A Bead Biofilm Reactor for High-Throughput Growth and Translational Applications
Abstract
1. Introduction
2. Materials and Methods
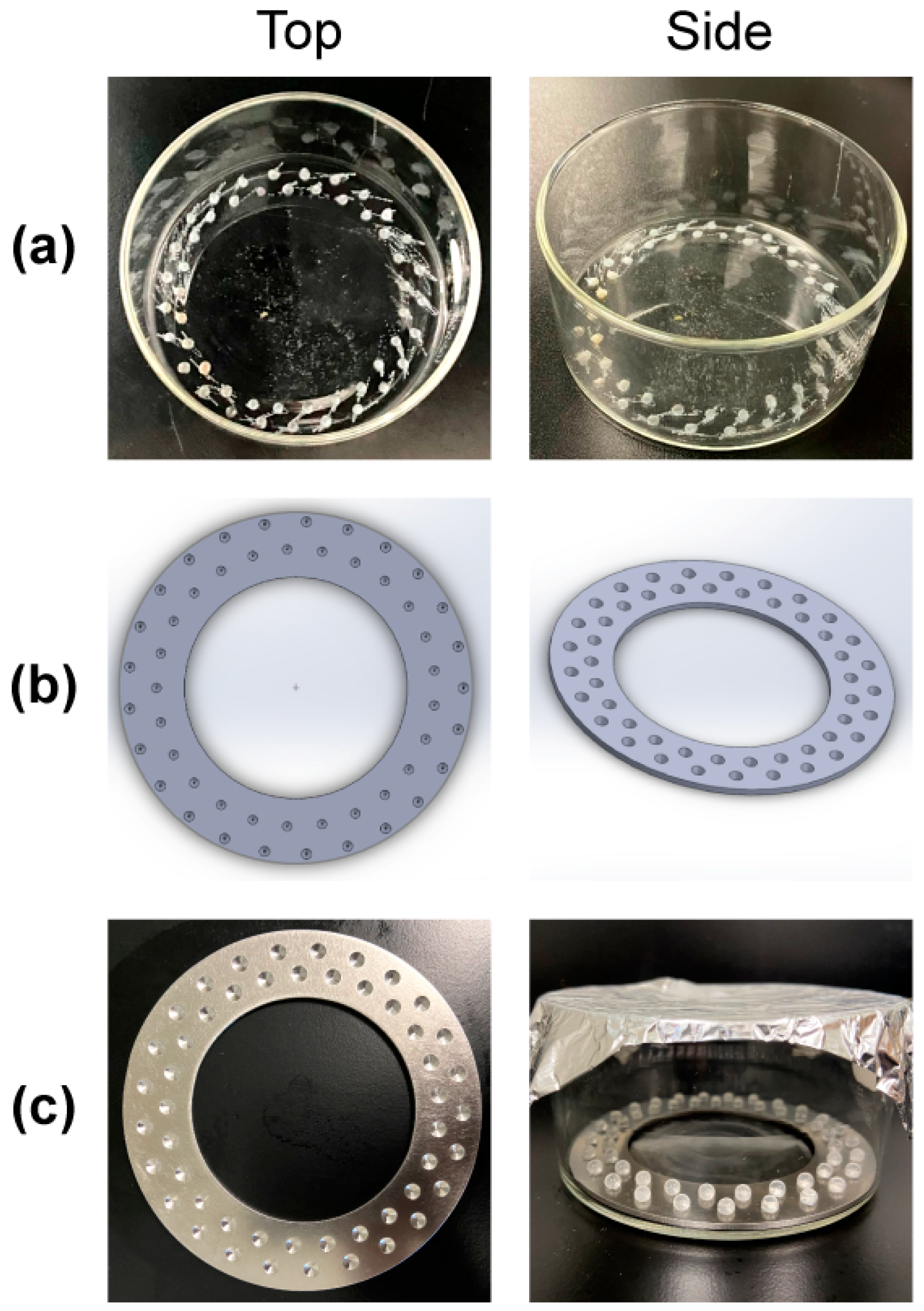
2.1. Reactor Design and Prototyping
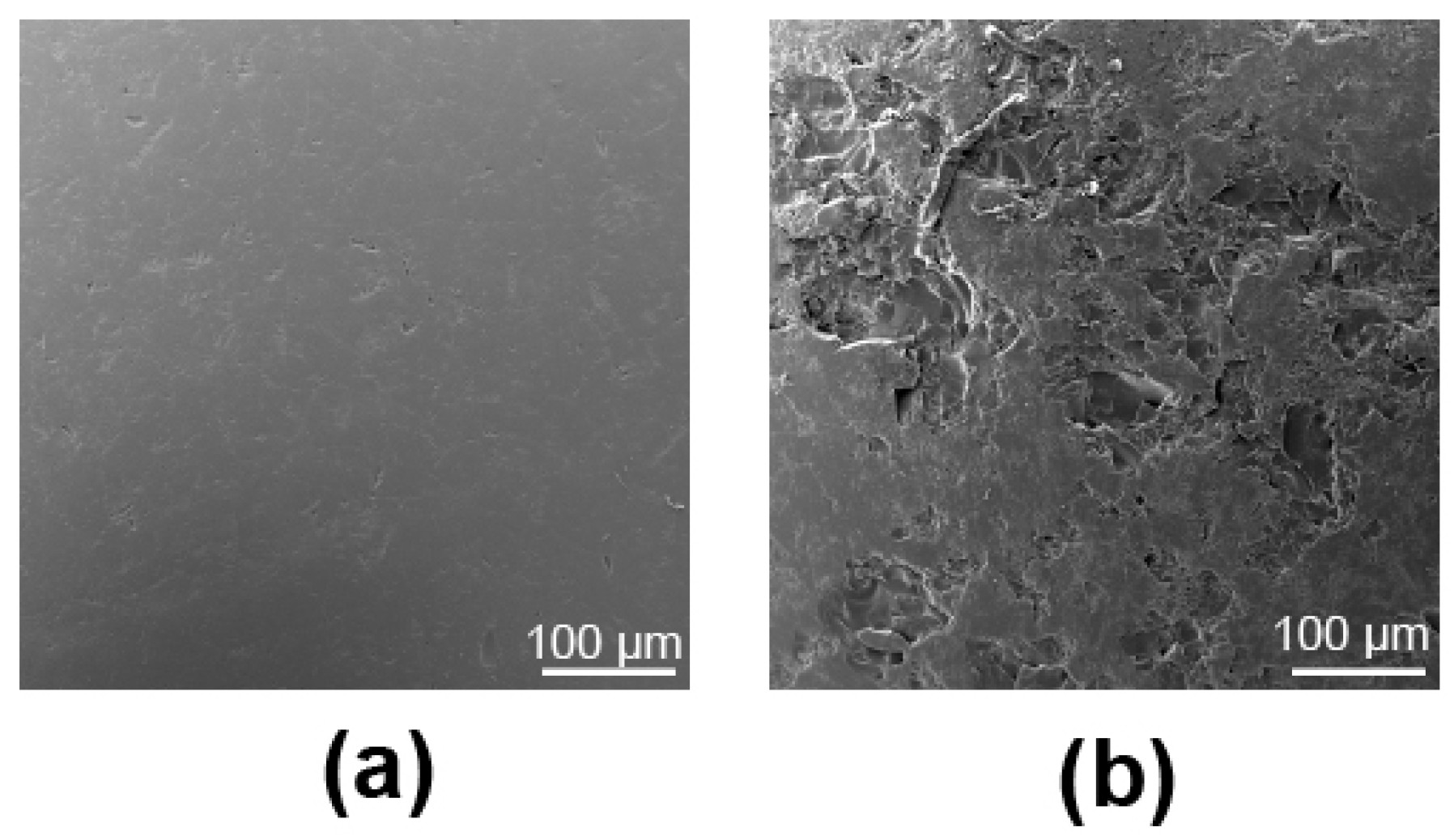
2.2. Reactor Substrate Preparation and Roughness
2.3. Bacterial Isolates and Supplies
2.4. Biofilm Growth
2.5. Quantification and Statistical Analysis
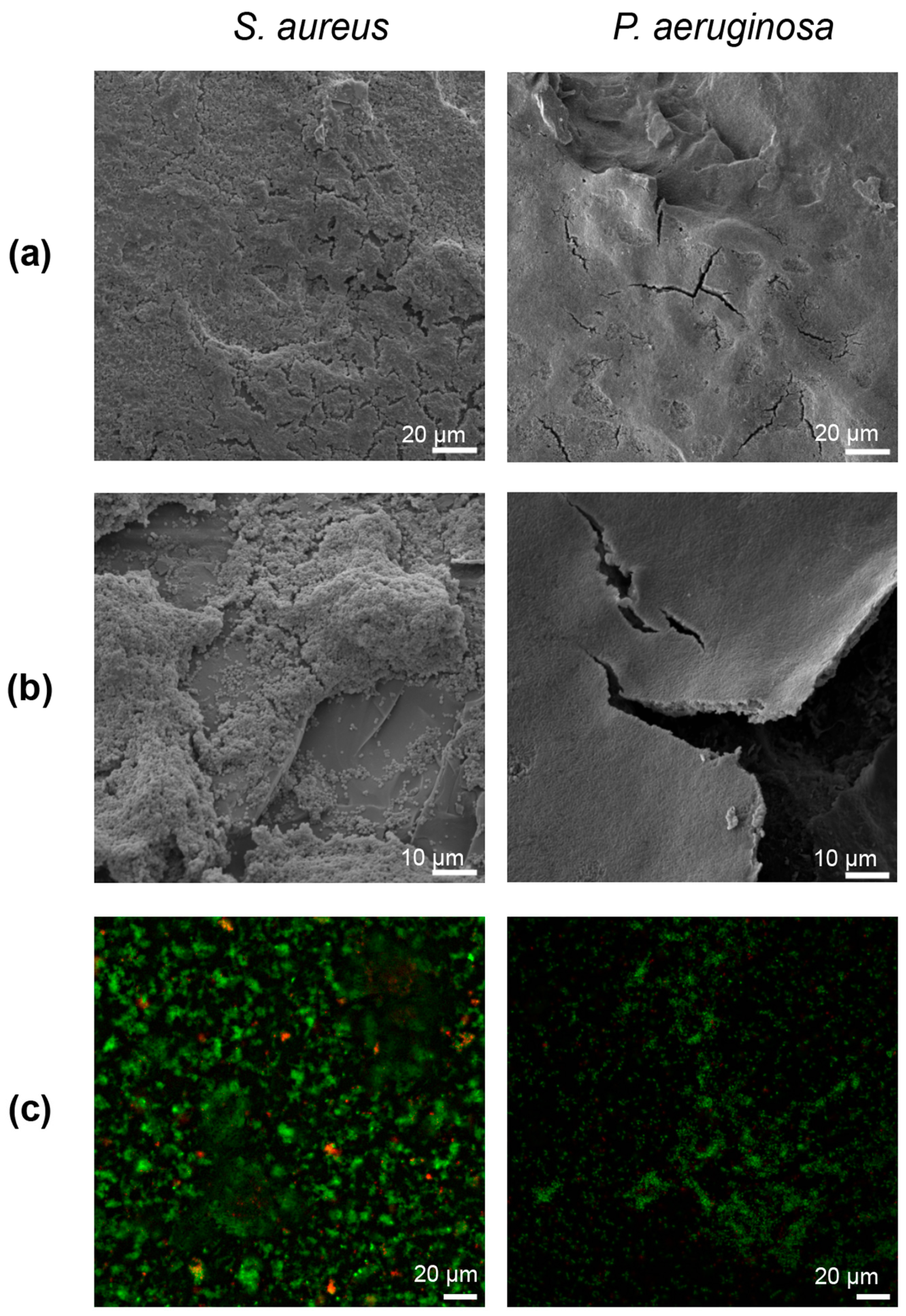
2.6. Fixation and Imaging
3. Results
3.1. Substrate Roughness Characterization
3.2. Biofilm Growth
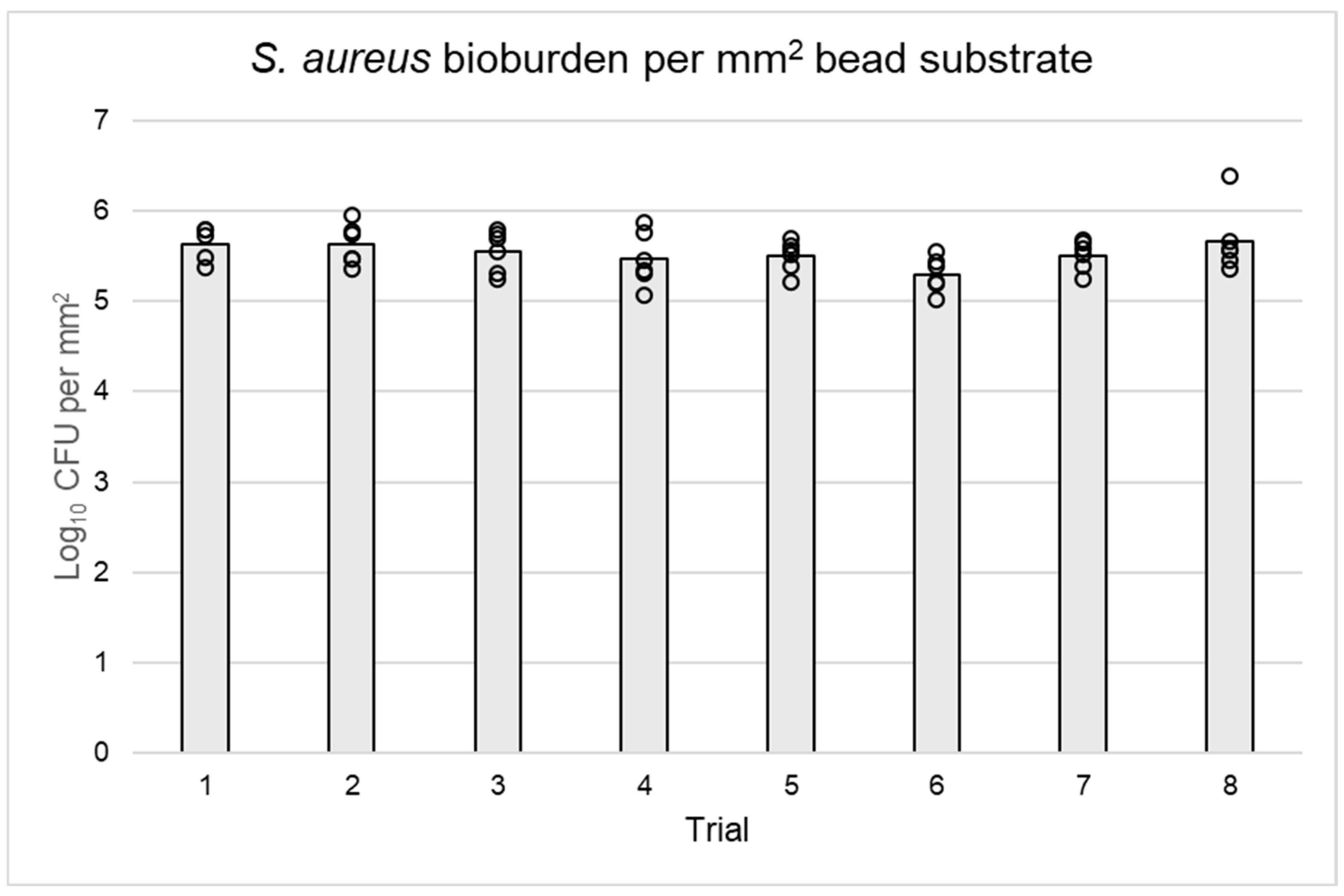
3.3. Bacterial Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial Biofilms in Nature and Disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wuertz, S. Bacteria and archaea on earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick. Sci. Am. 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells: Molecular mechanisms related to antibiotic tolerance. Antibiot. Resist. 2012, 211, 121–133. [Google Scholar]
- Costerton, J.W. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin. Orthop. Relat. Res. 2005, 437, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.; Leid, J.G.; Shirtliff, M. The Role of Biofilms in Device-Related Infections; Springer: Manhattan, NY, USA, 2009; Volume 2. [Google Scholar]
