Antimicrobial Effect of Chitosan Nanoparticles and Allium Species on Mycobacterium tuberculosis and Several Other Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Chitosan Nanoparticles
2.2. Physicochemical Properties of CNPs
2.3. Water-Based Extraction of Garlic
2.4. Antimicrobial Activity Assays
3. Results
3.1. Synthesis and Characterization of Chitosan Nanoparticles
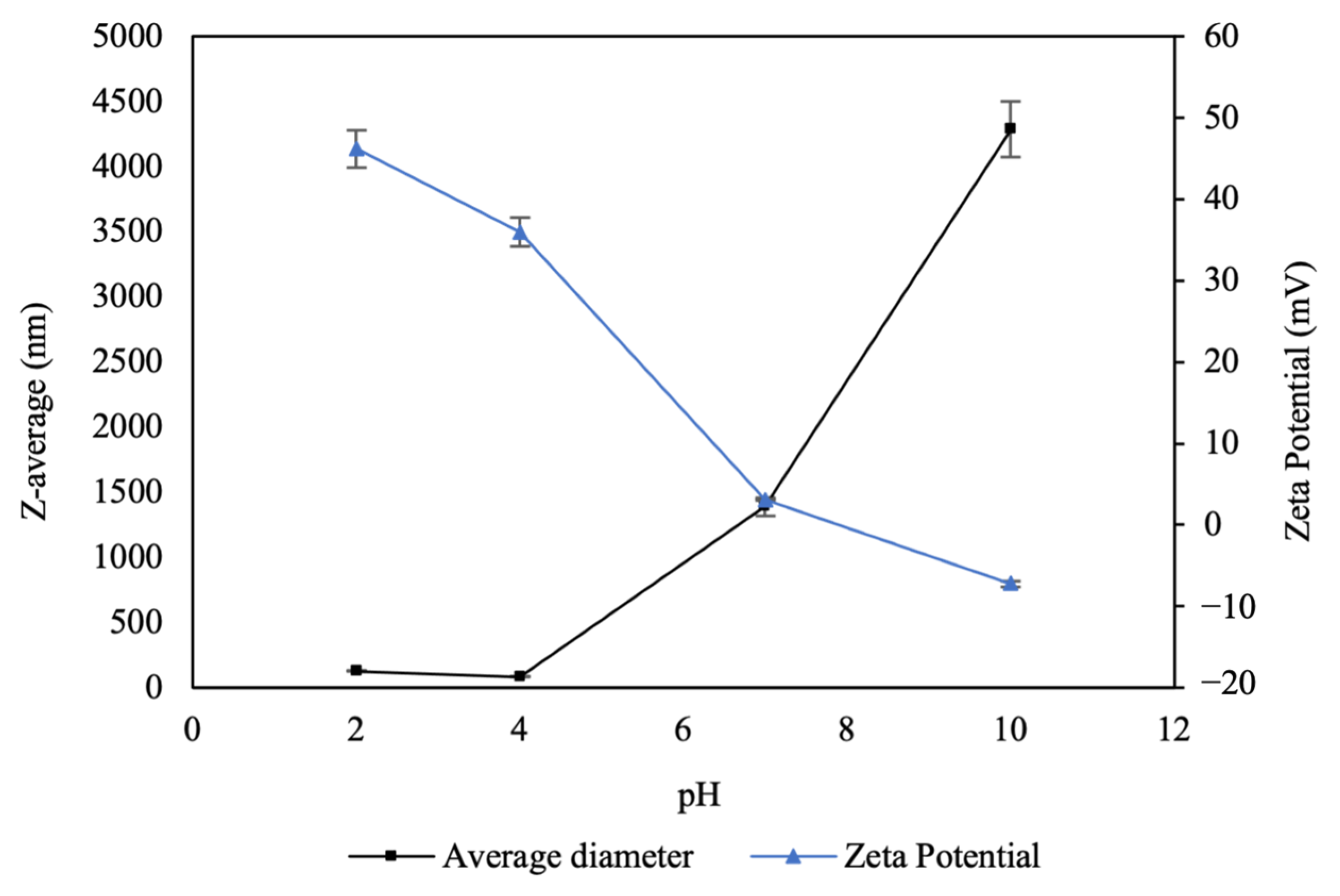
3.1.1. Effect of pH on Surface Charge and Size of CNPs
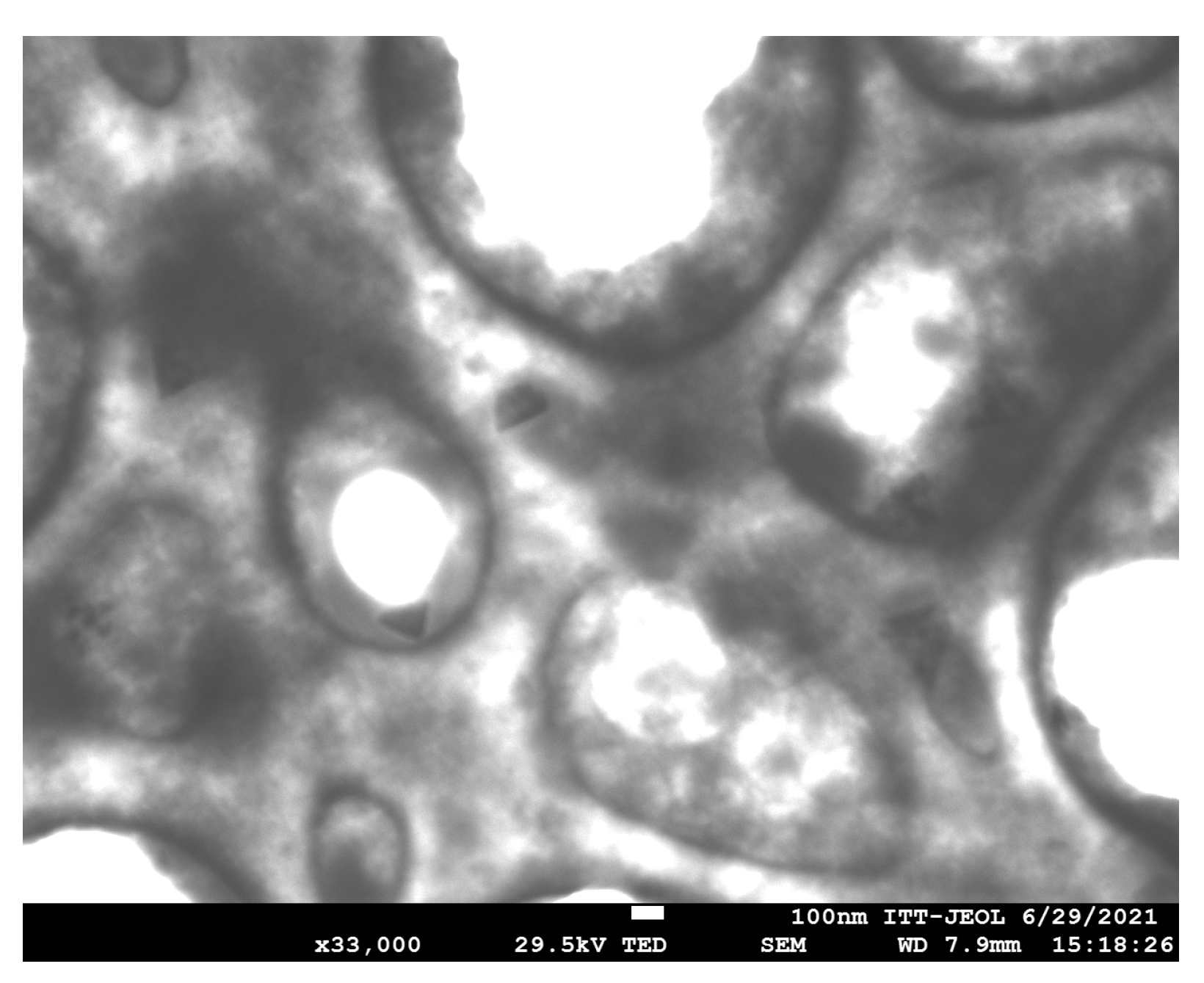
3.1.2. Morphological Analysis of CNPs
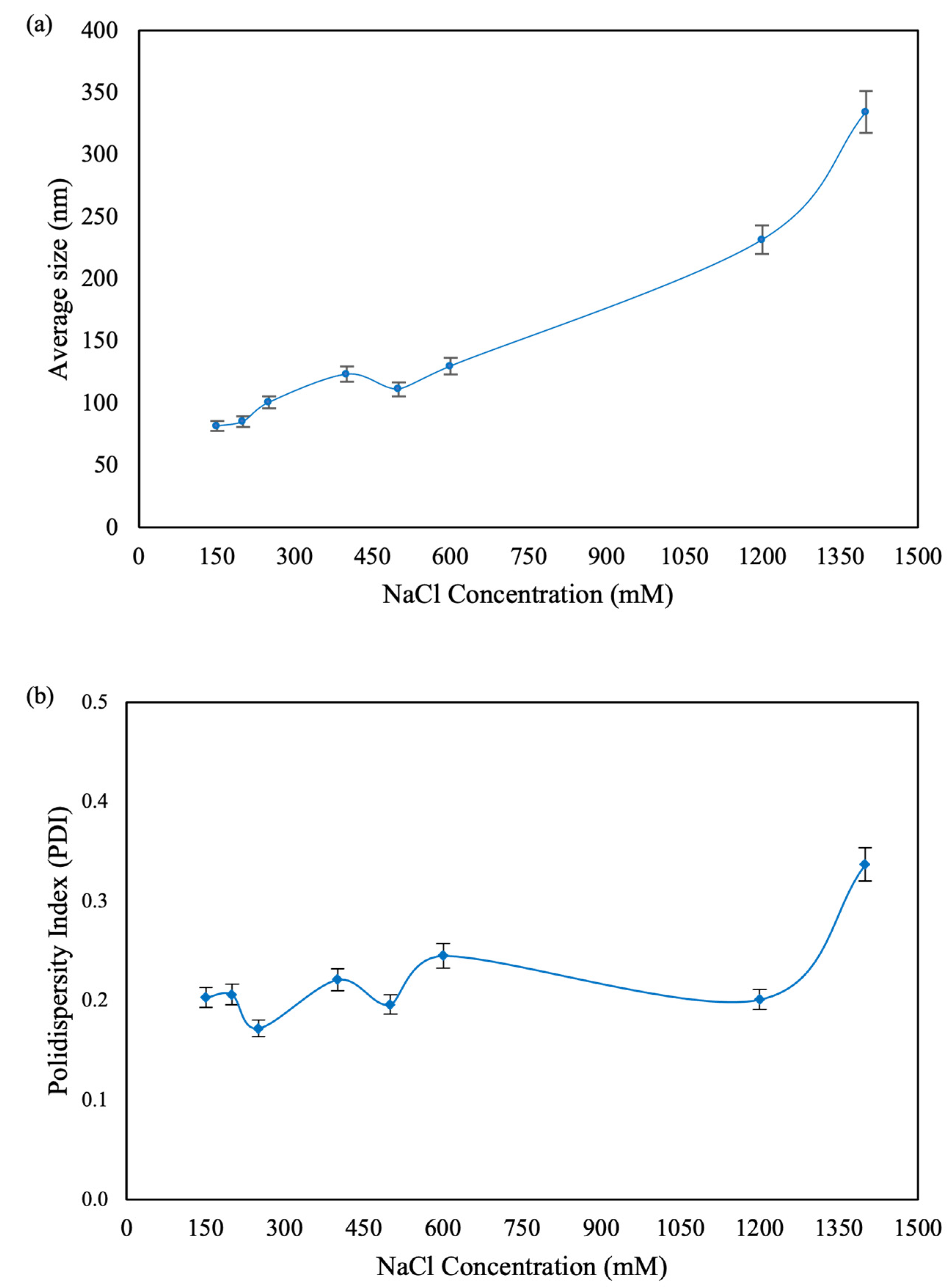
3.1.3. Stability and Size Variation of Stored Nanoparticles
3.2. Antimicrobial Activity
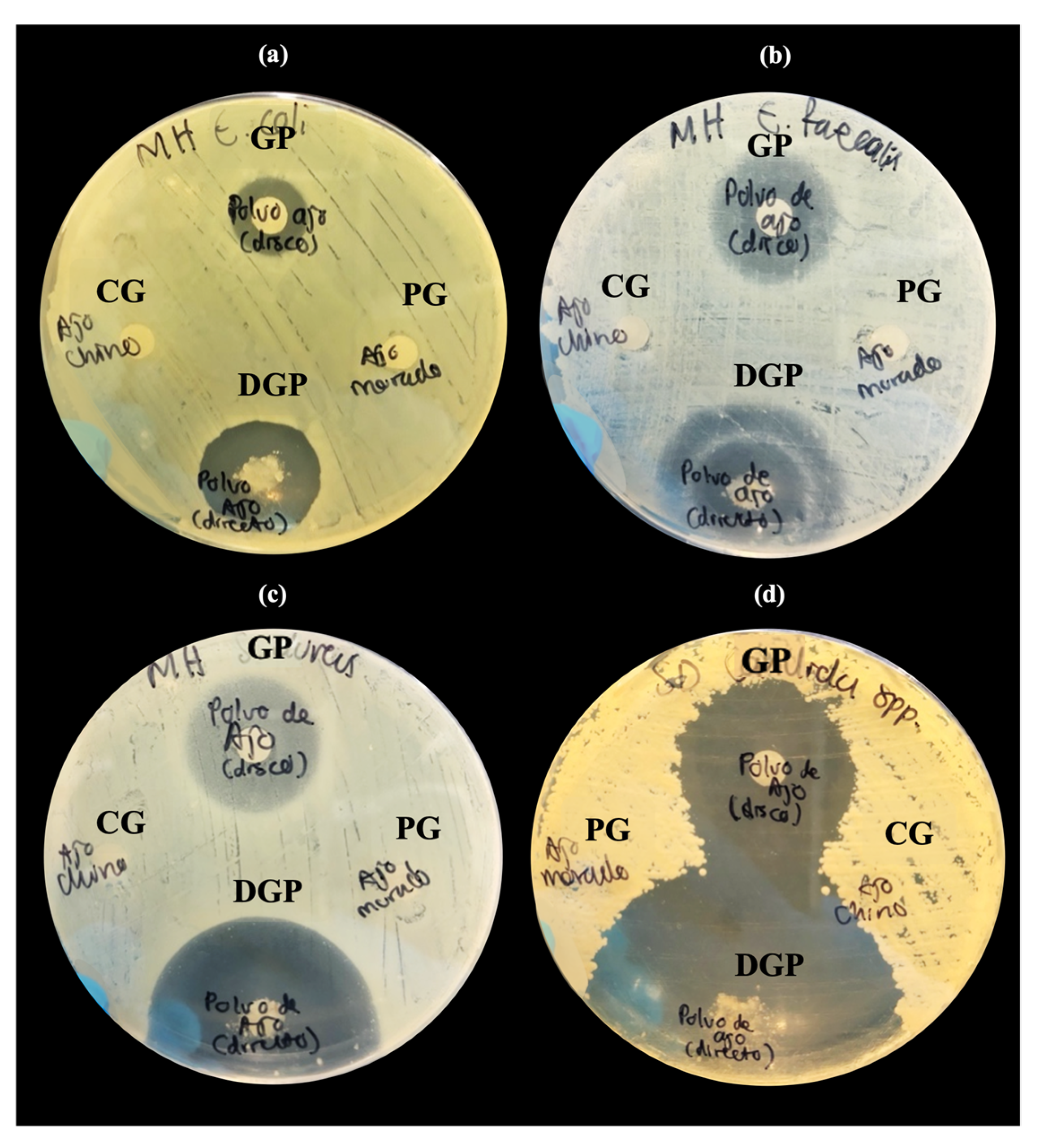
3.2.1. Inhibition Tests of Natural Extracts by Disk Diffusion
3.2.2. Antimicrobial Activity of CNPs Assessed by Broth Dilution Method
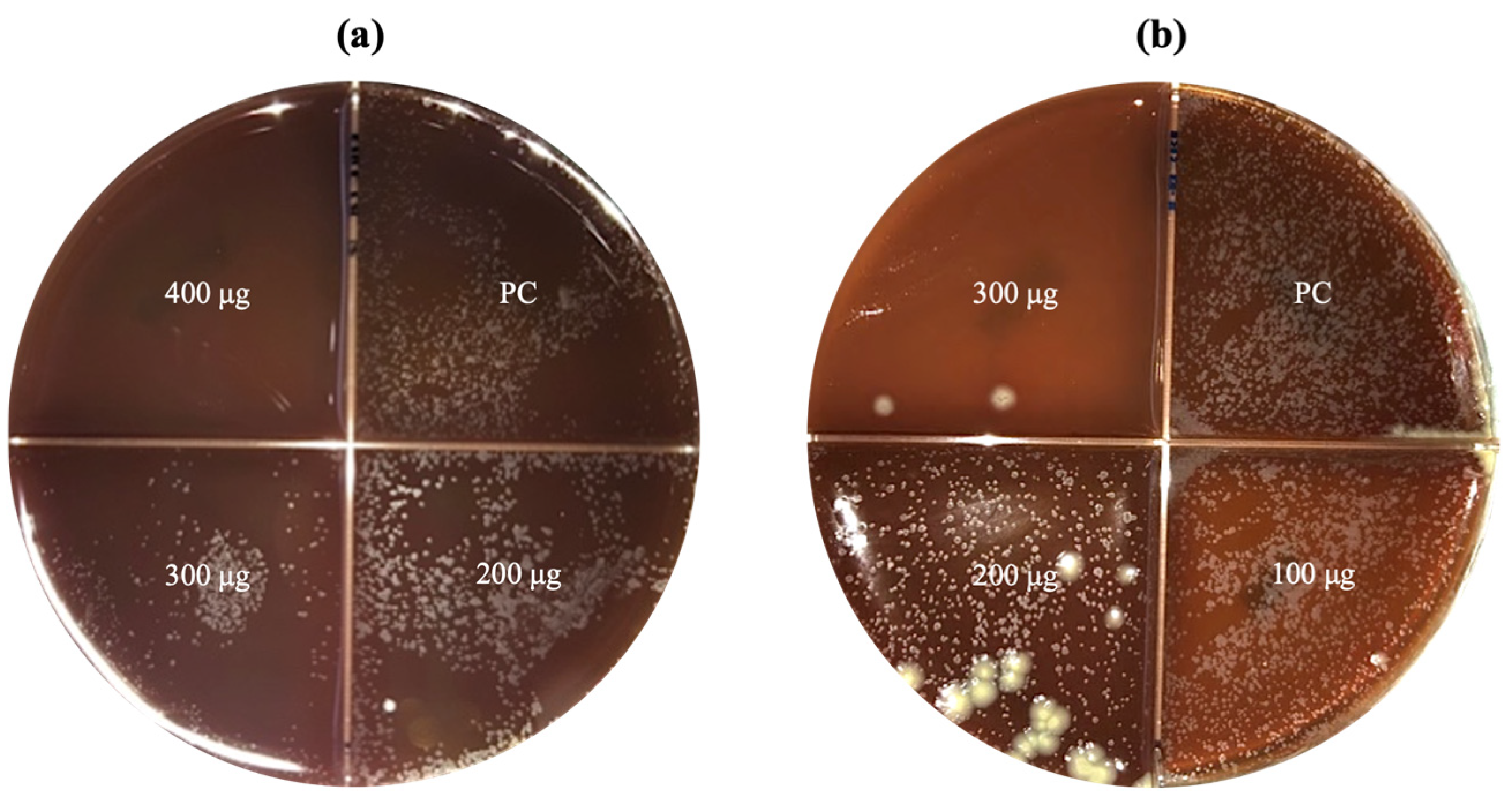
