The Effects of Aspirin Intervention on Inflammation-Associated Lingual Bacteria: A Pilot Study from a Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Parent Study Design
2.2. Data Collection
2.3. Sample Collection
2.4. DNA Extraction
2.5. DNA Amplification and Sequencing
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
2.8. Primary Analyses
2.9. Differential Abundance Analysis
3. Results
3.1. Parent Study Cohort
3.2. Analyses of α- and β-Diversity
3.3. Analysis of a Priori Selected Taxa
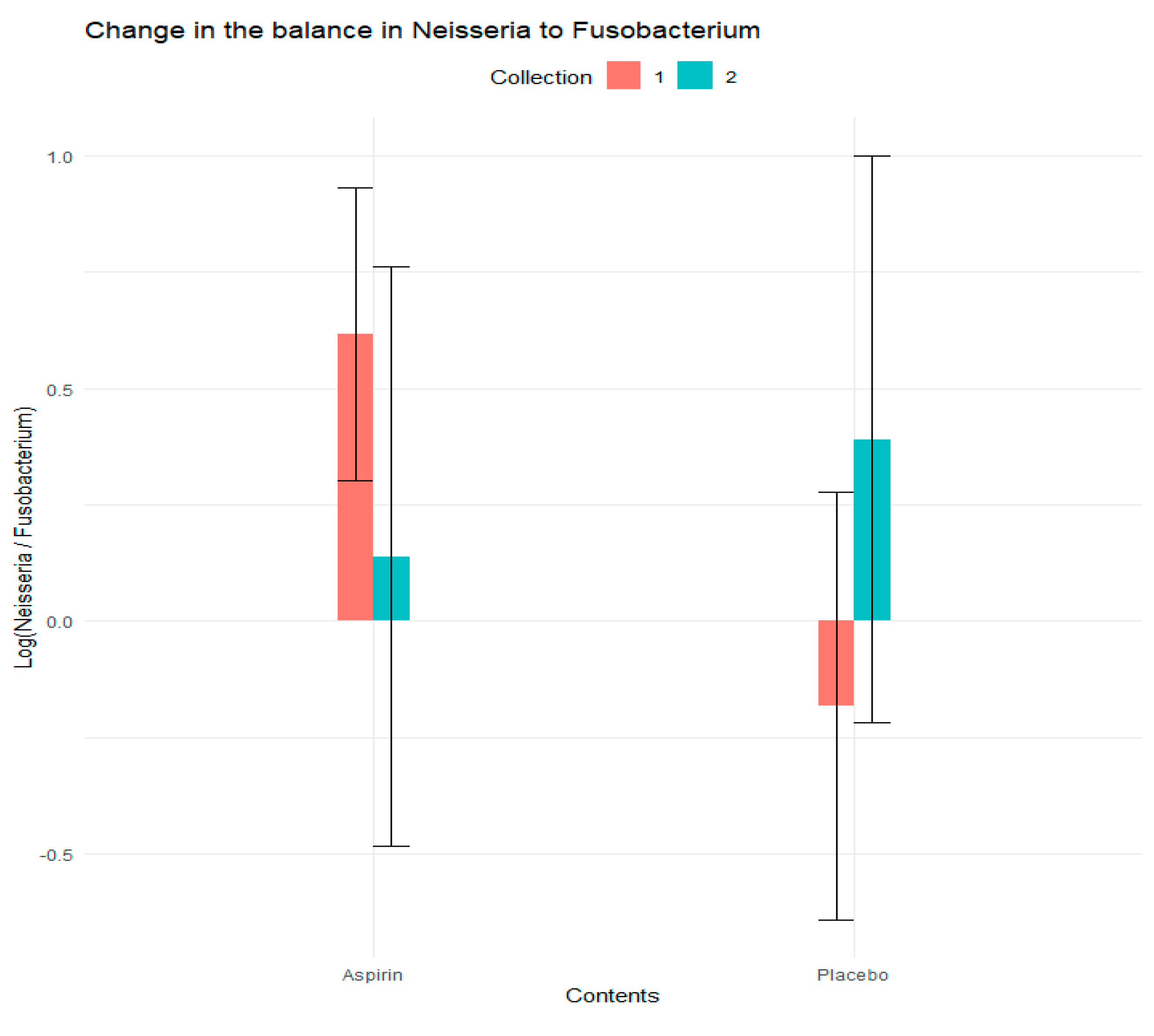
3.4. Analysis of Log Ratios
3.5. Differential Analysis Based on DESeq2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2018; pp. 1–101, Based on November 2016 SEER Data Submission, Posted to the SEER Web Site, April 2017. Available online: http://seer.cancer.gov/csr/1975_2014/ (accessed on 1 July 2020).
