Resistance and Co-Resistance of Metallo-Beta-Lactamase Genes in Diarrheal and Urinary-Tract Pathogens in Bangladesh
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Plan and Sample Collections
2.2. Isolation and Identification of Bacteria
2.3. Antimicrobial Susceptibility Test
2.4. Determining Minimum Inhibitory Concentrations (MICs)
2.5. Molecular Detection of Metallo-Beta-Lactamase (blaNDM-1 and blaVIM) Genes
2.6. Data Analysis
2.7. Ethical Clearance
3. Results
3.1. Study Population
3.2. Bacterial Prevalence
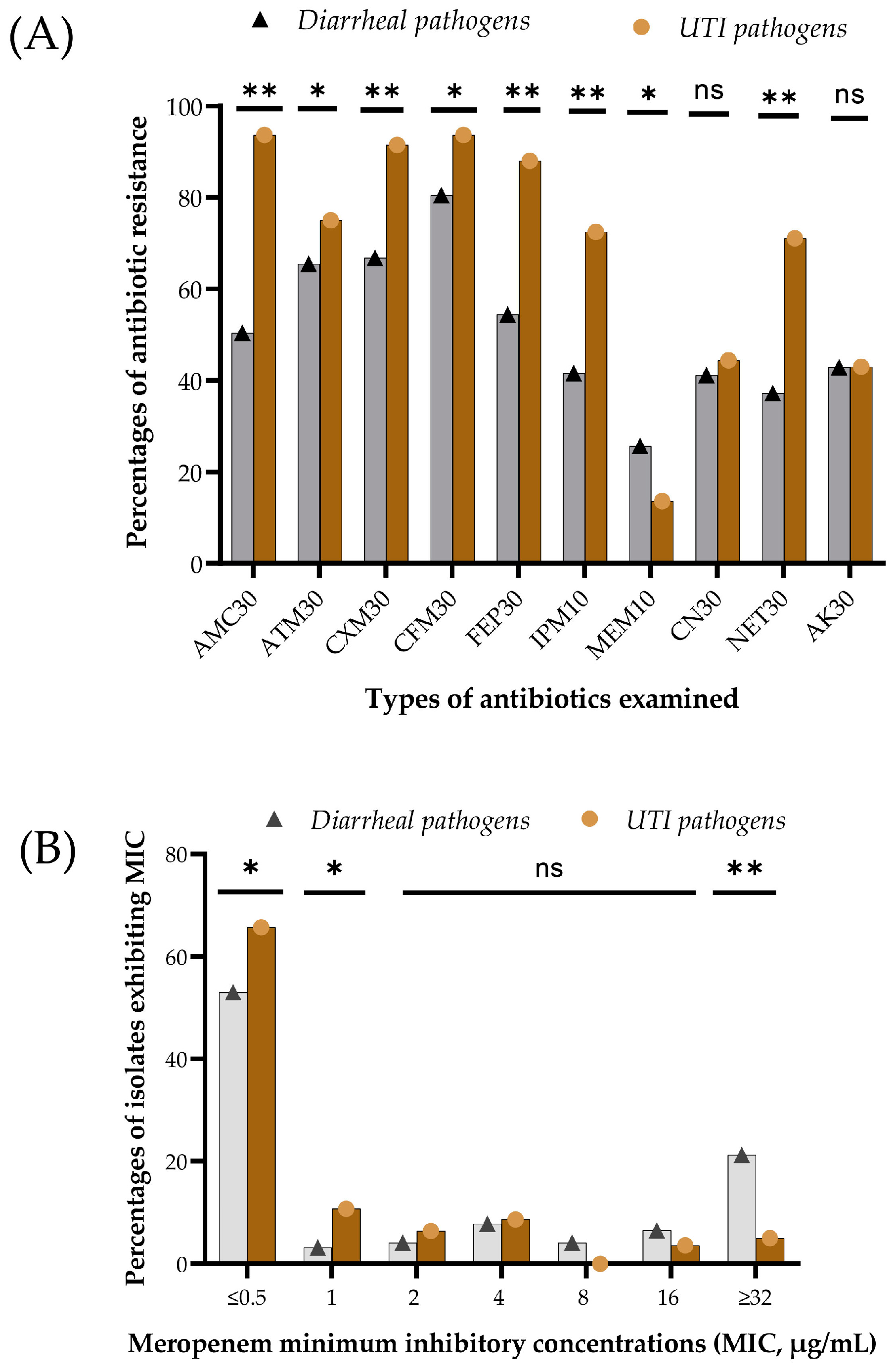
3.3. Phenotypic Resistance and Minimum Inhibitory Concentration
3.4. Prevalence of MBL and ESBL Genes
3.5. Association of Phenotypic and Genotypic (blaNDM-1 and blaVIM Gene) Resistance
3.6. Phenotype Resistance for Co-Occurance of MBL Genes
4. Discussion
5. Conclusions and Next Steps
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Papp-Wallace, K.M.; Endimiani, A.; Taracila, M.A.; Bonomo, R.A. Carbapenems: Past, present, and future. Antimicrob. Agents Chemother. 2011, 55, 4943–4960. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M.; Zanichelli, V.; Ombajo, L.A.; Bazira, J.; Cappello, B.; Chitatanga, R.; Chuki, P.; Gandra, S.; Getahun, H.; Harbarth, S. The WHO essential medicines list AWaRe book: From a list to a quality improvement system. Clin. Microbiol. Infect. 2022, 28, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Rabbi, M.B.; Sultana, S. Antibiotic resistance in Bangladesh: A systematic review. Int. J. Infect. Dis. 2019, 80, 54–61. [Google Scholar] [CrossRef]
- Islam, S.; Urmi, U.L.; Rana, M.; Sultana, F.; Jahan, N.; Hossain, B.; Iqbal, S.; Hossain, M.M.; Mosaddek, A.S.M.; Nahar, S. High abundance of the colistin resistance gene mcr-1 in chicken gut-bacteria in Bangladesh. Sci. Rep. 2020, 10, 17292. [Google Scholar] [CrossRef]
- Hoque, R.; Ahmed, S.M.; Naher, N.; Islam, M.A.; Rousham, E.K.; Islam, B.Z.; Hassan, S. Tackling antimicrobial resistance in Bangladesh: A scoping review of policy and practice in human, animal and environment sectors. PLoS ONE 2020, 15, e0227947. [Google Scholar] [CrossRef]
- Monira, S.; Shabnam, S.A.; Ali, S.I.; Sadique, A.; Johura, F.-T.; Rahman, K.Z.; Alam, N.H.; Watanabe, H.; Alam, M. Multi-drug resistant pathogenic bacteria in the gut of young children in Bangladesh. Gut Pathog. 2017, 9, 19. [Google Scholar] [CrossRef]
- Haque, T.A.; Urmi, U.L.; Islam, A.B.M.M.K.; Ara, B.; Nahar, S.; Mosaddek, A.S.M.; Lugova, H.; Kumar, S.; Jahan, D.; Rahman, N.A.A. Detection of qnr genes and gyrA mutation to quinolone phenotypic resistance of UTI pathogens in Bangladesh and the implications. J. Appl. Pharm. Sci. 2022, 12, 185–198. [Google Scholar] [CrossRef]
- Begum, N.; Shamsuzzaman, S. Emergence of carbapenemase-producing urinary isolates at a tertiary care hospital in Dhaka, Bangladesh. Tzu Chi Med. J. 2016, 28, 94–98. [Google Scholar] [CrossRef]
- Islam, M.A.; Akhtar, Z.; Hassan, M.Z.; Chowdhury, S.; Rashid, M.M.; Aleem, M.A.; Ghosh, P.K.; Mah-E-Muneer, S.; Parveen, S.; Ahmmed, M.K. Pattern of antibiotic dispensing at pharmacies according to the WHO Access, Watch, Reserve (AWaRe) classification in Bangladesh. Antibiotics 2022, 11, 247. [Google Scholar] [CrossRef]
