Based Interventions as Therapy for Inflammatory Bowel Disease: A Recent Update. Saudi J. Biol. Sci. 2022, 29, 3546–3567. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An Updated Review on Prebiotics: Insights on Potentials of Food Seeds Waste as Source of Potential Prebiotics. Molecules 2022, 27, 5947. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; Cruz-Martins, N.; Kuča, K.; Chopra, C.; Singh, R.; Kumar, H.; Șen, F.; et al. Plant Prebiotics and Their Role in the Amelioration of Diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Guarino, M.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.; Murphy, R.; Butts, C.; Brough, L.; Whelan, K.; Coad, J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: A randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br. J. Nutr. 2018, 119, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Culp, E.J.; Goodman, A.L. Cross-feeding in the gut microbiome: Ecology and mechanisms. Cell Host Microbe 2023, 31, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Thomson, J.M.; Flint, H.J. The role of pH in determining the species composition of the human colonic microbiota. Environ. Microbiol. 2009, 11, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Jin, Y.-T.; Duan, Y.; Deng, X.-K.; Lin, J. Prevention of necrotizing enterocolitis in premature infants—An updated review. WJCP 2019, 8, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Roy, B.; Khan, S.; Septer, S.; Umar, S. Microbiome, Metabolome and Inflammatory Bowel Disease. Microorganisms 2016, 4, 20. [Google Scholar] [CrossRef]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: Mechanisms and regulation. Nat. Rev. Microbiol. 2023, 21, 347–360. [Google Scholar] [CrossRef]
- Darb Emamie, A.; Rajabpour, M.; Ghanavati, R.; Asadolahi, P.; Farzi, S.; Sobouti, B.; Darbandi, A. The effects of probiotics, prebiotics and synbiotics on the reduction of IBD complications, a periodic review during 2009–2020. J. Appl. Microbiol. 2021, 130, 1823–1838. [Google Scholar] [CrossRef]
- Naseer, M.; Poola, S.; Ali, S.; Samiullah, S.; Tahan, V. Prebiotics and Probiotics in Inflammatory Bowel Disease: Where are we now and where are we going? CCP 2020, 15, 216–233. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Prebiotics: Preferential substrates for specific germs? Am. J. Clin. Nutr. 2001, 73, 406s–409s. [Google Scholar] [CrossRef]
- Hu, M.; Li, M.; Li, C.; Miao, M.; Zhang, T. Effects of Human Milk Oligosaccharides in Infant Health Based on Gut Microbiota Alteration. J. Agric. Food Chem. 2023, 71, 994–1001. [Google Scholar] [CrossRef]
- Chong, H.-Y.; Tan, L.T.-H.; Law, J.W.-F.; Hong, K.-W.; Ratnasingam, V.; Ab Mutalib, N.-S.; Lee, L.-H.; Letchumanan, V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef] [PubMed]
- Ben, X.-M.; Li, J.; Feng, Z.-T.; Shi, S.-Y.; Lu, Y.-D.; Chen, R.; Zhou, X.-Y. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal Bifidobacteria and Lactobacilli. World J. Gastroenterol. 2008, 14, 6564–6568. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.A. In Vitro and Clinical Experiences with a Human Milk Oligosaccharide, Lacto-N- neoTetraose, and Fructooligosaccharides. Foods Food Ingred. J. Jpn. 2005, 210, 1018. [Google Scholar]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Verkhnyatskaya, S.; Ferrari, M.; de Vos, P.; Walvoort, M.T.C. Shaping the Infant Microbiome With Non-digestible Carbohydrates. Front. Microbiol. 2019, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Chen, X.; Bi, Q.; Xiang, Y.; Jin, R.; Ai, H.; Nie, Y. A parallel and cascade control system: Magnetofection of miR125b for synergistic tumor-association macrophage polarization regulation and tumor cell suppression in breast cancer treatment. Nanoscale 2020, 12, 22615–22627. [Google Scholar] [CrossRef]
