A Holistic Approach to Producing Anti-Vibrio Metabolites by an Endosymbiotic Dinoflagellate Using Wastewater from Shrimp Rearing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Shrimp Production
2.2. Dinoflagellate Strain and Culture Conditions
2.3. Experimental Design
2.4. Biological, Chemical, and Biochemical Analyses
2.4.1. Growth Evaluation
2.4.2. Nitrogen and Phosphorus Analyses
2.4.3. Pigment Analysis
2.4.4. Lipid Extraction and Fatty Acid Composition
2.5. Antibacterial Activity
2.6. Economic Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
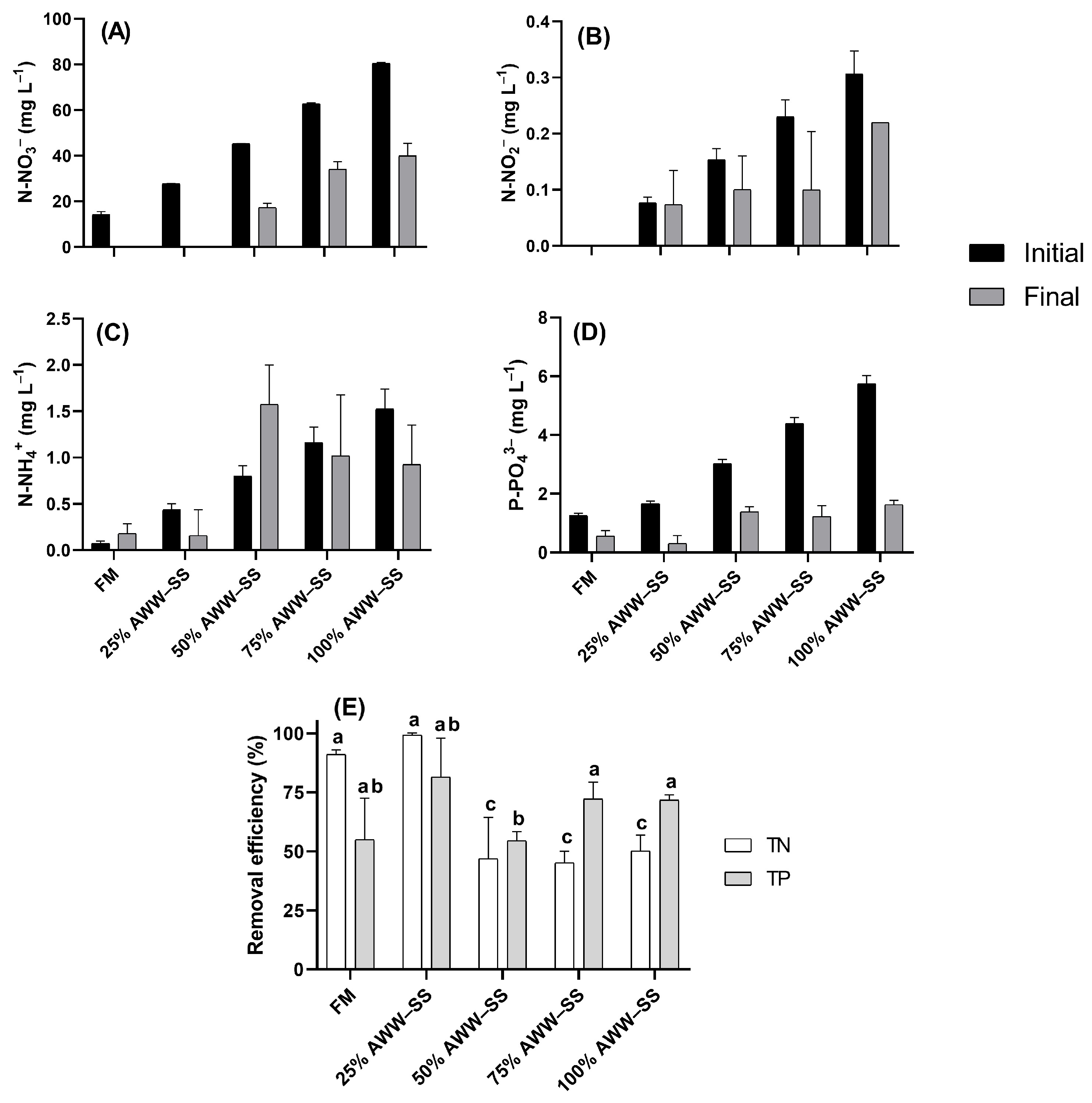
3.2. Nutrient Removal
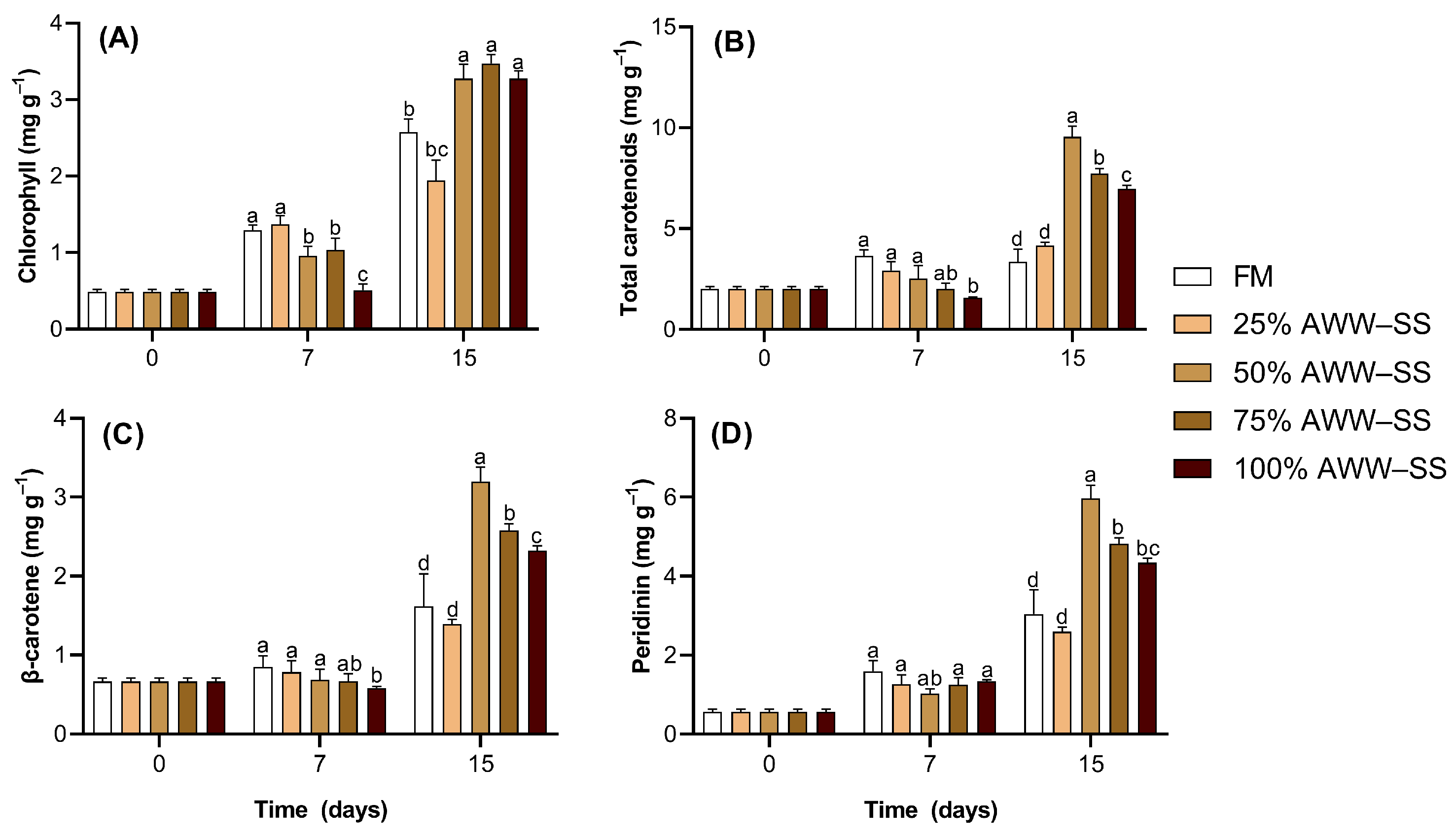
3.3. Pigments Composition
3.4. Fatty Acids Profile
3.5. Antibacterial Activity
3.6. Economic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hambrey, J. The 2030 Agenda and the Sustainable Development Goals: The Challenge for Aquaculture Development and Management; FAO Fisheries and Aquaculture Circular (C1141); FAO: Rome, Italy, 2017. [Google Scholar]
- Calijuri, M.L.; Silva, T.A.; Magalhães, I.B.; de Paula Pereira, A.S.A.; Marangon, B.B.; de Assis, L.R.; Lorentz, J.F. Bioproducts from microalgae biomass: Technology, sustainability, challenges and opportunities. Chemosphere 2022, 291, 135508. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture (SOFIA) 2022; Food and Agricultura Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Alhazzaa, R.; Nichols, P.D.; Carter, C.G. Sustainable alternatives to dietary fish oil in tropical fish aquaculture. Rev. Aquac. 2019, 11, 1195–1218. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent advances in single cell protein use as a feed ingredient in aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Maigual-Enriquez, Y.A.; Maia, A.A.D.; Guerrero-Romero, C.L.; Matsumoto, T.; Rangel, E.C.; de Morais, L.C. Comparison of sludges produced from two different recirculating aquaculture systems (RAS) for recycle and disposal. Aquaculture 2019, 502, 87–96. [Google Scholar] [CrossRef]
- Farzana, S.; Cheung, S.G.; Kong, R.Y.C.; Wong, Y.S.; Tam, N.F.Y. Enhanced remediation of BDE-209 in contaminated mangrove sediment by planting and aquaculture effluent. Sci. Total Environ. 2021, 754, 142094. [Google Scholar] [CrossRef]