- Orubu, E.; Samad, M.; Rahman, M.; Zaman, M.; Wirtz, V. Mapping the antimicrobial supply chain in Bangladesh: A scoping-review-based ecological assessment approach. Glob. Health Sci. Pract. 2021, 9, 532–547. [Google Scholar] [CrossRef]
- Nizame, F.A.; Shoaib, D.M.; Rousham, E.K.; Akter, S.; Islam, M.A.; Khan, A.A.; Rahman, M.; Unicomb, L. Barriers and facilitators to adherence to national drug policies on antibiotic prescribing and dispensing in Bangladesh. J. Pharm. Policy Pract. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Kabir, H.; Hasan, M.K.; Akter, N.; Tassdik, H.; Islam, M.F.; Jannat, H.; Tutul, A.H.; Akter, O.; Ara, R.; Islam, M.D. Antibiotics administration without prescription in Bangladesh. IJID Reg. 2023, 7, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-producing organisms: A global scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Wilson, H.; Török, M.E. Extended-spectrum β-lactamase-producing and carbapenemase-producing Enterobacteriaceae. Microb. Genom. 2018, 4, e000197. [Google Scholar] [CrossRef]
- Van Duin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet Infect. Dis. 2020, 20, 731–741. [Google Scholar] [CrossRef]
- Goodman, K.; Simner, P.; Tamma, P.; Milstone, A. Infection control implications of heterogeneous resistance mechanisms in carbapenem-resistant Enterobacteriaceae (CRE). Expert Rev. Anti-Infect. Ther. 2016, 14, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, D.; Gill, M.; Wise, R. Mechanisms of resistance to the carbapenems. J. Antimicrob. Chemother. 1995, 35, 1–5. [Google Scholar] [CrossRef]
- Tamma, P.D.; Goodman, K.E.; Harris, A.D.; Tekle, T.; Roberts, A.; Taiwo, A.; Simner, P.J. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin. Infect. Dis. 2017, 64, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, F.S.; Donkor, E.S. Carbapenem resistance: A review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Macesic, N.; Hawkey, J.; Vezina, B.; Wisniewski, J.A.; Cottingham, H.; Blakeway, L.V.; Harshegyi, T.; Pragastis, K.; Badoordeen, G.Z.; Dennison, A. Genomic dissection of endemic carbapenem resistance reveals metallo-beta-lactamase dissemination through clonal, plasmid and integron transfer. Nat. Commun. 2023, 14, 4764. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Walsh, T.R.; Toleman, M.A.; Poirel, L.; Nordmann, P. Metallo-β-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005, 18, 306–325. [Google Scholar] [CrossRef]
- Reddy, K.S. Global Burden of Disease Study 2015 provides GPS for global health 2030. Lancet 2016, 388, 1448–1449. [Google Scholar] [CrossRef]
- Mokomane, M.; Kasvosve, I.; Melo, E.d.; Pernica, J.M.; Goldfarb, D.M. The global problem of childhood diarrhoeal diseases: Emerging strategies in prevention and management. Ther. Adv. Infect. Dis. 2018, 5, 29–43. [Google Scholar] [CrossRef]