- Torres, P.J.; Fletcher, E.M.; Gibbons, S.M.; Bouvet, M.; Doran, K.S.; Kelley, S.T. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ 2015, 3, e1373. [Google Scholar] [CrossRef] [PubMed]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Vogtmann, E.; Goedert, J.J. Epidemiologic studies of the human microbiome and cancer. Br. J. Cancer 2016, 114, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Al-hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Vasquez, A.A.; Moyerbrailean, G.; Land, S.; Sun, J.; Lin, H.-S.; Ram, J.L. Oral microbiome and history of smoking and colorectal cancer. J. Epidemiol. Res. 2016, 2, 92. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Chen, C.Y.; Hayes, R.B. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control 2012, 23, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018, 67, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Nagy, K.N.; Sonkodi, I.; Szöke, I.; Nagy, E.; Newman, H.N. The microflora associated with human oral carcinomas. Oral. Oncol. 1998, 34, 304–308. [Google Scholar] [CrossRef]
- Mason, M.R.; Preshaw, P.M.; Nagaraja, H.N.; Dabdoub, S.M.; Rahman, A.; Kumar, P.S. The subgingival microbiome of clinically healthy current and never smokers. ISME J. 2015, 9, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L.; Garrett, W.S. Microbes, microbiota, and colon cancer. Cell Host Microbe 2014, 15, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Sarafidou, K.; Alexakou, E.; Talioti, E.; Bakopoulou, A.; Anastassiadou, V. The oral microbiome in older adults—A state-of-the-art review. Arch. Gerontol. Geriatr. Plus 2024, 1, 100061. [Google Scholar] [CrossRef]
- Marotz, C.; Molinsky, R.; Martino, C.; Bohn, B.; Roy, S.; Rosenbaum, M.; Desvarieux, M.; Yuzefpolskaya, M.; Paster, B.J.; Jacobs, D.R.; et al. Early microbial markers of periodontal and cardiometabolic diseases in ORIGINS. NPJ Biofilms Microbiomes 2022, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.A.; Saunders, N.J. The majority of genes in the pathogenic Neisseria species are present in non-pathogenic Neisseria lactamica, including those designated as “virulence genes”. BMC Genom. 2006, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Tramacere, I.; Negri, E.; Pelucchi, C.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; La Vecchia, C.; Boffetta, P. A meta-analysis on alcohol drinking and gastric cancer risk. Ann. Oncol. 