- Williams, D.L.; Costerton, J.W. Using biofilms as initial inocula in animal models of biofilm-related infections. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1163–1169. [Google Scholar] [CrossRef]
- Morgenstern, M.; Vallejo, A.; McNally, M.A.; Moriarty, T.F.; Ferguson, J.Y.; Nijs, S.; Metsemakers, W. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Jt. Res. 2018, 7, 447–456. [Google Scholar] [CrossRef]
- Tuon, F.F.; Cieslinski, J.; Ono, A.F.M.; Goto, F.L.; Machinski, J.M.; Mantovani, L.K.; Kosop, L.R.; Namba, M.S.; Rocha, J.L. Microbiological profile and susceptibility pattern of surgical site infections related to orthopaedic trauma. Int. Orthop. 2019, 43, 1309–1313. [Google Scholar] [CrossRef]
- ASTM E2871-21; Standard Test Method For Determining Disinfectant Efficacy against Biofilm Grown in the CDC Biofilm Reactor Using the Single Tube Method. ASTM International: West Conshohocken, PA, USA, 2012.
- EPA. Single tube method for determining the efficacy of disinfectants against bacterial biofilm. In Environmental Protection Agency; MB-20-04; U.S. Environmental Protection Agency Headquarters: Washington, DC, USA, 2022. [Google Scholar]
- Rasmussen, R.M.; Epperson, R.T.; Taylor, N.B.; Williams, D.L. Plume height and surface coverage analysis of methicillin-resistant Staphylococcus aureus isolates grown in a CDC biofilm reactor. Biofouling 2019, 35, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Kay, W.; Hunt, C.; Nehring, L.; Barnum, B.; Ashton, N.; Williams, D. Biofilm