- WHO. Diarrhoeal Disease; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Troeger, C.E.; Khalil, I.A.; Blacker, B.F.; Biehl, M.H.; Albertson, S.B.; Zimsen, S.R.; Rao, P.C.; Abate, D.; Ahmadi, A.; Brahim Ahmed, M.L.C. Quantifying risks and interventions that have affected the burden of diarrhoea among children younger than 5 years: An analysis of the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2020, 20, 37–59. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, L.; Hu, Y.; Dottorini, T.; Fanning, S.; Xu, J.; Li, F. Epidemiological study on prevalence, serovar diversity, multidrug resistance, and CTX-M-type extended-spectrum β-lactamases of Salmonella spp. from patients with diarrhea, food of animal origin, and pets in several provinces of China. Antimicrob. Agents Chemother. 2020, 64, e00092-20. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Xu, H.; Lv, T.; Guo, L.; Xiao, Y.; Huang, C.; Zhang, S.; Chen, Y.; Han, H.; Shen, P. Stool samples of acute diarrhea inpatients as a reservoir of ST11 hypervirulent KPC-2-producing Klebsiella pneumoniae. MSystems 2020, 5, e00498-20. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Guo, Y.; Moran, R.A.; Doughty, E.L.; Liu, B.; Yao, L.; Li, J.; He, N.; Shen, S.; Li, Y. Global emergence of a hypervirulent carbapenem-resistant Escherichia coli ST410 clone. Nat. Commun. 2024, 15, 494. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113, 5–13. [Google Scholar] [CrossRef]
- Eshetie, S.; Unakal, C.; Gelaw, A.; Ayelign, B.; Endris, M.; Moges, F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. Antimicrob. Resist. Infect. Control 2015, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.; Rafi, M.A.; Salam, M.A. High prevalence of multidrug resistant uropathogens: A recent audit of antimicrobial susceptibility testing from a tertiary care hospital in Bangladesh. Pak. J. Med. Sci. 2020, 36, 1297. [Google Scholar] [CrossRef] [PubMed]
- Urmi, U.L.; Nahar, S.; Rana, M.; Sultana, F.; Jahan, N.; Hossain, B.; Alam, M.S.; Mosaddek, A.S.M.; McKimm, J.; Rahman, N.A.A. Genotypic to Phenotypic Resistance Discrepancies Identified Involving β-Lactamase Genes, bla KPC, bla IMP, bla NDM-1, and bla VIM in Uropathogenic Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 2863–2875. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, K.; van Duin, D. Treatment for carbapenem-resistant Enterobacterales infections: Recent advances and future directions. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Potoski, B.A.; Buehrle, D.; Nguyen, M.H. Estimating the treatment of carbapenem-resistant Enterobacteriaceae infections in the United States using antibiotic prescription data. Open Forum Infect. Dis. 2019, 6, ofz344. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.T. Continuous evolution: Perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect. Dis. Ther. 2021, 10, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Löfmark, S.; Sjöström, K.; Mäkitalo, B.; Edquist, P.; Wisell, K.T.; Giske, C.G. Carbapenemase-producing Enterobacteriaceae in Sweden 2007–2013: Experiences from seven years of systematic surveillance and mandatory reporting. Drug Resist. Updates 2015, 20, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Miriagou, V.; Cornaglia, G.; Edelstein, M.; Galani, I.; Giske, C.; Gniadkowski, M.; Malamou-Lada, E.; Martinez-Martinez, L.; Navarro, F.; Nordmann, P. Acquired carbapenemases in Gram-negative bacterial pathogens: Detection and surveillance issues. Clin. Microbiol. Infect. 2010, 16, 112–122. [Google Scholar] [CrossRef]