- Heal, R.D.; Hasan, N.A.; Haque, M.M. Increasing disease burden and use of drugs and chemicals in Bangladesh shrimp aquaculture: A potential menace to human health. Mar. Pollut. Bull. 2021, 172, 112796. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Panda, S.K.; Luyten, W. Anti-vibrio and immune-enhancing activity of medicinal plants in shrimp: A comprehensive review. Fish Shellfish Immunol. 2021, 117, 192–210. [Google Scholar] [CrossRef]
- Kumar, V.; Baruah, K.; Nguyen, D.V.; Smagghe, G.; Vossen, E.; Bossier, P. Phloroglucinol-mediated Hsp70 production in crustaceans: Protection against Vibrio parahaemolyticus in Artemia franciscana and Macrobrachium rosenbergii. Front. Immunol. 2018, 9, 1091. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An overview on microalgae as renewable resources for meeting sustainable development goals. J. Environ. Manag. 2022, 320, 115897. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Y.; Guo, H.; Yan, S.; Mu, J. Microalgae cultivation using an aquaculture wastewater as growth medium for biomass and biofuel production. J. Environ. Sci. 2013, 25, S85–S88. [Google Scholar] [CrossRef]
- Tejido-Nuñez, Y.; Aymerich, E.; Sancho, L.; Refardt, D. Treatment of aquaculture effluent with Chlorella vulgaris and Tetradesmus obliquus: The effect of pretreatment on microalgae growth and nutrient removal efficiency. Ecol. Eng. 2019, 136, 1–9. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- Regueiro, L.; Newton, R.; Soula, M.; Mendez, D.; Kok, B.; Little, D.C.; Pastres, R.; Johansen, J.; Ferreira, M. Opportunities and limitations for the introduction of circular economy principles in EU aquaculture based on the regulatory framework. J. Ind. Ecol. 2022, 26, 2033–2044. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Feng, L.J.; Liu, J.Z.; Liu, M.; Cai, H.W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Duarte, J.H.; Andrade, B.B.; Lemos, P.V.F.; Costa, J.A.V.; Druzian, J.I.; Chinalia, F.A. Spirulina sp. LEB 18 cultivation in outdoor pilot scale using aquaculture wastewater: High biomass, carotenoid, lipid and carbohydrate production. Aquaculture 2020, 525, 735272. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Abreu, J.K.; Oliveira, C.D.L.; Celes, P.; Gálvez, A.O.; Dantas, D.M.M. Growth of Chlorella vulgaris using wastewater from Nile tilapia (Oreochromis niloticus) farming in a low-salinity biofloc system. Acta Sci. Technol. 2020, 42, e46232. [Google Scholar] [CrossRef]