growth on simulated fracture fixation plates using a customized CDC biofilm reactor for a sheep model of biofilm-related infection. Microorganisms 2022, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.; Malone, M.; Hu, H.; Deva, A.; Vickery, K. The effect of negative pressure wound therapy with and without instillation on mature biofilms in vitro. Materials 2018, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Espada, R.; Wang, Y.; Goh, X.S.; Dai, T. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers Surg. Med. 2020, 52, 472–478. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2799-22; Standard Test Method for Testing Disinfectant Efficacy against Pseudomonas aeruginosa Biofilm Using the MBEC Assay. ASTM International: West Conshohocken, PA, USA, 2011.
- Ceri, H.; Olson, M.; Morck, D.; Storey, D.; Read, R.; Buret, A.; Olson, B. The MBEC assay system: Multiple equivalent biofilms for antibiotic and biocide susceptibility testing. Methods Enzym. 2001, 337, 377–385. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary biofilm device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Epperson, R.T.; Isaacson, B.M.; Rothberg, D.L.; Olsen, R.E.; Kawaguchi, B.; Maxwell, J.M.; Dickerson, M.; Pasquina, P.F.; Shero, J.; Williams, D.L. Developing a combat-relevant translatable large animal model of heterotopic ossification. Bone Rep. 2021, 15, 101127. [Google Scholar] [CrossRef] [PubMed]