2012, 23, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Moritani, K.; Takeshita, T.; Shibata, Y.; Ninomiya, T.; Kiyohara, Y.; Yamashita, Y. Acetaldehyde production by major oral microbes. Oral Dis. 2015, 21, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Persaud, V.; Vanegas, S.; Gautier, G.; Esiobu, N. Analysis of the Human Oral Microbiome of Smokers and Non-Smokers Using PCR-RFLP and Ribotyping. Adv. Microbiol. 2014, 4, 681–691. [Google Scholar] [CrossRef]
- Brook, I.; Gober, A.E. Effect of smoking cessation on the microbial flora. Arch. Otolaryngol. Head Neck Surg. 2007, 133, 135–138. [Google Scholar] [CrossRef]
- Freedman, N.D.; Abnet, C.C.; Leitzmann, M.F.; Mouw, T.; Subar, A.F.; Hollenbeck, A.R.; Schatzkin, A. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am. J. Epidemiol. 2007, 165, 1424–1433. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Whitney, A.K.; Weir, T.L. Cancer-promoting effects of microbial dysbiosis. Curr. Oncol. Rep. 2014, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.J.; Baxter, N.T.; Schloss, P.D. Metabolic and Community Synergy of Oral Bacteria in Colorectal Cancer. MSphere 2016, 1, e00102-16. [Google Scholar] [CrossRef] [PubMed]
- Coppenhagen-Glazer, S.; Sol, A.; Abed, J.; Naor, R.; Zhang, X.; Han, Y.W.; Bachrach, G. Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth. Infect. Immun. 2015, 83, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Eun, C.S.; Lee, A.R.; Park, C.H.; Han, D.S. Fusobacterium isolates recovered from colonic biopsies of inflammatory bowel disease patients in Korea. Ann. Lab. Med. 2016, 36, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 2017, 23, 4548–4558. [Google Scholar] [CrossRef] [PubMed]
- Kummen, M.; Holm, K.; Anmarkrud, J.A.; Nygård, S.; Vesterhus, M.; Høivik, M.L.; Trøseid, M.; Marschall, H.-U.; Schrumpf, E.; Moum, B.; et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 2017, 66, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.A.; Bertha, M.; Hofmekler, T.; Chopra, P.; Vatanen, T.; Srivatsa, A.; Prince, J.; Kumar, A.; Sauer, C.; Zwick, M.E.; et al. Dysbiosis, inflammation, and response to treatment: A longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016, 8, 75. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Cao, Y.; Nishihara, R.; Wu, K.; Wang, M.; Ogino, S.; Willett, W.C.; Spiegelman, D.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016, 2, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T. Aspirin USPSTF-What about Cancer? JAMA Oncol. 2022, 8, 1392–1394. [Google Scholar] [CrossRef] [PubMed]