- Lopes da Silva, T.; Moniz, P.; Silva, C.; Reis, A. The dark side of microalgae biotechnology: A heterotrophic biorefinery platform directed to ω-3 rich lipid production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; van Staden, J. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; García-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Supasri, K.M.; Kumar, M.; Segečová, A.; McCauley, J.I.; Herdean, A.; Padula, M.P.; O’Meara, T.; Ralph, P.J. Characterisation and bioactivity analysis of peridinin-chlorophyll a-Protein (PCP) Isolated from Symbiodinium tridacnidorum CS-73. J. Mar. Sci. Eng. 2021, 9, 1387. [Google Scholar] [CrossRef]
- Andrade, R.J.V.; dos Santos, E.P.; de Almeida Costa, G.K.; da Silva Campos, C.V.F.; da Silva, S.M.B.C.; Gálvez, A.O.; Brito, L.O. Effect of different frequencies of the addition of Brachionus plicatilis on the performance of Litopenaeus vannamei in a nursery biofloc system with rice bran (anaerobic and aerobic) as an organic carbon source. Aquaculture 2021, 540, 736669. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Abreu, J.L.; Santos, E.P.; Matos, Â.P.; Tribuzi, G.; Oliveira, C.D.L.; Veras, B.O.; Bezerra, R.S.; Müller, M.N.; Gálvez, A.O. Light induces peridinin and docosahexaenoic acid accumulation in the dinoflagellate Durusdinium glynnii. Appl. Microbiol. Biotechnol. 2022, 106, 6263–6276. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Fries, J. Análisis de Trazas. Métodos Fotométricos Comprobados; Merck: Darmstadt, Germany, 1971; 130p. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Carreto, J.I.; Catoggio, J.A. An indirect method for the rapid estimation of carotenoid contents in Phaeodactylum tricornutum: Possible application to other marine algae. Mar. Biol. 1977, 40, 109–116. [Google Scholar] [CrossRef]
- Prézelin, B.B. The role of peridinin-chlorophyll a-proteins in the photosynthetic light adaption of the marine dinoflagellate, Glenodinium sp. Planta 1976, 1303, 225–233. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Faé Neto, W.A.; Borges Mendes, C.R.; Abreu, P.C. Carotenoid production by the marine microalgae Nannochloropsis oculata in different low-cost culture media. Aquac. Res. 2018, 49, 2527–2535. [Google Scholar] [CrossRef]