- Konrat, K.; Schwebke, I.; Laue, M.; Dittmann, C.; Levin, K.; Andrich, R.; Arvand, M.; Schaudinn, C. The bead assay for biofilms: A quick, easy and robust method for testing disinfectants. PLoS ONE 2016, 11, e0157663. [Google Scholar] [CrossRef] [PubMed]
- Gries, C.M.; Kielian, T. Staphylococcal biofilms and immune polarization during prosthetic joint infection. J. Am. Acad. Orthop. Surg. 2017, 25 (Suppl. S1), S20–S24. [Google Scholar] [CrossRef]
- Murray, C.K. Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. J. Trauma 2008, 64, S232–S238. [Google Scholar] [CrossRef]
- De-La-Pinta, I.; Cobos, M.; Ibarretxe, J.; Montoya, E.; Eraso, E.; Guraya, T.; Quindós, G. Effect of biomaterials hydrophobicity and roughness on biofilm development. J. Mater. Sci. Mater. Med. 2019, 30, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, W.; Fu, H.; Yao, A.; Wang, D.; Pan, H.; Lu, W.W. Bioactive borosilicate glass scaffolds: Improvement on the strength of glass-based scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Smith, S.R.; Peterson, B.R.; Allyn, G.; Cadenas, L.; Epperson, R.T.; Looper, R.E. Growth substrate may influence biofilm susceptibility to antibiotics. PLoS ONE 2019, 14, e0206774. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Biswas, N.; Datta, A.; Maiti, P.K. Periodicities in the roughness and biofilm growth on glass substrate with etching time: Hydrofluoric acid etchant. PLoS ONE 2019, 14, e0214192. [Google Scholar] [CrossRef] [PubMed]