- WHO. Antimicrobial Stewardship Programs in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Pierce, J.; Apisarnthanarak, A.; Schellack, N.; Cornistein, W.; Al Maani, A.; Adnan, S.; Stevens, M.P. Global Antimicrobial Stewardship with a Focus on Low-and Middle-Income Countries: A position statement for the international society for infectious diseases. Int. J. Infect. Dis. 2020, 96, 621–629. [Google Scholar] [CrossRef]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Hara, G.L. Antibiotic stewardship in low-and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Harun, M.G.D.; Anwar, M.M.U.; Sumon, S.A.; Hassan, M.Z.; Mohona, T.M.; Rahman, A.; Abdullah, S.A.H.M.; Islam, M.S.; Kaydos-Daniels, S.C.; Styczynski, A.R. Rationale and guidance for strengthening infection prevention and control measures and antimicrobial stewardship programs in Bangladesh: A study protocol. BMC Health Serv. Res. 2022, 22, 1239. [Google Scholar] [CrossRef] [PubMed]
- Akpan, M.R.; Isemin, N.U.; Udoh, A.E.; Ashiru-Oredope, D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Otieno, P.A.; Campbell, S.; Maley, S.; Obinju Arunga, T.; Otieno Okumu, M. A Systematic Review of Pharmacist-Led Antimicrobial Stewardship Programs in Sub-Saharan Africa. Int. J. Clin. Pract. 2022, 2022, 3639943. [Google Scholar] [CrossRef] [PubMed]
- Sumon, S.; Anwar, M.; Akther, F.; Priyanka, A.; Tamanna, T.; Rahman, A.; Islam, M.; Harun, M.D. Perceptions of antibiotic stewardship programmes and determinants of antibiotic prescribing patterns among physicians in tertiary hospitals in Bangladesh: Implications for future policy and practice. J. Hosp. Infect. 2024, 144, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Godman, B. Potential strategies to improve antimicrobial utilisation in hospitals in Bangladesh building on experiences across developing countries. Bangladesh J. Med. Sci. 2021, 20, 469–477. [Google Scholar] [CrossRef]
- M. Kurdi Al-Dulaimi, M.; Abd. Mutalib, S.; Abd. Ghani, M.; Mohd. Zaini, N.A.; Ariffin, A.A. Multiple antibiotic resistance (MAR), plasmid profiles, and DNA polymorphisms among Vibrio vulnificus isolates. Antibiotics 2019, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.C.; Fauntleroy, K.A.; Jenkins, S.G.; Abuali, M.; LaBombardi, V.J.; Nicolau, D.P.; Kuti, J.L. Comparison of meropenem MICs and susceptibilities for carbapenemase-producing Klebsiella pneumoniae isolates by various testing methods. J. Clin. Microbiol. 2010, 48, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Hindler, J.A.; Epson, E.; Horwich-Scholefield, S.; Miller, L.G.; Mendez, J.; Martinez, J.B.; Sinkowitz, J.; Sinkowtiz, D.; Hershey, C. Carbapenem-resistant Enterobacteriaceae detection practices in California: What are we missing? Clin. Infect. Dis. 2018, 66, 1061–1067. [Google Scholar] [CrossRef]