- Sun, W.; Tao, L.; Qian, C.; Xue, P.; Tong, X.; Yang, L.; Lu, F.; Wan, H.; Tao, Y. Human milk oligosaccharides and the association with microbiota in colostrum: A pilot study. Arch. Microbiol. 2024, 206, 58. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef] [PubMed]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; Da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Development of new probiotics by strain combinations: Is it possible to improve the adhesion to intestinal mucus? J. Dairy Sci. 2007, 90, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sood, U.; Gupta, V.; Singh, M.; Scaria, J.; Lal, R. Recent Advancements in the Development of Modern Probiotics for Restoring Human Gut Microbiome Dysbiosis. Indian J. Microbiol. 2020, 60, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the Microbiota-Gut-Brain Axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Konstantakis, C.; Assimakopoulos, S.F.; Triantos, C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb. Pathog. 2019, 137, 103774. [Google Scholar] [CrossRef] [PubMed]
- Štofilová, J.; Kvaková, M.; Kamlárová, A.; Hijová, E.; Bertková, I.; Guľašová, Z. Probiotic-Based Intervention in the Treatment of Ulcerative Colitis: Conventional and New Approaches. Biomedicines 2022, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wang, C.; Xia, Y.; Tang, J.; Wang, J.; Shen, L. Clostridioides difficile infection in inflammatory bowel disease: A clinical review. Expert Rev. Anti Infect. Ther. 2024, 22, 297–306. [Google Scholar] [CrossRef]
- Siddiqui, A.; Haider, R.; Aaqil, S.I.; Vohra, L.I.; Qamar, K.; Jawed, A.; Fatima, N.; Adnan, A.; Parikh, V.; Ochani, S.; et al. Probiotic formulations and gastro-intestinal diseases in the paediatric population: A narrative review. Ann. Med. Surg. 2024, 86, 2836–2847. [Google Scholar] [CrossRef]
- Turner, D.; Levine, A.; Escher, J.C.; Griffiths, A.M.; Russell, R.K.; Dignass, A.; Dias, J.A.; Bronsky, J.; Braegger, C.P.; Cucchiara, S.; et al. Management of pediatric ulcerative colitis: Joint ECCO and ESPGHAN evidence-based consensus guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 340–361. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar] [CrossRef]
- Huynh, H.Q.; de Bruyn, J.; Guan, L.; Diaz, H.; Li, M.; Girgis, S.; Turner, J.; Fedorak, R.; Madsen, K. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: A pilot study. Inflamm. Bowel Dis. 2009, 15, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns. Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef] [PubMed]
- Corsello, A.; Scatigno, L.; Fiore, G.; Baresi, S.; Eletti, F.; Zuccotti, G.; Strisciuglio, C.; Dilillo, D.; Verduci, E. Nutraceuticals and biotics in pediatric gastrointestinal disorders. Eur. J. Clin. Nutr. 2024, 78, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, S.; Sansotta, N. Probiotics in the Treatment of Inflammatory Bowel Disease. Adv. Exp. Med. Biol. 2019, 1125, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H. What are the indications for using probiotics in children? Arch. Dis. Child. 2016, 101, 398–403. [Google Scholar] [CrossRef]
- Szajewska, H.; Berni Canani, R.; Domellöf, M.; Guarino, A.; Hojsak, I.; Indrio, F.; Lo Vecchio, A.; Mihatsch, W.A.; Mosca, A.; Orel, R.; et al. Probiotics for the Management of Pediatric Gastrointestinal Disorders: Position Paper of the ESPGHAN Special Interest Group on Gut Microbiota and Modifications. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef]
- Steed, H.; Macfarlane, G.T.; Blackett, K.L.; Bahrami, B.; Reynolds, N.; Walsh, S.V.; Cummings, J.H.; Macfarlane, S. Clinical trial: The microbiological and immunological effects of synbiotic consumption—A randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment. Pharmacol. Ther. 2010, 32, 872–883. [Google Scholar] [CrossRef]
- Jiménez-Villeda, B.E.; Falfán-Cortés, R.N.; Rangel-Vargas, E.; Santos-López, E.M.; Gómez-Aldapa, C.A.; Torres-Vitela, M.R.; Villarruel-López, A.; Castro-Rosas, J. Synbiotic Encapsulation: A Trend towards Increasing Viability and Probiotic Effect. J. Food Process. Preserv. 2023, 2023, 1–20. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- Lemoine, A.; Tounian, P.; Adel-Patient, K.; Thomas, M. Pre-, pro-, syn-, and Postbiotics in Infant Formulas: What Are the Immune Benefits for Infants? Nutrients 2023, 15, 1231. [Google Scholar] [CrossRef] [PubMed]