- Buckingham-Meyer, K.; Goeres, D.M.; Hamilton, M.A. Comparative evaluation of biofilm disinfectant efficacy tests. J. Microbiol. Methods 2007, 70, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Buckner, E.; Buckingham-Meyer, K.; Miller, L.A.; Parker, A.E.; Jones, C.J.; Goeres, D.M. Coupon position does not affect Pseudomonas aeruginosa and Staphylococcus aureus biofilm densities in the CDC biofilm reactor. J. Microbiol. Methods 2024, 223, 106960. [Google Scholar] [CrossRef] [PubMed]
- Carson, L.; Chau, P.K.W.; Earle, M.J.; Gilea, M.A.; Gilmore, B.F.; Gorman, S.P.; McCann, M.T.; Seddon, K.R. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009, 11, 492–497. [Google Scholar] [CrossRef]
- Harrison, J.J.; Turner, R.J.; Ceri, H. High-throughput metal susceptibility testing of microbial biofilms. BMC Microbiol. 2005, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Gomes, I.B.; Meireles, A.; Gonçalves, A.L.; Goeres, D.M.; Sjollema, J.; Simões, L.C.; Simões, M. Standardized reactors for the study of medical biofilms: A review of the principles and latest modifications. Crit. Rev. Biotechnol. 2018, 38, 657–670. [Google Scholar] [CrossRef]

| Organism | Mean Log10 CFU/mm2 | SE 1 | Repeatability SD 2 | % Contribution between/within Runs |
|---|---|---|---|---|