- Cucco, M.; Guasco, B.; Malacarne, G.; Ottonelli, R. Effects of β-carotene on adult immune condition and antibacterial activity in the eggs of the Grey Partridge, Perdix perdix. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2007, 147, 1038–1046. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, C.; Lopes, R.; Derner, R.; Vinatea, L. Using residual water from a marine shrimp farming BFT system. Part I: Nutrient removal and marine microalgae biomass production. Aquac. Res. 2016, 47, 2435–2443. [Google Scholar] [CrossRef]
- Morando-Grijalva, C.A.; Vázquez-Larios, A.L.; Alcántara-Hernández, R.J.; Ortega-Clemente, L.A.; Robledo-Narváez, P.N. Isolation of a freshwater microalgae and its application for the treatment of wastewater and obtaining fatty acids from tilapia cultivation. Environ. Sci. Pollut. Res. 2020, 27, 28575–28584. [Google Scholar] [CrossRef] [PubMed]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M.; Zając, G.; Szyszlak-Bargłowicz, J. Production of microalgal biomass using aquaculture wastewater as growth medium. Water 2019, 12, 106. [Google Scholar] [CrossRef]
- Andreotti, V.; Chindris, A.; Brundu, G.; Vallainc, D.; Francavilla, M.; García, J. Bioremediation of aquaculture wastewater from Mugil cephalus (Linnaeus, 1758) with different microalgae species. Chem. Ecol. 2017, 33, 750–761. [Google Scholar] [CrossRef]
- Dinesh Kumar, S.; Santhanam, P.; Prabhavathi, P.; Kanimozhi, B.; Abirami, M.; Park, M.S.; Kim, M.K. Optimal conditions for the treatment of shrimp culture effluent using immobilized marine microalga Picochlorum maculatum (PSDK01). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 1177–1185. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquac. 2021, 13, 676–705. [Google Scholar] [CrossRef]
- Kuhn, D.D.; Smith, S.A.; Boardman, G.D.; Angier, M.W.; Marsh, L.; Flick, G.J., Jr. Chronic toxicity of nitrate to Pacific white shrimp, Litopenaeus vannamei: Impacts on survival, growth, antennae length, and pathology. Aquaculture 2010, 309, 109–114. [Google Scholar] [CrossRef]
- Murphy, A.P. Chemical removal of nitrate from water. Nature 1991, 350, 223–225. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, J. A critical review of various adsorbents for selective removal of nitrate from water: Structure, performance and mechanism. Chemosphere 2021, 291, 132728. [Google Scholar] [CrossRef]