- Barbosa, C.; Nogueira, S.; Gadanho, M.; Chaves, S. DNA extraction: Finding the most suitable method. In Molecular Microbial Diagnostic Methods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 135–154. [Google Scholar]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- Monstein, H.J.; Östholm-Balkhed, Å.; Nilsson, M.; Nilsson, M.; Dornbusch, K.; Nilsson, L. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. Apmis 2007, 115, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Aryal, S.C.; Rai, G.; Rai, K.R.; Pyakurel, S.; Bhandari, B.; Sah, A.K.; Rai, S.K. Prevalence of multidrug-resistance and bla VIM and bla IMP genes among gram-negative clinical isolates in tertiary care hospital, Kathmandu, Nepal. Iran. J. Microbiol. 2021, 13, 303. [Google Scholar]
- Al Jarousha, A.M.K.; El Jarou, M.A.; El Qouqa, I.A. Bacterial enteropathogens and risk factors associated with childhood diarrhea. Indian J. Pediatr. 2011, 78, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Chander, A.; Shrestha, C.D. Prevalence of extended spectrum beta lactamase producing Escherichia coli and Klebsiella pneumoniae urinary isolates in a tertiary care hospital in Kathmandu, Nepal. BMC Res. Notes 2013, 6, 487. [Google Scholar] [CrossRef] [PubMed]
- Ronald, A. The etiology of urinary tract infection: Traditional and emerging pathogens. Am. J. Med. 2002, 113, 14–19. [Google Scholar] [CrossRef]
- Mirbagheri, S.Z.; Meshkat, Z.; Naderinasab, M.; Rostami, S.; Nabavinia, M.S.; Rahmati, M. Study on imipenem resistance and prevalence of blaVIM1 and blaVIM2 metallo-beta lactamases among clinical isolates of Pseudomonas aeruginosa from Mashhad, Northeast of Iran. Iran. J. Microbiol. 2015, 7, 72. [Google Scholar]
- Kumari, M.; Verma, S.; Venkatesh, V.; Gupta, P.; Tripathi, P.; Agarwal, A.; Siddiqui, S.S.; Arshad, Z.; Prakash, V. Emergence of blaNDM-1 and blaVIM producing Gram-negative bacilli in ventilator-associated pneumonia at AMR Surveillance Regional Reference Laboratory in India. PLoS ONE 2021, 16, e0256308. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Family Welfare (MoHFW). National Action Plan: Antimicrobial Resistance Containment in Bangladesh 2017–2022; Government of the People’s Republic of Bangladesh: Dhaka, Bangladesh, 2017.
- Mishra, S.; Acharya, J.; Kattel, H.; Koirala, J.; Rijal, B.; Pokhrel, B. Metallo-beta-lactamase producing gram-negative bacterial isolates. J. Nepal Health Res. Counc. 2012, 10, 208–213. [Google Scholar]
- Emeraud, C.; Escaut, L.; Boucly, A.; Fortineau, N.; Bonnin, R.A.; Naas, T.; Dortet, L. Aztreonam plus Clavulanate, Tazobactam, or Avibactam for Treatment of Infections Caused by Metallo-beta-Lactamase-Producing Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Canton, R.; Ruiz-Garbajosa, P. Co-resistance: An opportunity for the bacteria and resistance genes. Curr. Opin. Pharmacol. 2011, 11, 477–485. [Google Scholar] [CrossRef]
- Palzkill, T. Metallo-β-lactamase structure and function. Ann. N. Y. Acad. Sci. 2013, 1277, 91–104. [Google Scholar] [CrossRef]