- Ferro, L.E.; Crowley, L.N.; Bittinger, K.; Friedman, E.S.; Decker, J.E.; Russel, K.; Katz, S.; Kim, J.K.; Trabulsi, J.C. Effects of prebiotics, probiotics, and synbiotics on the infant gut microbiota and other health outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2023, 63, 5620–5642. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Schnorr, C.E.; Pasquali, M.A.d.B. Paraprobiotics and Postbiotics-Current State of Scientific Research and Future Trends toward the Development of Functional Foods. Foods 2023, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Dahiya, D.K.; Renuka; Dangi, A.K.; Shandilya, U.K.; Puniya, A.K.; Shukla, P. New-Generation Probiotics. In Microbiome and Metabolome in Diagnosis, Therapy, and other Strategic Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 417–424. ISBN 9780128152492. [Google Scholar]
- Hizay, A.; Dag, K.; Oz, N.; Comak-Gocer, E.M.; Ozbey-Unlu, O.; Ucak, M.; Keles-Celik, N. Lactobacillus acidophilus regulates abnormal serotonin availability in experimental ulcerative colitis. Anaerobe 2023, 80, 102710. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics-A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, S.; Jia, J.; Huang, L.; Li, F.; Jin, F.; Ren, Z.; Wang, Y. Intestinal Microbiota-Associated Metabolites: Crucial Factors in the Effectiveness of Herbal Medicines and Diet Therapies. Front. Physiol. 2019, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. A Narrative Review on the Advance of Probiotics to Metabiotics. J. Microbiol. Biotechnol. 2024, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kang, D.-K.; Paik, H.-D.; Park, Y.-S. Beyond probiotics: A narrative review on an era of revolution. Food Sci. Biotechnol. 2023, 32, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Levy, M. New Approaches to Microbiome-Based Therapies. mSystems 2019, 4, e00122-19. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; de Paepe, K.; van de Wiele, T. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar] [CrossRef]
- Shen, G.; Wu, J.; Ye, B.-C.; Qi, N. Gut Microbiota-Derived Metabolites in the Development of Diseases. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6658674. [Google Scholar] [CrossRef]
- Morniroli, D.; Vizzari, G.; Consales, A.; Mosca, F.; Giannì, M.L. Postbiotic Supplementation for Children and Newborn’s Health. Nutrients 2021, 13, 781. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for Preterm Neonates-The Next Frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for Preventing and Treating Common Infectious Diseases in Children: A Systematic Review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef]
- Zagato, E.; Mileti, E.; Massimiliano, L.; Fasano, F.; Budelli, A.; Penna, G.; Rescigno, M. Lactobacillus paracasei CBA L74 metabolic products and fermented milk for infant formula have anti-inflammatory activity on dendritic cells in vitro and protective effects against colitis and an enteric pathogen in vivo. PLoS ONE 2014, 9, e87615. [Google Scholar] [CrossRef]
- Athalye-Jape, G.; Rao, S.; Simmer, K.; Patole, S. Bifidobacterium breve M-16V as a Probiotic for Preterm Infants: A Strain-Specific Systematic Review. JPEN J. Parenter. Enter. Nutr. 2018, 42, 677–688. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Bistoletti, M.; Bosi, A.; Banfi, D.; Giaroni, C.; Baj, A. The microbiota-gut-brain axis: Focus on the fundamental communication pathways. Prog. Mol. Biol. Transl. Sci. 2020, 176, 43–110. [Google Scholar] [CrossRef]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of Microbial Metabolites on Microbiota-Gut-Brain Axis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Annison, G.; Topping, D.L. Nutritional role of resistant starch: Chemical structure vs. physiological function. Annu. Rev. Nutr. 1994, 14, 297–320. [Google Scholar] [CrossRef]