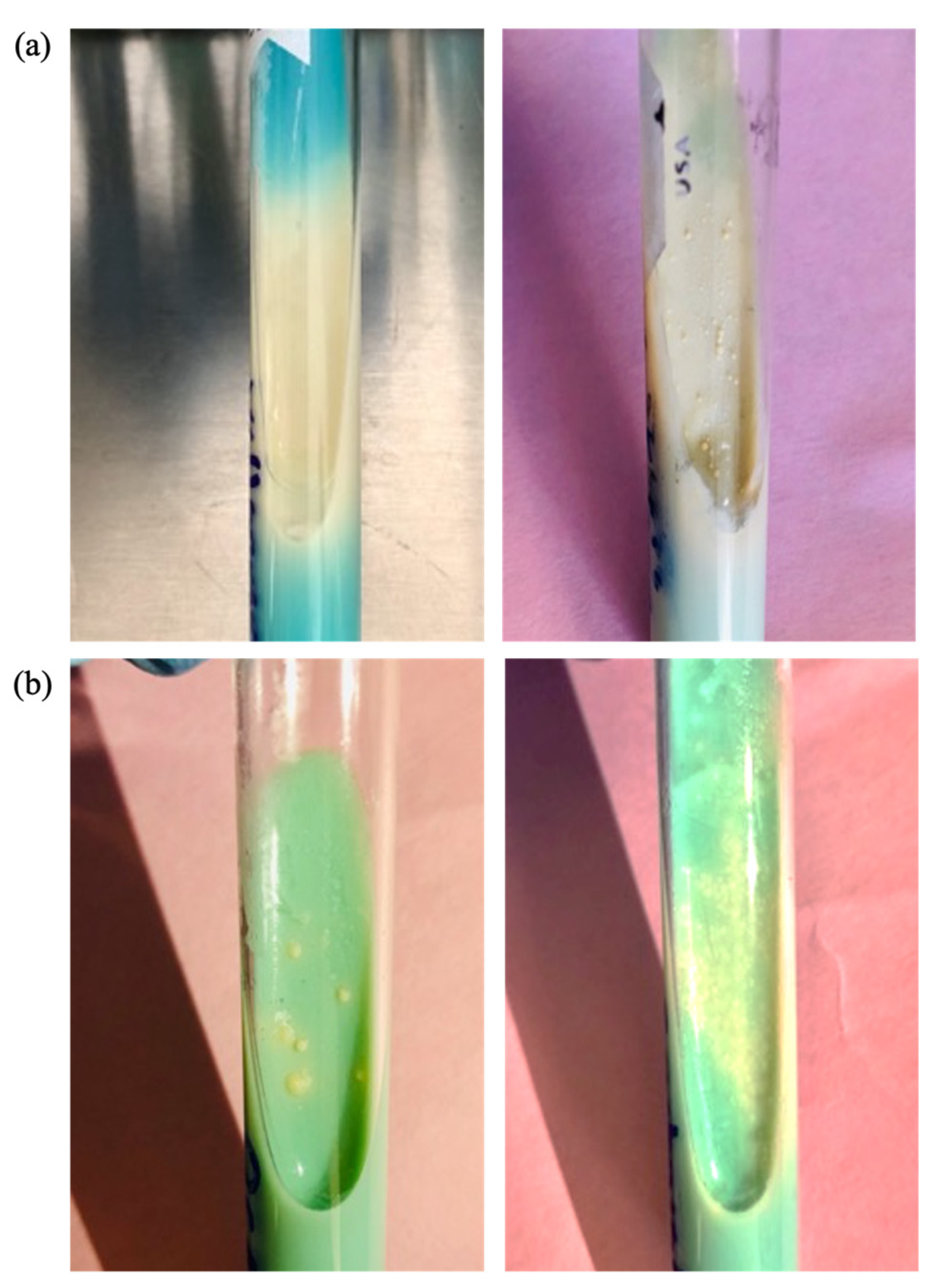
3.2.3. Antimycobacterial Activity of CNPs Assessed by Agar Proportion Method
3.3. Morphological Comparison of Clinical Strain Colonies of MTB
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| BAB | Blood agar- based |
| CNPs | Chitosan nanoparticles |
| CS | Chitosan |
| DLS | Dynamic light scattering |
| LJ | Lowenstein–Jensen |
| MBC | Minimum bactericidal concentration |
| MDR-TB | Multidrug-resistant tuberculosis |
| MHA | Mueller–Hinton agar |
| MIC | Minimum inhibitory concentration |
| MTB | Mycobacterium tuberculosis |
| MWCO | Molecular weight cut-off |
| NaCl | Sodium chloride |
| NaOH | Sodium hydroxide |
| PDI | Polydispersity index |
| SEM | Scanning electron microscopy |
| TB | Tuberculosis |
| TMD | Glycolipid trehalose 6,6-dimycolate |
| TPP | Sodium pentabasic tripolyphosphate |