- Qian, Z.; Na, L.; Wang, B.-L.; Tao, Z.; Ma, P.-F.; Zhang, W.-X.; Sraboni, N.Z.; Zheng, M.; Zhang, Y.-Q.; Liu, Y. Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J. Environ. Manag. 2022, 320, 115673. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.; Wenley, J.; Currie, K.; Hepburn, C.; Herndl, G.J.; Baltar, F. Resolving the paradox: Continuous cell-free alkaline phosphatase activity despite high phosphate concentrations. Mar. Chem. 2019, 214, 103671. [Google Scholar] [CrossRef]
- Mo, Y.; Ou, L.; Lin, L.; Huang, B. Temporal and spatial variations of alkaline phosphatase activity related to phosphorus status of phytoplankton in the East China Sea. Sci. Total Environ. 2020, 731, 139192. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.; Hansen, P.J.; Moestrup, Ø. Feeding mechanism, prey specificity and growth in light and dark of the plastidic dinoflagellate Karlodinium armiger. Aquat. Microb. Ecol. 2008, 50, 279–288. [Google Scholar] [CrossRef]
- Müller, M.N.; Dorantes-Aranda, J.J.; Seger, A.; Botana, M.T.; Brandini, F.P.; Hallegraeff, G.M. Ichthyotoxicity of the Dinoflagellate Karlodinium veneficum in Response to Changes in Seawater pH. Front. Mar. Sci. 2019, 6, 82. [Google Scholar] [CrossRef]
- Sheikh, H.; John, A.; Musa, N.; Alfatama, M.; Fadhlina, A. Vibrio spp. and Their Vibriocin as a Vibriosis Control Measure in Aquaculture. Appl. Biochem. Biotechnol. 2022, 194, 4477–4491. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rodriguez, S.A.; Magallón-Servín, P.; López-Vela, M.; Nieves Soto, M. Inhibitory effect of marine microalgae used in shrimp hatcheries on Vibrio parahaemolyticus responsible for acute hepatopancreatic necrosis disease. Aquac. Res. 2022, 53, 1337–1347. [Google Scholar] [CrossRef]
- Esquer-Miranda, E.; Nieves-Soto, M.; Rivas-Vega, M.E.; Miranda-Baeza, A.; Pi, P. Effects of methanolic macroalgae extracts from Caulerpa sertularioides and Ulva lactuca on Litopenaeus vannamei survival in the presence of Vibrio bacteria. Fish Shellfish Immunol. 2016, 51, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Boonsri, N.; Rudtanatip, T.; Withyachumnarnkul, B.; Wongprasert, K. Protein extract from red seaweed Gracilaria fisheri prevents acute hepatopancreatic necrosis disease (AHPND) infection in shrimp. J. Appl. Phycol. 2017, 29, 1597–1608. [Google Scholar] [CrossRef]