- Cherbut, C.; Ferrier, L.; Rozé, C.; Anini, Y.; Blottière, H.; Lecannu, G.; Galmiche, J.P. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am. J. Physiol. 1998, 275, G1415–G1422. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-H.; Park, B.O.; Kwak, Y.S. Perspectives on the therapeutic potential of short-chain fatty acid receptors. BMB Rep. 2014, 47, 173–178. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133, 2485S–2493S. [Google Scholar] [CrossRef]
- Vinolo, M.A.R.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef]
- Li, M.; van Esch, B.C.A.M.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide- or Tumor Necrosis Factor α-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef]
- Weng, M.; Walker, W.A.; Sanderson, I.R. Butyrate regulates the expression of pathogen-triggered IL-8 in intestinal epithelia. Pediatr. Res. 2007, 62, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.M.; Koetsier, M.A.; van Deventer, S.J.H.; van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Rudiansyah, M.; Abdalkareem Jasim, S.; Azizov, B.S.; Samusenkov, V.; Kamal Abdelbasset, W.; Yasin, G.; Mohammad, H.J.; Jawad, M.A.; Mahmudiono, T.; Hosseini-Fard, S.R.; et al. The emerging microbiome-based approaches to IBD therapy: From SCFAs to urolithin A. J. Dig. Dis. 2022, 23, 412–434. [Google Scholar] [CrossRef]
- Sugihara, K.; Kamada, N. Diet-Microbiota Interactions in Inflammatory Bowel Disease. Nutrients 2021, 13, 1533. [Google Scholar] [CrossRef] [PubMed]
- Olendzki, B.; Bucci, V.; Cawley, C.; Maserati, R.; McManus, M.; Olednzki, E.; Madziar, C.; Chiang, D.; Ward, D.V.; Pellish, R.; et al. Dietary manipulation of the gut microbiome in inflammatory bowel disease patients: Pilot study. Gut Microbes 2022, 14, 2046244. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.R.; Celiberto, L.S.; Lee, S.M.; Jacobson, K. Fiber and Prebiotic Interventions in Pediatric Inflammatory Bowel Disease: What Role Does the Gut Microbiome Play? Nutrients 2020, 12, 3204. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The Therapeutic Role of Short-Chain Fatty Acids Mediated Very Low-Calorie Ketogenic Diet-Gut Microbiota Relationships in Paediatric Inflammatory Bowel Diseases. Nutrients 2022, 14, 4113. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef] [PubMed]
- Matuszczyk, M.; Meglicka, M.; Landowski, P.; Czkwianianc, E.; Sordyl, B.; Szymańska, E.; Kierkuś, J. Oral exclusive enteral nutrition for induction of clinical remission, mucosal healing, and improvement of nutritional status and growth velocity in children with active Crohn’s disease—A prospective multicentre trial. Prz. Gastroenterol. 2021, 16, 346–351. [Google Scholar] [CrossRef]
- Niseteo, T.; Sila, S.; Trivić, I.; Mišak, Z.; Kolaček, S.; Hojsak, I. Modified Crohn’s disease exclusion diet is equally effective as exclusive enteral nutrition: Real-world data. Nutr. Clin. Pract. 2022, 37, 435–441. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Surono, I.S.; Jalal, F.; Bahri, S.; Romulo, A.; Kusumo, P.D.; Manalu, E.; Yusnita; Venema, K. Differences in immune status and fecal SCFA between Indonesian stunted children and children with normal nutritional status. PLoS ONE 2021, 16, e0254300. [Google Scholar] [CrossRef]
- Śliżewska, K.; Włodarczyk, M.; Sobczak, M.; Barczyńska, R.; Kapuśniak, J.; Socha, P.; Wierzbicka-Rucińska, A.; Kotowska, A. Comparison of the Activity of Fecal Enzymes and Concentration of SCFA in Healthy and Overweight Children. Nutrients 2023, 15, 987. [Google Scholar] [CrossRef] [PubMed]
- Gyarmati, P.; Song, Y.; Dotimas, J.; Yoshiba, G.; Christison, A. Cross-sectional comparisons of gut microbiome and short-chain fatty acid levels among children with varied weight classifications. Pediatr. Obes. 2021, 16, e12750. [Google Scholar] [CrossRef] [PubMed]