- Dehmer, S.P.; O’Keefe, L.R.; Evans, C.V.; Guirguis-Blake, J.M.; Perdue, L.A.; MacIosek, M.V. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Updated Modeling Study for the US Preventive Services Task Force. JAMA 2022, 327, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Henley, S.J.; Patrono, C. Nonsteroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. JNCI J. Natl. Cancer Inst. 2002, 94, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, Cellular, and Molecular Biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Antimicrobial effects of antipyretics. Antimicrob. Agents Chemother. 2017, 61, e02268-16. [Google Scholar] [CrossRef]
- Wang, Y.; Boland, C.R.; Goel, A.; Wodarz, D.; Komarova, N.L. Aspirin’s effect on kinetic parameters of cells contributes to its role in reducing incidence of advanced colorectal adenomas, shown by a multiscale computational study. eLife 2022, 11, e71953. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Nakatsu, G.; Comeau, C.A.G.; Drew, D.A.; Glickman, J.N.; Schoen, R.E.; Chan, A.T.; Garrett, W.S. Aspirin Modulation of the Colorectal Cancer-Associated Microbe Fusobacterium nucleatum. mBio 2021, 12, e00547-21. [Google Scholar] [CrossRef] [PubMed]
- Prizment, A.E.; Staley, C.; Onyeaghala, G.C.; Vivek, S.; Thyagarajan, B.; Straka, R.J.; Demmer, R.T.; Knights, D.; Meyer, K.A.; Shaukat, A.; et al. Randomised clinical study: Oral aspirin 325 mg daily vs placebo alters gut microbial composition and bacterial taxa associated with colorectal cancer risk. Aliment. Pharmacol. Ther. 2020, 52, 976–987. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Mohebati, A.; Milne, G.L.; Zhou, X.K.; Duffield-Lillico, A.J.; Boyle, J.O.; Knutson, A.; Bosworth, B.P.; Kingsley, P.J.; Marnett, L.J.; Brown, P.H.; et al. Effect of zileuton and celecoxib on urinary LTE4 and PGE-M levels in smokers. Cancer Prev. Res. 2013, 6, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Schuck, M.M.; Magicheva-Gupta, M.V.; Stewart, K.O.; Gilpin, K.K.; Miller, P.; Parziale, M.P.; Pond, E.N.; Takacsi-Nagy, O.; Zerjav, D.C.; et al. Effect of Low-dose and Standard-dose Aspirin on PGE2 Biosynthesis Among Individuals with Colorectal Adenomas: A Randomized Clinical Trial. Cancer Prev. Res. 2020, 13, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Walker, A.W.; Berry, S.H.; Duncan, S.H.; Farquarson, F.M.; Louis, P.; Thomson, J.M. The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS ONE 2014, 9, e88982. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.J.; Rutherford, K.M.; Berriman, M.; Rajandream, M.-A.; Barrell, B.G.; Parkhill, J. Qubit dsDNA HS Assay Kit. Bioinformatics 2016, 21, 3422–3423. [Google Scholar] [CrossRef] [PubMed]
- Brauge, T.; Faille, C.; Inglebert, G.; Dubois, T.; Morieux, P.; Slomianny, C.; Midelet-Bourdin, G. Comparative evaluation of DNA extraction methods for amplification by qPCR of superficial vs intracellular DNA from Bacillus spores. Int. J. Food Microbiol. 2018, 266, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.D.; Morrow, J.B. DNA extract characterization process for microbial detection methods development and validation. BMC Res. Notes 2012, 5, 668. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Vrbanac, A.; Taylor, B.C.; Aksenov, A.; Callewaert, C.; Debelius, J.; Gonzalez, A.; Kosciolek, T.; McCall, L.-I.; McDonald, D.; et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 2018, 16, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, R.; Sugawara, H.; Shumway, M. The sequence read archive. Nucleic Acids Res. 2011, 39, D19–D21. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An effective distance metric for microbial community comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef]
- Pragman, A.; Issacson, R.; Wendt, C.; Reilly, C. A Method for Determining Taxonomical Contributions to Group Differences in Microbiomic Investigations. J. Comput. Biol. 2015, 22, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The gut microbiome modulates colon tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.T.; Sanders, J.; Quinn, R.A.; McDonald, D.; Gonzalez, A.; Vázquez-Baeza, Y.; Navas-Molina, J.A.; Song, S.J.; Metcalf, J.L.; Hyde, E.R.; et al. Balance Trees Reveal Microbial Niche Differentiation. MSystems 2017, 2, e00162-16. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; DeSantis, T.Z.; Weinmaier, T.; McMurdie, P.J.; Cope, J.L.; Altrichter, A.; Yamal, J.-M.; Hollister, E.B. Leveraging sequence-based faecal microbial community survey data to identify a composite biomarker for colorectal cancer. Gut 2018, 67, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Rogers, M.A.M.; Ruffin, M.T.; Schloss, P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014, 7, 1112–1121. [Google Scholar] [CrossRef]