- Rudi, M.; Sukenda, S.; Wahjuningrum, D.; Pasaribu, W.; Hidayatullah, D. Seaweed extract of Gracilaria verrucosa as an antibacterial and treatment against Vibrio harveyi infection of Litopenaeus vannamei. J. Akuak. Indones. 2019, 18, 120–129. [Google Scholar] [CrossRef]
- Suhartono, S.; Ismail, Y.S.; Muhayya, S.R.; Husnah, M. Ethanolic extracts of Moringa oleifera leaves inhibit biofilm formation of Vibrio alginolyticus in vitro. IOP Conf. Ser. Earth Environ. Sci. 2019, 348, 012018. [Google Scholar] [CrossRef]
- Rattanavichai, W.; Cheng, W. Effects of hot-water extract of banana (Musa acuminata) fruit’s peel on the antibacterial activity, and anti-hypothermal stress, immune responses and disease resistance of the giant freshwater prawn, Macrobrachium rosenbegii. Fish Shellfish Immunol. 2014, 39, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acid | FM | 25% AWW-SS | 50% AWW-SS | 75% AWW-SS | 100% AWW-SS |
|---|---|---|---|---|---|
| C10:0 | 0.22 ± 0.10 | 0.56 ± 0.07 | 0.48 ± 0.10 | 0.70 ± 0.09 | 0.60 ± 0.12 |
| C12:0 | 2.28 ± 0.23 | 4.50 ± 0.14 | 3.02 ± 0.19 | 4.48 ± 0.20 | 3.73 ± 0.22 |
| C14:0 | 6.42 ± 0.32 | 9.73 ± 0.27 | 7.66 ± 0.44 | 12.10 ± 0.37 | 9.00 ± 0.57 |
| C15:0 | 0.12 ± 0.05 | 0.18 ± 0.10 | 0.18 ± 0.10 | 0.21 ± 0.05 | 0.21 ± 0.10 |
| C16:0 | 39.90 ± 0.47 | 34.16 ± 0.55 | 32.20 ± 0.25 | 33.46 ± 0.35 | 28.57 ± 0.65 |
| C17:0 | 0.15 ± 0.03 | 0.17 ± 0.04 | 0.28 ± 0.05 | 0.34 ± 0.04 | 0.31 ± 0.07 |
| C18:0 | 1.32 ± 0.13 | 1.28 ± 0.10 | 1.60 ± 0.15 | 1.75 ± 0.17 | 1.65 ± 0.12 |
| C21:0 | 6.55 ± 0.22 | 8.92 ± 0.41 | 14.38 ± 0.37 | 11.52 ± 0.31 | 13.00 ± 0.18 |
| Ʃ SFA | 56.96 ± 0.78 | 59.50 ± 0.70 | 59.80 ± 0.69 | 64.56 ± 0.84 | 57.70 ± 077 |
| C14:1 | 0.20 ± 0.05 | 0.15 ± 0.02 | 0.30 ± 0.04 | 0.23 ± 0.02 | 0.23 ± 0.01 |
| C15:1 | 0.62 ± 0.11 | 0.51 ± 0.10 | 1.38 ± 0.12 | 1.24 ± 0.15 | 1.10 ± 0.10 |
| C16:1 | 14.39 ± 0.11 | 9.36 ± 0.21 | 10.31 ± 0.12 | 10.18 ± 0.22 | 11.07 ± 0.18 |
| C17:1 | 0.20 ± 0.05 | 0.15 ± 0.01 | 0.25 ± 0.02 | 0.26 ± 0.05 | 0.10 ± 0.04 |
| C18:1 | 16.53 ± 0.47 | 15.03 ± 0.22 | 7.48 ± 0.14 | 6.58 ± 0.22 | 7.40 ± 0.17 |