- Holmes, Z.C.; Silverman, J.D.; Dressman, H.K.; Wei, Z.; Dallow, E.P.; Armstrong, S.C.; Seed, P.C.; Rawls, J.F.; David, L.A. Short-Chain Fatty Acid Production by Gut Microbiota from Children with Obesity Differs According to Prebiotic Choice and Bacterial Community Composition. mBio 2020, 11, e00914-20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Chan, J.C.Y.; Yap, G.C.; Huang, C.-H.; Kioh, D.Y.Q.; Tham, E.H.; Loo, E.X.L.; Shek, L.P.C.; Karnani, N.; Goh, A.; et al. Evaluation of Stool Short Chain Fatty Acids Profiles in the First Year of Life With Childhood Atopy-Related Outcomes. Front. Allergy 2022, 3, 873168. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, U.; Ludvigsson, J. The concentrations of short-chain fatty acids and other microflora-associated characteristics in faeces from children with newly diagnosed Type 1 diabetes and control children and their family members. Diabet. Med. 2004, 21, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Ivanko, O.H.; Bilykh, V.M. Fecal concentrations of lactic acid and short-chain fatty acids in young children hospitalized in an infectious-diagnostic hospital with diarrhea. ZMJ 2022, 24, 332–337. [Google Scholar] [CrossRef]
- Demehri, F.R.; Frykman, P.K.; Cheng, Z.; Ruan, C.; Wester, T.; Nordenskjöld, A.; Kawaguchi, A.; Hui, T.T.; Granström, A.L.; Funari, V.; et al. Altered fecal short chain fatty acid composition in children with a history of Hirschsprung-associated enterocolitis. J. Pediatr. Surg. 2016, 51, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rotondo-Trivette, S.; Wang, B.; Luan, Y.; Fiehn, O.; Sun, F.; Michail, S. Reduced fecal short-chain fatty acids in hispanic children with ulcerative colitis. Physiol. Rep. 2021, 9, e14918. [Google Scholar] [CrossRef] [PubMed]
- Vernero, M.; de Blasio, F.; Ribaldone, D.G.; Bugianesi, E.; Pellicano, R.; Saracco, G.M.; Astegiano, M.; Caviglia, G.P. The Usefulness of Microencapsulated Sodium Butyrate Add-On Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 2020, 9, 3941. [Google Scholar] [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases-Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019, 12, 1178646919852996. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; de Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased Tryptophan Metabolism Is Associated with Activity of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Berlanga, M.; Miñana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Brooks, A.K.; Lawson, M.A.; Smith, R.A.; Janda, T.M.; Kelley, K.W.; McCusker, R.H. Interactions between inflammatory mediators and corticosteroids regulate transcription of genes within the Kynurenine Pathway in the mouse hippocampus. J. Neuroinflammation 2016, 13, 98. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; La Pérez-de Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxid. Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef]
- Grant, R.S.; Coggan, S.E.; Smythe, G.A. The physiological action of picolinic Acid in the human brain. Int. J. Tryptophan Res. 2009, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Dudzińska, E.; Szymona, K.; Kloc, R.; Gil-Kulik, P.; Kocki, T.; Świstowska, M.; Bogucki, J.; Kocki, J.; Urbanska, E.M. Increased expression of kynurenine aminotransferases mRNA in lymphocytes of patients with inflammatory bowel disease. Therap. Adv. Gastroenterol. 2019, 12, 1756284819881304. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, D.; Troost, F.J.; Masclee, A.A.M. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil. 2009, 21, 1239–1249. [Google Scholar] [CrossRef]
- Desmons, A.; Humbert, L.; Eguether, T.; Krasniqi, P.; Rainteau, D.; Mahdi, T.; Kapel, N.; Lamazière, A. High performance liquid chromatography-tandem mass spectrometry quantification of tryptophan metabolites in human serum and stool—Application to clinical cohorts in Inflammatory Bowel Diseases. J. Chromatogr. A 2022, 1685, 463602. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, X.; Su, D.; Ren, Y.; Cheng, F.; Yao, Y.; Shi, G.; Ji, Y.; Chen, S.; Shi, P.; et al. Therapeutic efficacy of human adipose mesenchymal stem cells in Crohn’s colon fibrosis is improved by IFN-γ and kynurenic acid priming through indoleamine 2,3-dioxygenase-1 signaling. Stem Cell Res. Ther. 2022, 13, 465. [Google Scholar] [CrossRef]