- Perez, F.; Endimiani, A.; Hujer, K.M.; Bonomo, R.A. The continuing challenge of ESBLs. Curr. Opin. Pharmacol. 2007, 7, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F. Other Beta-lactam Antibiotics: Beta-lactamase Inhibitors, Carbapenems, and Monobactams. Antimicrob. Ther. Vet. Med. 2013, 175–187. [Google Scholar] [CrossRef]
- Sabbagh, P.; Rajabnia, M.; Maali, A.; Ferdosi-Shahandashti, E. Integron and its role in antimicrobial resistance: A literature review on some bacterial pathogens. Iran. J. Basic Med. Sci. 2021, 24, 136. [Google Scholar] [PubMed]
- Rice, L.B. Antimicrobial stewardship and antimicrobial resistance. Med. Clin. 2018, 102, 805–818. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, F.R.T.; Telles, J.P.; Tuon, F.F.B.; Rabello Filho, R.; Caruso, P.; Correa, T.D. Antimicrobial stewardship programs: A review of strategies to avoid polymyxins and carbapenems misuse in low middle-income countries. Antibiotics 2022, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Sono, T.M.; Yeika, E.; Cook, A.; Kalungia, A.; Opanga, S.A.; Acolatse, J.E.E.; Sefah, I.A.; Jelić, A.G.; Campbell, S.; Lorenzetti, G. Current rates of purchasing of antibiotics without a prescription across sub-Saharan Africa; rationale and potential programmes to reduce inappropriate dispensing and resistance. Expert Rev. Anti-Infect. Ther. 2023, 21, 1025–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, X.; Hou, H.; Lu, Y.; Yu, J.; Mao, L.; Mao, L.; Sun, Z. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: A hospital based study. BMC Infect. Dis. 2018, 18, 63. [Google Scholar] [CrossRef]
- Meyer, E.; Schwab, F.; Schroeren-Boersch, B.; Gastmeier, P. Dramatic increase of third-generation cephalosporin-resistant E. coli in German intensive care units: Secular trends in antibiotic drug use and bacterial resistance, 2001 to 2008. Crit. Care 2010, 14, R113. [Google Scholar] [CrossRef]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book and Prevention of Antimicrobial Resistance; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Duffy, E.; Ritchie, S.; Metcalfe, S.; Van Bakel, B.; Thomas, M. Antibacterials dispensed in the community comprise 85%–95% of total human antibacterial consumption. J. Clin. Pharm. Ther. 2018, 43, 59–64. [Google Scholar] [CrossRef]
| Age Group (Years) | Diarrheal Pathogens (n = 228) | Urinary-Tract-Infection Pathogens (n = 142) | ||
|---|---|---|---|---|
| Frequency | Percentage | Frequency | Percentage | |
| 1–10 | 191 a | 83.8 | 5 | 3.5 |
| 11–20 | 5 | 2.2 | 22 | 15.5 |
| 21–30 | 8 | 3.5 | 50 b | 35.2 |
| 31–40 | 10 | 4.4 | 19 b | 13.4 |
| 41–50 | 0 | 0 | 27 b | 19.0 |
| 51–60 | 5 | 2.2 | 8 | 5.6 |
| 61–70 | 0 | 0 | 9 | 6.3 |
| 71–80 | 9 | 3.9 | 2 | 1.4 |
| Total | 228 | 100 | 142 | 100 |
| Phenotypic Susceptibility | Presence of β-Lactamase Genes in Diarrheal and UTI Isolates (n = 370) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| blaTEM, No (%) | p Value | blaOXA, No (%) | p Value | blaSHV, No (%) | p Value | |||||
| Positive (n = 165) | Negative (n = 205) | Positive (n = 97) | Negative (n = 273) | Positive (n = 30) | Negative (n = 340) | |||||
| AMC 30 | Sensitive | 50 (30.3) | 73 (35.6) | 0.318 | 23 (23.7) | 100 (36.6) | 0.024 | 13 (43.3) | 110 (32.4) | 0.230 |