| C20:1 | - | - | 0.20 ± 0.03 | 0.14 ± 0.03 | - |
| C22:1 | - | 0.11 ± 0.01 | 0.25 ± 0.03 | 0.20 ± 0.04 | - |
| Ʃ MUFA | 31.94 ± 0.41 | 25.20 ± 0.76 | 20.17 ± 0.29 | 18.83 ± 0.10 | 19.90 ± 0.22 |
| C18:3 ω3 (ALA) | 0.24 ± 0.05 | 0.43 ± 0.06 | 0.43 ± 0.08 | 0.53 ± 0.09 | 0.46 ± 0.05 |
| C20:5 ω3 (EPA) | 0.77 ± 0.03 | 0.90 ± 0.05 | 0.70 ± 0.03 | 0.81 ± 0.06 | 1.76 ± 0.06 |
| C22:6 ω3 (DHA) | 5.38 ± 0.21 | 7.70 ± 0.11 | 8.20 ± 0.18 | 6.81 ± 0.27 | 11.14 ± 0.16 |
| Ʃ PUFA–ω3 | 6.40 ± 0.11 | 9.00 ± 0.09 | 9.33 ± 0.13 | 8.15 ± 0.16 | 13.36 ± 0.17 |
| C18:2 ω6 (LA) | 1.27 ± 0.05 | 1.16 ± 0.02 | 1.26 ± 0.02 | 1.00 ± 0.04 | 0.73 ± 0.02 |
| C18:3 ω6 (GLA) | 0.74 ± 0.02 | 0.18 ± 0.03 | 0.78 ± 0.07 | 0.46 ± 0.05 | 0.31 ± 0.03 |
| C20:3 ω6 | 0.14 ± 0.05 | 0.16 ± 0.02 | - | 0.42 ± 0.03 | 0.30 ± 0.05 |
| C20:4 ω6 (AA) | - | - | 0.28 ± 0.02 | 0.21 ± 0.03 | 0.45 ± 0.04 |
| Ʃ PUFA–ω6 | 2.15 ± 0.03 | 1.50 ± 0.02 | 2.32 ± 0.04 | 2.09 ± 0.03 | 1.79 ± 0.03 |
| ω3/ω6 | 2.97 | 6.00 | 4.02 | 3.89 | 7.46 |
| Treatment | Culture Medium Cost (USD m−3) | Biomass Production (g m−3) | Peridinin Content (g kg−1) | USD per kg Biomass | USD per g Peridinin | Production Time (Days) * |
|---|---|---|---|---|---|---|
| FM | 15.6 | 266.0 ± 39.7 | 3.0 ± 0.6 | 59.5 ± 8.7 | 20.2 ± 5.3 | 37.4 ± 4.1 |
| 25% AWW–SS | 11.7 | 340.0 ± 52.9 | 2.6 ± 0.1 | 35.0 ± 5.9 | 13.5 ± 2.6 | 30.6 ± 2.7 |
| 50% AWW–SS | 7.8 | 426.7 ± 23.1 | 6.0 ± 0.3 | 18.3 ± 1.0 | 3.1 ± 0.0 | 21.1 ± 1.2 |
| 75% AWW–SS | 3.9 | 513.3 ± 41.6 | 4.8 ± 0.2 | 7.6 ± 0.6 | 1.6 ± 0.2 | 17.6 ± 1.4 |
| 100% AWW–SS | 0 | 453.3 ± 11.5 | 4.3 ± 0.1 | 0 | 0 | 19.9 ± 0.5 |
| Microalgae | Systems | Target Species | TN (%) | TP (%) | Refs. |
|---|---|---|---|---|---|
| Durusdinium glynnii | SS | Pacific white shrimp | 50.1 | 71.7 | This study |
| Chaetoceros muelleri | BFT | Pacific white shrimp | - | 100 | [37] |
| Chlamydomonas sp. | - | Tilapia | 79.6 | 96.0 | [38] |
| Chlorella minutissima | RAS | Salmon | 88.0 | 99.0 | [39] |
| Chlorella vulgaris | BFT | Tilapia | 84.3 | 48.3 | [18] |
| RAS | Tilapia | 99.8 | 82.7 | [16] | |
| - | Pacific white shrimp | 86.1 | 82.7 | [16] | |
| - | Flathead grey mullet | 95.4 | 92.0 | [40] | |
| Isochrysis galbana | - | Flathead grey mullet | 66.0 | 91.9 | [40] |
| Nannochloropsis oculata | BFT | Pacific white shrimp | 83.0 | 100 | [37] |