- Clarke, G.; McKernan, D.P.; Gaszner, G.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. A Distinct Profile of Tryptophan Metabolism along the Kynurenine Pathway Downstream of Toll-Like Receptor Activation in Irritable Bowel Syndrome. Front. Pharmacol. 2012, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Di Meglio, P.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Yan, J.; Kothur, K.; Mohammad, S.; Chung, J.; Patel, S.; Jones, H.F.; Keating, B.A.; Han, V.X.; Webster, R.; Ardern-Holmes, S.; et al. CSF neopterin, quinolinic acid and kynurenine/tryptophan ratio are biomarkers of active neuroinflammation. eBioMedicine 2023, 91, 104589. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J.; Chirumbolo, S.; Bjørklund, G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab. Brain Dis. 2017, 32, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, K.; Guo, X.; Liao, S.; Zhang, Q.; Xu, Y.; Cui, H.; Zheng, L.; Xu, M. Metabolic profiling reveals altered tryptophan metabolism in patients with kawasaki disease. Front. Mol. Biosci. 2023, 10, 1180537. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.M.-L.; Tint, M.-T.; Kothandaraman, N.; Michael, N.; Sadananthan, S.A.; Velan, S.S.; Fortier, M.V.; Yap, F.; Tan, K.H.; Gluckman, P.D.; et al. The Kynurenine Pathway Metabolites in Cord Blood Positively Correlate With Early Childhood Adiposity. J. Clin. Endocrinol. Metab. 2022, 107, e2464–e2473. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Griffiths, A.M.; Leder, O.; Turner, D. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2014, 2014, CD006320. [Google Scholar] [CrossRef] [PubMed]
- Jayapala, H.P.S.; Lim, S.Y. N-3 Polyunsaturated Fatty Acids and Gut Microbiota. Comb. Chem. High Throughput Screen. 2023, 26, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, B.; Dong, L.; Chang, P. Potential of Omega-3 Polyunsaturated Fatty Acids in Managing Chemotherapy- or Radiotherapy-Related Intestinal Microbial Dysbiosis. Adv. Nutr. 2019, 10, 133–147. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun. Signal. 2022, 20, 64. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Bhan, A.K.; Kang, J.X. Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int. J. Obes. 2016, 40, 1039–1042. [Google Scholar] [CrossRef]
- Hollander, D.; Truscott, T.C. Mechanism and site of small intestinal uptake of vitamin D3 in pharmacological concentrations. Am. J. Clin. Nutr. 1976, 29, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohns. Colitis 2018, 12, 963–972. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Rejnmark, L.; Moss, A.C. Role of Vitamin D in the Natural History of Inflammatory Bowel Disease. J. Crohns. Colitis 2018, 12, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E. Vitamin D and prebiotics may benefit the intestinal microbacteria and improve glucose homeostasis in prediabetes and type 2 diabetes. Endocr. Pract. 2013, 19, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Lin, Y.-D.; Arora, J.; Bora, S.; Tian, Y.; Nichols, R.G.; Patterson, A.D. Vitamin D Regulates the Microbiota to Control the Numbers of RORγt/FoxP3+ Regulatory T Cells in the Colon. Front. Immunol. 2019, 10, 1772. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.D.; Ferreira, S.R.G. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef]
- við Streym, S.; Højskov, C.S.; Møller, U.K.; Heickendorff, L.; Vestergaard, P.; Mosekilde, L.; Rejnmark, L. Vitamin D content in human breast milk: A 9-mo follow-up study. Am. J. Clin. Nutr. 2016, 103, 107–114. [Google Scholar] [CrossRef]
- Tan, M.L.; Abrams, S.A.; Osborn, D.A. Vitamin D supplementation for term breastfed infants to prevent vitamin D deficiency and improve bone health. Cochrane Database Syst. Rev. 2020, 12, CD013046. [Google Scholar] [CrossRef]
- Wagner, C.L.; Greer, F.R. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008, 122, 1142–1152. [Google Scholar] [CrossRef]