| XDR-TB | Extremely resistant tuberculosis |
References
- Fitzpatrick, M.C.; Bauch, C.T.; Townsend, J.P.; Galvani, A.P. Modelling Microbial Infection to Address Global Health Challenges. Nat. Microbiol. 2019, 4, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, R.; Hegde, S.; Manaktala, N. Chitosan Nanoparticle Applications in Dentistry: A Sustainable Biopolymer. Front. Chem. 2024, 12, 1362482. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial Food Packaging Based on Sustainable Bio-Based Materials for Reducing Foodborne Pathogens: A Review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Du, W.L.; Niu, S.S.; Xu, Y.L.; Xu, Z.R.; Fan, C.L. Antibacterial Activity of Chitosan Tripolyphosphate Nanoparticles Loaded with Various Metal Ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Liu, H. Preparation, Characterization and Antimicrobial Activity of Chitosan-Zn Complex. Carbohydr. Polym. 2004, 56, 21–26. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The Synthesis of Chitosan-Based Silver Nanoparticles and Their Antibacterial Activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef]
- de Paz, L.E.C.; Resin, A.; Howard, K.A.; Sutherland, D.S.; Wejse, P.L. Antimicrobial Effect of Chitosan Nanoparticles on Streptococcus Mutans Biofilms. Appl. Environ. Microbiol. 2011, 77, 3892–3895. [Google Scholar] [CrossRef]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida Albicans, Candida Tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Palma-Guerrero, J.; Lopez-Jimenez, J.A.; Pérez-Berná, A.J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Villalaín, J.; Read, N.D.; Lopez-Llorca, L.V. Membrane Fluidity Determines Sensitivity of Filamentous Fungi to Chitosan. Mol. Microbiol. 