- Dejea, C.; Wick, E.; Sears, C.L. Bacterial oncogenesis in the colon. Future Microbiol. 2013, 8, 445–460. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2010, 29, 781–788. [Google Scholar] [CrossRef]
- Sun, J.; Kato, I. Gut microbiota, inflammation and colorectal cancer. Genes Dis 2016, 3, 130–143. [Google Scholar] [CrossRef]
- Demmer, R.T.; Breskin, A.; Rosenbaum, M.; Zuk, A.; LeDuc, C.; Leibel, R.; Paster, B.; Desvarieux, M.; Jacobs, D.R., Jr.; Papapanou, P.N. The Subgingival Microbiome, Systemic Inflammation and Insulin Resistance: The Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS). J. Clin. Periodontol. 2016, 44, 255–265. [Google Scholar] [CrossRef]
- Demmer, R.T.; Squillaro, A.; Papapanou, P.N.; Rosenbaum, M.; Friedewald, W.T.; Jacobs, D.R.; Desvarieux, M. Periodontal infection, systemic inflammation, and insulin resistance: Results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care 2012, 35, 2235–2242. [Google Scholar] [CrossRef]
- Winning, L.; Patterson, C.C.; Cullen, K.M.; Stevenson, K.A.; Lundy, F.T.; Kee, F.; Linden, G.J. The association between subgingival periodontal pathogens and systemic inflammation. J. Clin. Periodontol. 2015, 42, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Zeng, X.T.; Deng, A.P.; Li, C.; Xia, L.Y.; Niu, Y.M.; Leng, W.D. Periodontal Disease and Risk of Head and Neck Cancer: A Meta-Analysis of Observational Studies. PLoS ONE 2013, 8, e79017. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Liu, Y.; Meyer, M.; Giovannucci, E.; Joshipura, K. Periodontal disease, tooth loss, and cancer risk in male health professionals: A prospective cohort study. Lancet Oncol. 2008, 9, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cai, Q.; Shu, X.; Steinwandel, M.D.; Blot, W.J.; Zheng, W.; Long, J. Prospective study of oral microbiome and colorectal cancer risk in low-income and African American populations. Int. J. Cancer 2019, 144, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 inhibitors in cancer: A review of oncology and medicinal chemistry literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Yamanaka, W.; Takeshita, T.; Shibata, Y.; Matsuo, K.; Eshima, N.; Yokoyama, T.; Yamashita, Y. Compositional stability of a salivary bacterial population against supragingival microbiota shift following periodontal therapy. PLoS ONE 2012, 7, e42806. [Google Scholar] [CrossRef]