- Lee, R.; Maltz, R.M.; Crandall, W.V.; Plogsted, S.W.; Shaikhkhalil, A.K.; Bowden, S.A.; Mezoff, E.A. Single High-dose Vitamin D3 Supplementation in Pediatric Patients with Inflammatory Bowel Disease and Hypovitaminosis D. J. Pediatr. Gastroenterol. Nutr. 2020, 70, e77–e80. [Google Scholar] [CrossRef]
- Simek, R.Z.; Prince, J.; Syed, S.; Sauer, C.G.; Martineau, B.; Hofmekler, T.; Freeman, A.J.; Kumar, A.; McElhanon, B.O.; Schoen, B.T.; et al. Pilot Study Evaluating Efficacy of 2 Regimens for Hypovitaminosis D Repletion in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 252–258. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; El Ashry, H.; Hodeib, H.; Hassan, S. Vitamin D in Children with Inflammatory Bowel Disease: A Randomized Controlled Clinical Trial. J. Clin. Gastroenterol. 2021, 55, 815–820. [Google Scholar] [CrossRef]
- Semrad, C.E. Zinc and intestinal function. Curr. Gastroenterol. Rep. 1999, 1, 398–403. [Google Scholar] [CrossRef]
- Duggan, C.; Gannon, J.; Walker, W.A. Protective nutrients and functional foods for the gastrointestinal tract. Am. J. Clin. Nutr. 2002, 75, 789–808. [Google Scholar] [CrossRef]
- Quarterman, J.; Jackson, F.A.; Morrison, J.N. The effect of zinc deficiency on sheep intestinal mucin. Life Sci. 1976, 19, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Keil, C.; Straubing, S.; Robbe-Masselot, C.; Haase, H. Zinc Deficiency Disturbs Mucin Expression, O-Glycosylation and Secretion by Intestinal Goblet Cells. Int. J. Mol. Sci. 2020, 21, 6149. [Google Scholar] [CrossRef]
- Chao, H.-C. Zinc Deficiency and Therapeutic Value of Zinc Supplementation in Pediatric Gastrointestinal Diseases. Nutrients 2023, 15, 4093. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chiu, C.-H. Current and future applications of fecal microbiota transplantation for children. Biomed. J. 2022, 45, 11–18. [Google Scholar] [CrossRef]
- Gu, X.; Chen, Z.-H.; Zhang, S.-C. Fecal microbiota transplantation in childhood: Past, present, and future. World J. Pediatr. 2023, 19, 813–822. [Google Scholar] [CrossRef]
- Elgarten, C.W.; Margolis, E.B.; Kelly, M.S. The Microbiome and Pediatric Transplantation. J. Pediatr. Infect. Dis. Soc. 2024, 13, S80–S89. [Google Scholar] [CrossRef]
- Karolewska-Bochenek, K.; Grzesiowski, P.; Banaszkiewicz, A.; Gawronska, A.; Kotowska, M.; Dziekiewicz, M.; Albrecht, P.; Radzikowski, A.; Lazowska-Przeorek, I. A Two-Week Fecal Microbiota Transplantation Course in Pediatric Patients with Inflammatory Bowel Disease. Adv. Exp. Med. Biol. 2018, 1047, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kunde, S.; Pham, A.; Bonczyk, S.; Crumb, T.; Duba, M.; Conrad, H.; Cloney, D.; Kugathasan, S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, L.d.F.; Borba, H.H.; Tonin, F.S.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Fecal microbiota transplantation in inflammatory bowel disease patients: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238910. [Google Scholar] [CrossRef]
- Hourigan, S.K.; Chen, L.A.; Grigoryan, Z.; Laroche, G.; Weidner, M.; Sears, C.L.; Oliva-Hemker, M. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment. Pharmacol. Ther. 2015, 42, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.; Tun, K.M.; Batra, K.; Haque, L.; Vongsavath, T.; Hong, A.S. Safety and Efficacy of Fecal Microbiota Transplantation in Treatment of Inflammatory Bowel Disease in the Pediatric Population: A Systematic Review and Meta-Analysis. Microorganisms 2023, 11, 1272. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Salge, M.M.H. The Importance of a Healthy Microbiome in Pregnancy and Infancy and Microbiota Treatment to Reverse Dysbiosis for Improved Health. Antibiotics 2023, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.B.; Jordan, S.; Baker, B.J.; Carlson, N.S. The Maternal Infant Microbiome: Considerations for Labor and Birth. MCN Am. J. Matern. Child Nurs. 2017, 42, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, X.; Zhou, L.; Xie, R.