| Resistant | 115 (69.7) | 132 (64.4) | 74 (76.3) | 173 (64.4) | 17 (56.7) | 230 (67.6) | ||||
| ATM 30 | Sensitive | 53 (32.1) | 63 (30.7) | 0.822 | 16 (16.5) | 100 (36.6) | 0.000 | 12 (40.0) | 104 (30.6) | 0.306 |
| Resistant | 112 (67.9) | 142 (69.3) | 81 (83.5) | 173 (64.4) | 18 (60.0) | 236 (69.4) | ||||
| CXM 30 | Sensitive | 38 (23.0) | 51 (24.9) | 0.715 | 14 (14.4) | 75 (27.5) | 0.012 | 7 (23.3) | 82 (24.1) | 1.00 |
| Resistant | 127 (77.0) | 154 (75.1) | 83 (85.6) | 198 (72.5) | 23 (76.7) | 258 (75.9) | ||||
| CFM 30 | Sensitive | 28 (17.0) | 27 (13.2) | 0.378 | 10 (10.3) | 45 (16.5) | 0.183 | 9 (30.0) | 46 (13.5) | 0.028 |
| Resistant | 137 (83.0) | 178 (86.7) | 87 (89.7) | 228 (83.5) | 21 (70.0) | 294 (86.5) | ||||
| FEP 30 | Sensitive | 57 (34.5) | 65 (31.7) | 0.580 | 23 (23.7) | 99 (36.3) | 0.024 | 8 (26.7) | 114 (33.5) | 0.545 |
| Resistant | 108 (65.5) | 140 (68.3) | 74 (76.3) | 174 (63.7) | 22 (73.3) | 226 (66.5) | ||||
| IMP 10 | Sensitive | 83 (50.3) | 90 (43.9) | 0.249 | 38 (39.2) | 135 (49.5) | 0.097 | 12 (40.0) | 161 (47.4) | 0.454 |
| Resistant | 82 (49.7) | 115 (56.1) | 59 (60.9) | 138 (50.5) | 18 (60.0) | 179 (52.6) | ||||
| MEP 10 | Sensitive | 130 (79.8) | 161 (78.5) | 0.798 | 77 (80.2) | 214 (78.7) | 0.884 | 27 (90.0) | 264 (78.1) | 0.161 |
| Resistant | 33 (20.2) | 44 (21.5) | 19 (19.8) | 58 (21.3) | 3 (10.0) | 74 (21.9) | ||||
| MIC Values (µg/mL) | Diarrheal Pathogens | Urinary-Tract-Infection Pathogen | ||||||
|---|---|---|---|---|---|---|---|---|
| blaNDM-1 Carriage | blaVIM Carriage | blaNDM-1 Carriage | blaVIM Carriage | |||||
| Yes (n = 12) ∆ | No (n = 216) | Yes (n = 12) | No (n = 216) | Yes (n = 13) | No (n = 129) | Yes (n = 23) | No (n = 119) | |
| ≤0.5 | 6 (50) | 109 (55) | 7 (58) | 108 (55) | 2 (15) | 90 (71) | 12 (52) | 80 (68) |
| 1.0 | 1 (8) | 7 (3.5) | 1 (8) | 7 (3.6) | 1 (7.7) | 14 (11) | 1 (4.3) | 14 (12) |
| 2.0 | 0 (0) | 9 (4.5) | 0 (0) | 9 (4.6) | 0 (0) | 9 (7.1) | 0 (0) | 9 (7.7) |
| 4.0 | 0 (0) | 17 (8.6) | 1 8) | 16 (8.1) | 6 (46) | 6 (4.7) | 7 (30) | 5 (4.3) |
| 8.0 | 1 (8) | 14 (7.1) | 0 (0) | 14 (7.1) | 1 (7.7) | 4 (3.1) | 0 (0) | 5 (4.3) |
| 16 | 1 (8) | 1 (0.5) | 0 (0) | 2 (1) | 0 (0) | 2 (1.6) | 0 (0) | 2 (1.7) |
| ≥32 | 3 (25) | 41 (21) | 3 (25) | 41 (21) | 3 (23) | 2 (1.6) | 3 (13) | 2 (1.7) |
| Types of MBL Gene Carriage a | Isolate ID | Phenotypic Susceptibility Assessment b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMC 30 | CXM 30 | CFM 30 | FEP 30 | IMP 10 | MRP 10 | CN 30 | NET 30 | AK 30 | ||
| blaNDM-1 | PBD20 | R | R | R | R | R | R | S | S | R |
| PBD24 | R | S | S | S | S | S | S | S | R | |
| PBD78 | R | S | R | S | S | S | S | S | S | |
| PBD78C1 | R | R | R | R | R | R | S | S | S | |
| PBD78C2 | R | R | R | R | R | R | S | S | S | |
| PBD86 | S | S | S | S | S | S | S | S | S | |
| PBD86C1 | R | R | R | R | R | R | R | R | R | |
| PBD86C2 | R | R | R | R | R | R | R | R | R | |
| PBD103 | S | R | R | S | S | S | R | S | R | |
| UJ8 | R | R | R | R | R | S | R | R | S | |
| UJ18C2 | R | R | R | R | R | R | R | R | R | |
| UJ29 | R | R | R | R | R | R | R | R | R | |