2010, 75, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Rath, G.; Goyal, A.K. Inhalable Chitosan Nanoparticles as Antitubercular Drug Carriers for an Effective Treatment of Tuberculosis. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scolari, I.R.; Páez, P.L.; Sánchez-Borzone, M.E.; Granero, G.E. Promising Chitosan-Coated Alginate-Tween 80 Nanoparticles as Rifampicin Coadministered Ascorbic Acid Delivery Carrier Against Mycobacterium Tuberculosis. Aaps Pharmscitech 2019, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Wardani, G.; Mahmiah; Sudjarwo, S.A. In Vitro Antibacterial Activity of Chitosan Nanoparticles against Mycobacterium Tuberculosis. Pharmacogn. J. 2018, 10, 162–166. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, R.S. Historical Perspective on the Use of Garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef]
- Goncagul, G.; Ayaz, E. Antimicrobial Effect of Garlic (Allium sativum) and Traditional Medicine. J. Anim. Vet. Adv. 2010, 9, 1–4. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Gebreyohannes, M. Medicinal Values of Garlic: A Review. Int. J. 2013, 5, 401–408. [Google Scholar] [CrossRef]
- Viswanathan, V.; Phadatare, A.; Mukne, A. Antimycobacterial and Antibacterial Activity of Allium sativum Bulbs. Indian J. Pharm. Sci. 2014, 76, 256. [Google Scholar] [PubMed]
- Nair, S.S.; Gaikwad, S.S.; Kulkarni, S.P.; Mukne, A.P. Allium sativum Constituents Exhibit Anti-Tubercular Activity In Vitro and in RAW 264.7 Mouse Macrophage Cells Infected with Mycobacterium Tuberculosis H37Rv. Pharmacogn. Mag. 2017, 13, S209–S215. [Google Scholar] [CrossRef]
- World Health Organization. Technical Manual for Drug Susceptibility Testing of Medicines Used in the Treatment of Tuberculosis. 2018. Available online: https://iris.who.int/handle/10665/275469 (accessed on 21 July 2024).