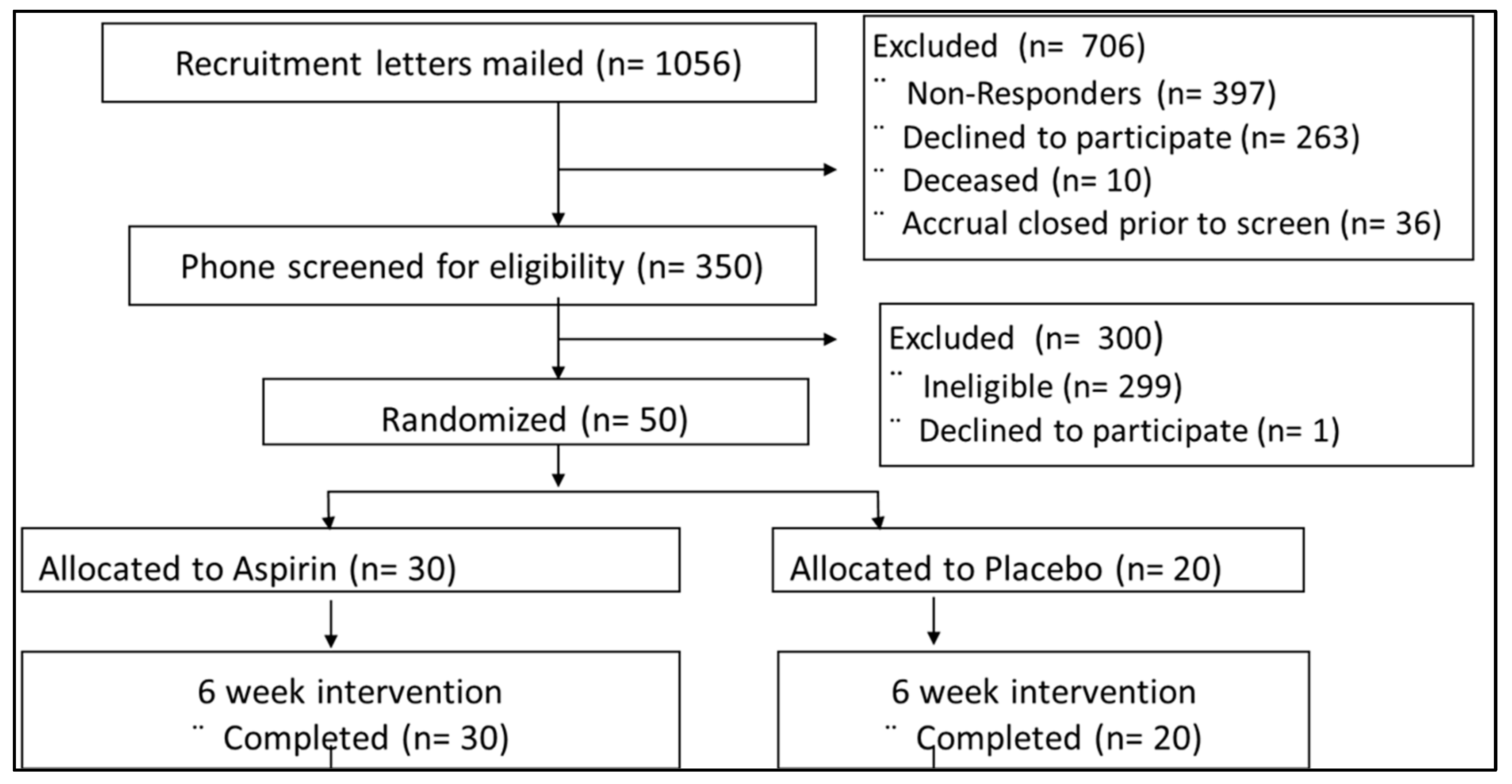

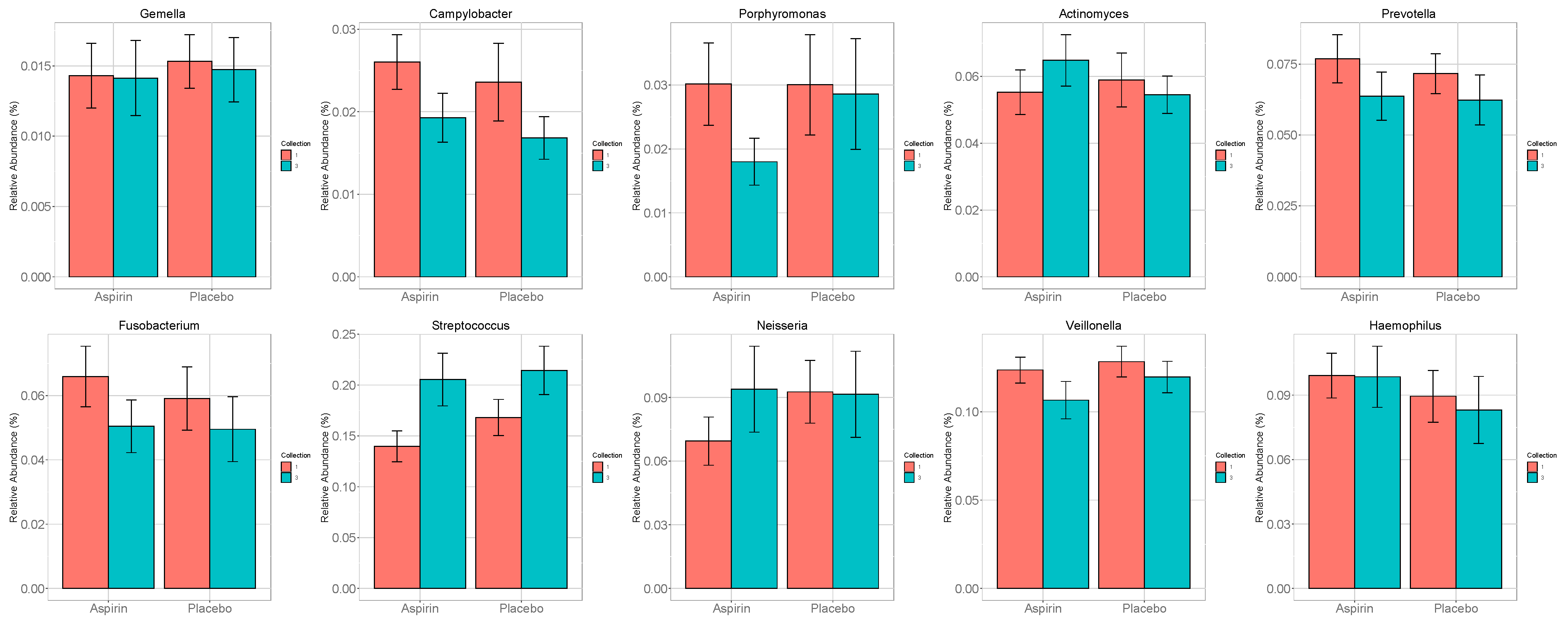
| Characteristics | Aspirin Group | Placebo Group | p-Value |
|---|---|---|---|
| n (%) | 30 (60.0%) | 20 (40.0%) | |
| Age, Mean (SD) y | 62.2 (5.1) | 61.2 (5.2) | 0.56 |
| Sex, Female% | 23 (76.7%) | 9 (45.0%) | 0.02 |
| BMI, Mean (SD), kg/m2 | 27.3 (4.3) | 28.2 (4.8) | 0.50 |
| Baseline urinary PGE-M (adjusted for creatinine, mg/dL) | 11.82 (13.59) | 12.94 (7.39) | 0.73 |
| Change in urinary PGE-M at 6 weeks (adjusted for creatinine, mg/dL) | −5.31 (0.96) | 0.86 (1.18) | <0.01 |
| Balance Ratio | Predictor | Average Change in Balance in the Aspiring Group | Average Change in Balance in the Placebo Group | Estimate | Std. Error | Z Value | Pr (>|z|) |
|---|---|---|---|---|---|---|---|
| Neisseria to Fusobacterium Ratio | Placebo (vs. Aspirin) | −1.060 | 0.508 | −0.80 | 0.36 | −2.20 | 0.03 |
| Collection 2 (vs. Collection 1) | −0.48 | 0.32 | −1.47 | 0.14 | |||
| Intervention X Collection Interaction | 1.05 | 0.51 | 2.05 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyeaghala, G.C.; Sharma, S.; Oyenuga, M.; Staley, C.M.; Milne, G.L.; Demmer, R.T.; Shaukat, A.; Thyagarajan, B.; Straka, R.J.; Church, T.R.; et al. The Effects of Aspirin Intervention on Inflammation-Associated Lingual Bacteria: A Pilot Study from a Randomized Clinical Trial. Microorganisms 2024, 12, 1609. https://doi.org/10.3390/microorganisms12081609
Onyeaghala GC, Sharma S, Oyenuga M, Staley CM, Milne GL, Demmer RT, Shaukat A, Thyagarajan B, Straka RJ, Church TR, et al. The Effects of Aspirin Intervention on Inflammation-Associated Lingual Bacteria: A Pilot Study from a Randomized Clinical Trial. Microorganisms. 2024; 12(8):1609. https://doi.org/10.3390/microorganisms12081609
Chicago/Turabian StyleOnyeaghala, Guillaume C., Shweta Sharma, Mosunmoluwa Oyenuga, Christopher M. Staley, Ginger L. Milne, Ryan T. Demmer, Aasma Shaukat, Bharat Thyagarajan, Robert J. Straka, Timothy R. Church, and et al. 2024. "The Effects of Aspirin Intervention on Inflammation-Associated Lingual Bacteria: A Pilot Study from a Randomized Clinical Trial" Microorganisms 12, no. 8: 1609. https://doi.org/10.3390/microorganisms12081609