| UJ49 | R | R | R | R | R | R | R | R | R | |
| UJ56C1 | R | R | R | R | S | R | R | R | R | |
| UJ73 | R | R | R | R | R | S | R | R | R | |
| blaVIM | PBD5 | R | S | R | R | R | R | S | S | S |
| PBD8 | R | R | R | S | R | S | S | S | S | |
| PBD40 | S | R | R | S | R | S | S | S | S | |
| PBD53C2 | R | R | S | S | R | S | S | S | R | |
| PBD55 | S | S | S | S | S | R | R | S | S | |
| PBD77 | S | S | S | S | S | S | R | R | S | |
| PBD80C2 | S | R | R | R | S | S | R | S | S | |
| PBD81 | R | R | R | S | S | S | S | S | S | |
| PBD87 | R | R | R | R | R | R | R | R | R | |
| UJ15 | R | R | R | R | R | R | R | R | S | |
| UJ19 | R | R | R | R | R | R | R | R | R | |
| UJ39 | R | R | R | R | R | S | R | R | R | |
| UJ57 | R | R | R | R | R | S | R | R | R | |
| UJ59 | R | R | R | R | R | S | R | R | R | |
| UJ62 | R | R | R | R | R | S | R | R | R | |
| UJ75 | R | R | R | R | R | S | R | R | S | |
| UJ76C1 | R | R | R | R | R | S | R | R | R | |
| UJ77 | R | R | R | R | R | S | R | R | S | |
| UJ78C2 | R | R | R | R | R | S | R | R | S | |
| UJ98 | R | R | R | R | S | R | S | S | S | |
| UJ108C1 | S | R | S | S | R | S | S | S | S | |
| UJ112 | R | R | R | R | R | S | R | R | R | |
| UJ116 | R | R | R | R | S | S | R | R | R | |
| UJ120 | R | R | R | R | R | S | R | R | R | |
| UJ123 | R | R | R | R | R | S | S | S | S | |
| blaNDM-1 + blaVIM | PBD048 | S | S | R | S | R | S | R | S | S |
| UJ48 | R | R | R | R | R | R | R | R | R | |
| UJ79C1 | R | R | R | R | R | R | R | R | R | |
| UJ79C2 | R | R | R | R | R | R | R | R | R | |
| UJ87C1 | R | R | R | R | R | R | R | R | R | |
| UJ87C2 | R | R | R | R | R | S | R | R | R | |
| UJ88 | R | R | R | R | R | R | R | R | R | |
| UJ90 | R | R | R | R | R | R | R | R | R | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanta, A.S.; Islam, N.; Al Asad, M.; Akter, K.; Habib, M.B.; Hossain, M.J.; Nahar, S.; Godman, B.; Islam, S. Resistance and Co-Resistance of Metallo-Beta-Lactamase Genes in Diarrheal and Urinary-Tract Pathogens in Bangladesh. Microorganisms 2024, 12, 1589. https://doi.org/10.3390/microorganisms12081589
Shanta AS, Islam N, Al Asad M, Akter K, Habib MB, Hossain MJ, Nahar S, Godman B, Islam S. Resistance and Co-Resistance of Metallo-Beta-Lactamase Genes in Diarrheal and Urinary-Tract Pathogens in Bangladesh. Microorganisms. 2024; 12(8):1589. https://doi.org/10.3390/microorganisms12081589
Chicago/Turabian StyleShanta, Ayasha Siddique, Nahidul Islam, Mamun Al Asad, Kakoli Akter, Marnusa Binte Habib, Md. Jubayer Hossain, Shamsun Nahar, Brian Godman, and Salequl Islam. 2024. "Resistance and Co-Resistance of Metallo-Beta-Lactamase Genes in Diarrheal and Urinary-Tract Pathogens in Bangladesh" Microorganisms 12, no. 8: 1589. https://doi.org/10.3390/microorganisms12081589
APA StyleShanta, A. S., Islam, N., Al Asad, M., Akter, K., Habib, M. B., Hossain, M. J., Nahar, S., Godman, B., & Islam, S. (2024). Resistance and Co-Resistance of Metallo-Beta-Lactamase Genes in Diarrheal and Urinary-Tract Pathogens in Bangladesh. Microorganisms, 12(8), 1589. https://doi.org/10.3390/microorganisms12081589