| S. aureus | 5.53 | 0.041 | 0.062 | 6.30/93.70 |
| P. aeruginosa | 6.21 | 0.11 | 0.23 | 33.96/66.04 |
| Organism | Biofilm Structure | S. aureus Mean Log10 CFU/mm2 | P. aeruginosa Mean Log10 CFU/mm2 | References |
|---|---|---|---|---|
| MBEC Peg | Single-layered | 3.83 | 4.83 | [31,32] |
| Glass bead | Multilayered | 5.53 | 6.21 | |
| Glass coupon | Multilayered | 6.10–6.30 | 6.23–6.5 | [29,30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilmore, A.; Badham, M.; Rudisin, W.; Ashton, N.; Williams, D. A Bead Biofilm Reactor for High-Throughput Growth and Translational Applications. Microorganisms 2024, 12, 1588. https://doi.org/10.3390/microorganisms12081588
Gilmore A, Badham M, Rudisin W, Ashton N, Williams D. A Bead Biofilm Reactor for High-Throughput Growth and Translational Applications. Microorganisms. 2024; 12(8):1588. https://doi.org/10.3390/microorganisms12081588
Chicago/Turabian StyleGilmore, Annika, Marissa Badham, Winston Rudisin, Nicholas Ashton, and Dustin Williams. 2024. "A Bead Biofilm Reactor for High-Throughput Growth and Translational Applications" Microorganisms 12, no. 8: 1588. https://doi.org/10.3390/microorganisms12081588
APA StyleGilmore, A., Badham, M., Rudisin, W., Ashton, N., & Williams, D. (2024). A Bead Biofilm Reactor for High-Throughput Growth and Translational Applications. Microorganisms, 12(8), 1588. https://doi.org/10.3390/microorganisms12081588