- Coban, A.Y. Blood Agar Validation for Susceptibility Testing of Isoniazid, Rifampicin, Ethambutol, and Streptomycin to Mycobacterium Tuberculosis Isolates. PLoS ONE 2013, 8, e55370. [Google Scholar] [CrossRef]
- Coban, A.Y.; Bilgin, K.; Uzun, M.; Akgunes, A.; Yusof, A.; Durupinar, B. Comparative Study for Determination of Mycobacterium Tuberculosis Susceptibility to First- and Second-Line Antituberculosis Drugs by the Etest Using 7H11, Blood, and Chocolate Agar. J. Clin. Microbiol. 2008, 46, 4095. [Google Scholar] [CrossRef] [PubMed]
- Satana, D.; Coban, A.Y.; Uzun, M. Testing Susceptibility of Multidrug-Resistant Mycobacterium Tuberculosis to Second-Line Drugs by Use of Blood Agar. J. Clin. Microbiol. 2010, 48, 4291–4293. [Google Scholar] [CrossRef]
- Yildiz, C.; Ulger, M.; Aslan, G. Isoniazid Susceptibilities of Mycobacterium Tuberculosis on Blood Agar. Saudi Med. J. 2007, 28, 975. [Google Scholar] [PubMed]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of Chitosan/Tripolyphosphate Nanoparticles with Highly Tunable Size and Low Polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef]
- Satti, L.; Ikram, A.; Coban, A.Y.; Martin, A. Rapid Direct Testing of Susceptibility of Mycobacterium Tuberculosis to Isoniazid and Rifampin on Nutrient and Blood Agar in Resource-Starved Settings. J. Clin. Microbiol. 2012, 50, 1659. [Google Scholar] [CrossRef]
- Huang, Y.; Lapitsky, Y. Salt-Assisted Mechanistic Analysis of Chitosan/Tripolyphosphate Micro- and Nanogel Formation. Biomacromolecules 2012, 13, 3868–3876. [Google Scholar] [CrossRef]
- Thakur, A. Taranjit Preparation of Chitosan Nanoparticles: A Study of Influencing Factors. AIP Conf. Proc. 2011, 1393, 299–300. [Google Scholar] [CrossRef]
- Phadatare, A.G.; Viswanathan, V.; Mukne, A. Novel Strategies for Optimized Delivery of Select Components of Allium sativum. Pharmacogn. Res. 2014, 6, 334–340. [Google Scholar] [CrossRef]
- Prasetyoputri, A.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A.T. The Eagle Effect and Antibiotic-Induced Persistence: Two Sides of the Same Coin? Trends Microbiol. 2018, 27, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the Mode of Action of Chitosan as an Antibacterial Compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Giovannini, D.; Cappelli, G.; Jiang, L.; Castilletti, C.; Colone, A.; Serafino, A.; Wannenes, F.; Giacò, L.; Quintiliani, G.; Fraziano, M.; et al. A New Mycobacterium Tuberculosis Smooth Colony Reduces Growth inside Human Macrophages and Represses PDIM Operon Gene Expression. Does an Heterogeneous Population Exist in Intracellular Mycobacteria? Microb. Pathog. 2012, 53, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Yang, Z. Comparison of Ambient Air Survival of Mycobacterium Tuberculosis Clinical Strains Associated with Different Epidemiological Phenotypes. Int. J. Mycobacteriol. 2014, 3, 211–213. [Google Scholar] [CrossRef]
- Egorov, A.R.; Kirichuk, A.A.; Rubanik, V.V.; Rubanik, V.V.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan and Its Derivatives: Preparation and Antibacterial Properties. Mater. 2023, 16, 6076. [Google Scholar] [CrossRef]
- Sankar, A.; Ramesh, S.; Rajeshkumar, S.; Arun, N. Anti Microbial Activity of Chitosan Nanoparticles with Chlorhexidine- An In Vitro Study. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 41–48. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Mattei, A.; Cristiano, L.; Mancini, L.; Torge, D.; Varvara, G.; Macchiarelli, G.; Marchetti, E. Morphological and Biological Evaluations of Human Periodontal Ligament Fibroblasts in Contact with Different Bovine Bone Grafts Treated with Low-Temperature Deproteinisation Protocol. Int. J. Mol. Sci. 2022, 23, 5273. [Google Scholar] [CrossRef] [PubMed]