| Picochlorum maculatum | - | Pacific white shrimp | 66.7 | 92.8 | [41] |
| Platymonas subcordiformi | - | Flounder | 100 | 100 | [12] |
| Spirulina sp. | - | Tilapia | 81.1 | 100 | [17] |
| Tetradesmus obliquus | RAS | Tilapia | 99.7 | 99.6 | [13] |
| RAS | Tilapia | 80.1 | ~100 | [27] | |
| Tetraselmis chuii | BFT | Pacific white shrimp | 87.0 | 100 | [37] |
| Source | Type of Inclusion | Dosage (μg mL−1) | Method | Vibrio Strain | Refs. |
|---|---|---|---|---|---|
| Microalgae | |||||
| Durusdinium glynnii | AcE | KBM | VP, VV | This study | |
| Chaetoceros calcitrans | AqE | 70 | LM | VP | [52] |
| Seaweeds | |||||
| Caulerpa sertularioides | ME | 1000 | MM | VA, VP | [53] |
| Gracilaria fisheri | CPE | 50 | LM | VP | [54] |
| Gracilaria verrucosa | EE | 2 | AD | VH | [55] |
| Ulva lactuca | ME | >1500 | MM | VA, VP | [53] |
| Plants | |||||
| Moringa oleifera | EE | 64 | BMPA | VA | [56] |
| Musa acuminata | AqE | 1560 | DD | VP, VA | [57] |
| Ocimum basilicum | AqE | 19 | KBM | VH, VP, VA | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.Y.B.; Abreu, J.L.; Brandão, B.C.; Oliveira, D.W.S.; de Sena, P.R.; da Silva, W.A.; Araújo, E.S.; Rörig, L.R.; de Almeida Costa, G.K.; Silva, S.M.B.C.; et al. A Holistic Approach to Producing Anti-Vibrio Metabolites by an Endosymbiotic Dinoflagellate Using Wastewater from Shrimp Rearing. Microorganisms 2024, 12, 1598. https://doi.org/10.3390/microorganisms12081598
Oliveira CYB, Abreu JL, Brandão BC, Oliveira DWS, de Sena PR, da Silva WA, Araújo ES, Rörig LR, de Almeida Costa GK, Silva SMBC, et al. A Holistic Approach to Producing Anti-Vibrio Metabolites by an Endosymbiotic Dinoflagellate Using Wastewater from Shrimp Rearing. Microorganisms. 2024; 12(8):1598. https://doi.org/10.3390/microorganisms12081598
Chicago/Turabian StyleOliveira, Carlos Yure B., Jéssika L. Abreu, Barbara C. Brandão, Deyvid Willame S. Oliveira, Pedro Rodrigues de Sena, Weverson Ailton da Silva, Evando S. Araújo, Leonardo R. Rörig, Gisely Karla de Almeida Costa, Suzianny Maria B. C. Silva, and et al. 2024. "A Holistic Approach to Producing Anti-Vibrio Metabolites by an Endosymbiotic Dinoflagellate Using Wastewater from Shrimp Rearing" Microorganisms 12, no. 8: 1598. https://doi.org/10.3390/microorganisms12081598