-H.; He, Y. Microbiota transplantation in restoring cesarean-related infant dysbiosis: A new frontier. Gut Microbes 2024, 16, 2351503. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Butler, É.M.; Grigg, C.P.; Derraik, J.G.B.; Chiavaroli, V.; Walker, N.; Thampi, S.; Creagh, C.; Reynolds, A.J.; Vatanen, T.; et al. Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial. EBioMedicine 2021, 69, 103443. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; de Jesus-Laboy, K.M.; Shen, N.; Cox, L.M.; Amir, A.; Gonzalez, A.; Bokulich, N.A.; Song, S.J.; Hoashi, M.; Rivera-Vinas, J.I.; et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 2016, 22, 250–253. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [PubMed]

| Reference | Population | Treatment | Effects | |
|---|---|---|---|---|
| Ben, X. et al. [69] | Prebiotics | 371 Term infants | Infants 4 weeks after birth randomly assigned to IF or IF + GOS for 3 months | HM and IF + GOS intestinal Bifidobacteria, Lactobacilli  Fecal pH  |
| Prieto, P.A. et al. [70] | Prebiotics | 84 Term infants | Infants within 11 days after birth, randomly assigned to IF or IF + FOS for 16 weeks | HM and IF + FOS Intestinal Lactobacilli  |
| Puccio, G. et al. [71] | Prebiotics | 175 Term infants | Infants within 14 days after birth, randomly assigned to IF or IF + 20FL + LNnT for 6 months | IF + 20FL + LNnT safe and well tolerated, morbidity (bronchitis) and medication  use (antipyretics and antibiotics) |
| Miele, E. et al. [92] | Probiotics | 29 patients (mean age: 9.8 years; female/male: 13/16), | Children newly diagnosed for UC, randomly assigned to VSL#3 weight-based dose, (range: 450–1800 billion bacteria/day) (n = 14) or placebo (n = 15) * | Endoscopic, histological scores  No biochemical or clinical adverse events |
| Huynh, H.Q. et al. [93] | Probiotics | 18 patients (mean age: 12.2 years; female/male: 7/11), | All UC patients received 3 g sachet of VSL#3 twice daily by mouth for 8 Weeks | 10 patients remission (SCCAI < 3); 1 patient response (decrease in SCCAI 2, final score 5); 7 patients no changes. Bacterial taxonomy changes VSL#3 well tolerated No adverse effects |
| Pietrzak, A. et al. [161] | Postbiotics | 72 patients (mean age: 13.5 years; female/male: 14/28): 42 Crohn’s disease, 30 mild conditions | Randomly assigned to sodium butyrate 150 mg twice a day for 12 weeks (n = 29) or placebo (n = 23) | Not effective as an adjunctive treatment |
| Kunde, S. et al. [213] | FMT | 9 patients (7–21 years) Mild to moderate UC | Freshly prepared fecal enemas daily for 5 days | 7 patients response within 1 week, 6 patients maintained response at 1 month. No adverse effects, good tolerability |
| Wilson, B.C. et al. [220] | Vaginal seeding | 47 newborns 22 Vaginal delivery (control) 12 Cesarean-seeded 13 Cesarean placebo | Newborns randomized to 3 mL solution of maternal vaginal microbes or sterile water; stool samples at 1 h, 1 month, and 3 months undergoing shotgun metagenomic sequencing | No differences in gut microbiome composition or functional potential were observed |
 = increase,
= increase,  = decrease.
= decrease.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottria, R.; Xynomilakis, O.; Casati, S.; Ciuffreda, P. Pre- to Postbiotics: The Beneficial Roles of Pediatric Dysbiosis Associated with Inflammatory Bowel Diseases. Microorganisms 2024, 12, 1582. https://doi.org/10.3390/microorganisms12081582
Ottria R, Xynomilakis O, Casati S, Ciuffreda P. Pre- to Postbiotics: The Beneficial Roles of Pediatric Dysbiosis Associated with Inflammatory Bowel Diseases. Microorganisms. 2024; 12(8):1582. https://doi.org/10.3390/microorganisms12081582
Chicago/Turabian StyleOttria, Roberta, Ornella Xynomilakis, Silvana Casati, and Pierangela Ciuffreda. 2024. "Pre- to Postbiotics: The Beneficial Roles of Pediatric Dysbiosis Associated with Inflammatory Bowel Diseases" Microorganisms 12, no. 8: 1582. https://doi.org/10.3390/microorganisms12081582