- Loo, H.L.; Goh, B.H.; Lee, L.H.; Chuah, L.H. Application of Chitosan-Based Nanoparticles in Skin Wound Healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Thandapani, G.; Supriya Prasad, P.; Sudha, P.N.; Sukumaran, A. Size Optimization and in Vitro Biocompatibility Studies of Chitosan Nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Bulbul, E.; Kasikci, E. The Toxic Effect Ways of Allicin on Different Cell Lines. J. Neurobehav. Sci. 2023, 10, 49. [Google Scholar] [CrossRef]
- Dutta, A.; Dahiya, A.; Prakash, A.; Agrawala, P.K. Acute Toxicity of Diallyl Sulfide Derived from Allium sativum (Garlic) in Mice and Its Possible Mechanisms. Phytomed. Plus 2021, 1, 100084. [Google Scholar] [CrossRef]
- Fowotade, A.; Fowotade, A.; Enaibe, B.; Avwioro, G. Evaluating Toxicity Profile of Garlic (Allium sativum) on the Liver, Kidney and Heart Using Wistar Rat Model. Int. J. Trop. Dis. Health 2017, 26, 1–12. [Google Scholar] [CrossRef]
- Kanabalan, R.D.; Lee, L.J.; Lee, T.Y.; Chong, P.P.; Hassan, L.; Ismail, R.; Chin, V.K. Human Tuberculosis and Mycobacterium Tuberculosis Complex: A Review on Genetic Diversity, Pathogenesis and Omics Approaches in Host Biomarkers Discovery. Microbiol. Res. 2021, 246, 126674. [Google Scholar] [CrossRef]
| Organism | Diameter of Zone of Inhibition (mm) | ||
|---|---|---|---|
| Allium sativum (300 μg) | Allium neapolitanum (300 μg) | Allium sphaerocephalon (300 μg) | |
| E. coli ATCC 25922 | 8 | 5 | 0 |
| E. faecalis ATCC 29212 | 8 | 5 | 0 |
| S. aureus ATCC 25923 | 12 | 9 | 0 |
| C. albicans ATCC 14053 | 10 | 7 | 0 |
| Organism | Diameter of Zone of Inhibition (mm) | |
|---|---|---|
| Allium sativum (100 μg) | Allium sativum (Direct Contact Testing of Powder) | |
| E. coli ATCC 25922 | 9 | 13 |
| E. faecalis ATCC 29212 | 12 | 14 |
| S. aureus ATCC 25923 | 12 | 21 |
| C. albicans ATCC 14053 | 15 | 25 |
| CNPs | Size (nm) | MIC and MBC (μg/mL) of Nanoparticles against Organisms | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | E. faecalis | S. aureus | C. albicans | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| CNP100 | 134.6 | 0.25 | 0.5 | 0.5 | 1 | 0.25 | 0.5 | 2 | - |
| CNP600 | 164.6 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | - |
| CNP1200 | 216.2 | 1 | 1 | 0.125 | 0.25 | 2 | 2 | 0.25 | 0.5 |
| CNP1400 | 280.0 | 0.125 | 0.25 | 0.125 | 0.5 | 2 | 2 | - | - |
| CNPs | Size (nm) | Nanoparticle Concentration (μg) | |||
|---|---|---|---|---|---|
| 100 | 200 | 300 | 400 | ||
| CNP100 | 116.6 | R | R | S | NT |
| CNP600 | 144.3 | NT | R | R | R |
| CNP1200 | 279.1 | R | R | R | NT |
| CNP1400 | 364.4 | NT | R | R | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivas-Flores, J.; Chávez-Méndez, J.R.; Castillo-Martínez, N.A.; Sánchez-Pérez, H.J.; Serrano-Medina, A.; Cornejo-Bravo, J.M. Antimicrobial Effect of Chitosan Nanoparticles and Allium Species on Mycobacterium tuberculosis and Several Other Microorganisms. Microorganisms 2024, 12, 1605. https://doi.org/10.3390/microorganisms12081605
Olivas-Flores J, Chávez-Méndez JR, Castillo-Martínez NA, Sánchez-Pérez HJ, Serrano-Medina A, Cornejo-Bravo JM. Antimicrobial Effect of Chitosan Nanoparticles and Allium Species on Mycobacterium tuberculosis and Several Other Microorganisms. Microorganisms. 2024; 12(8):1605. https://doi.org/10.3390/microorganisms12081605
Chicago/Turabian StyleOlivas-Flores, Jocelyn, José Román Chávez-Méndez, Nydia Alejandra Castillo-Martínez, Héctor Javier Sánchez-Pérez, Aracely Serrano-Medina, and José Manuel Cornejo-Bravo. 2024. "Antimicrobial Effect of Chitosan Nanoparticles and Allium Species on Mycobacterium tuberculosis and Several Other Microorganisms" Microorganisms 12, no. 8: 1605. https://doi.org/10.3390/microorganisms12081605





