Clostridium perfringens in the Intestine: Innocent Bystander or Serious Threat?
Abstract
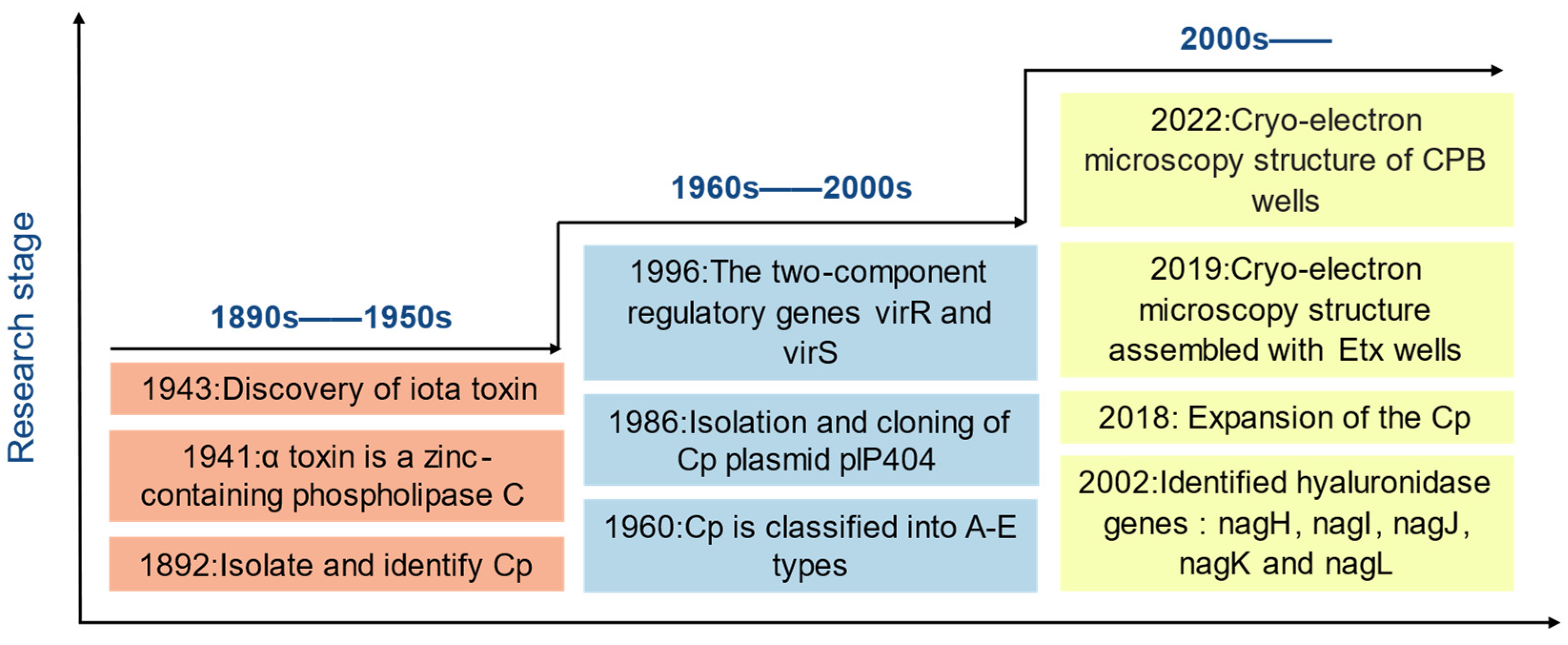
1. Introduction
1.1. C. perfringens Threatens Global Biological Public Safety
1.1.1. Humans
1.1.2. Animal
1.1.3. Economy
2. Virulence Mechanisms
2.1. Enzymes
2.2. Toxin
2.2.1. Alpha Toxin
2.2.2. Beta Toxin
2.2.3. Epsilon Toxin
2.2.4. Iota Toxin
2.2.5. Enterotoxins and Necrotizing Enterocolitis B-Like Toxins
3. Prevention and Treatment of C. perfringens
3.1. Vaccines
3.2. Antibiotics
3.3. Probiotics
3.4. Alternative Treatment
4. Conclusions and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orrell, K.E.; Melnyk, R.A. Large Clostridial Toxins: Mechanisms and Roles in Disease. Microbiol. Mol. Biol. Rev. 2021, 85. [Google Scholar] [CrossRef] [PubMed]
- Bergogne-Bérézin, E. Treatment and Prevention of Antibiotic Associated Diarrhea. Int. J. Antimicrob. Agents 2000, 16, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, S.; Hutton, M.L.; Lyras, D. Involvement of Bacteria Other Than Clostridium difficile in Antibiotic-Associated Diarrhoea. Trends Microbiol. 2016, 24, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liang, T.; Jin, Q.; Shen, C.; Zhang, Y.; Jing, P. Chinese Yam (Dioscorea opposita Thunb.) Alleviates Antibiotic-Associated Diarrhea, Modifies Intestinal Microbiota, and Increases the Level of Short-Chain Fatty Acids in Mice. Food Res. Int. 2019, 122, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Alimolaei, M.; Afzali, S. Prevalence of Clostridium perfringens Toxinotypes in Antibiotic-Associated Diarrheal (AAD) Patients in Iranian Hospitals; Can Toxinotype D Serve as a Possible Zoonotic Agent for Humans? Acta Trop. 2023, 247, 107002. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Uzal, F.A.; McClane, B.A. Enterotoxic Clostridia: Clostridium perfringens Enteric Diseases. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Finnie, J.W.; Navarro, M.A.; Uzal, F.A. Pathogenesis and Diagnostic Features of Brain and Ophthalmic Damage Produced by Clostridium perfringens Type D Epsilon Toxin. J. Vet. Diagn. Investig. 2020, 32, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Fancher, C.A.; Thames, H.T.; Colvin, M.G.; Zhang, L.; Nuthalapati, N.; Kiess, A.; Dinh, T.T.N.; Sukumaran, A.T. Research Note: Prevalence and Molecular Characteristics of Clostridium perfringens in “No Antibiotics Ever” Broiler Farms. Poult. Sci. 2021, 100, 101414. [Google Scholar] [CrossRef] [PubMed]
- Rumah, K.R.; Linden, J.; Fischetti, V.A.; Vartanian, T. Isolation of Clostridium perfringens Type B in an Individual at First Clinical Presentation of Multiple Sclerosis Provides Clues for Environmental Triggers of the Disease. PLoS ONE 2013, 8, e76359. [Google Scholar] [CrossRef]
- Chen, J.; Ma, M.; Uzal, F.A.; McClane, B.A. Host Cell-Induced Signaling Causes Clostridium perfringens to Upregulate Production of Toxins Important for Intestinal Infections. Gut Microbes 2014, 5, 96–107. [Google Scholar] [CrossRef]
- Wang, Y.-H. Sialidases From Clostridium perfringens and Their Inhibitors. Front. Cell. Infect. Microbiol. 2019, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Bendary, M.M.; Abd El-Hamid, M.I.; El-Tarabili, R.M.; Hefny, A.A.; Algendy, R.M.; Elzohairy, N.A.; Ghoneim, M.M.; Al-Sanea, M.M.; Nahari, M.H.; Moustafa, W.H. Clostridium perfringens Associated with Foodborne Infections of Animal Origins: Insights into Prevalence, Antimicrobial Resistance, Toxin Genes Profiles, and Toxinotypes. Biology 2022, 11, 551. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Z.; Ma, Y.; Yang, Z.; Liu, G.; Fang, J. Effects of Lactobacillus Plantarum and Weissella Viridescens on the Gut Microbiota and Serum Metabolites of Mice with Antibiotic-Associated Diarrhea. Nutrients 2023, 15, 4603. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Barış, E.; Go, D.S.; Lofgren, H.; Osorio-Rodarte, I.; Thierfelder, K. Assessing the Global Poverty Effects of Antimicrobial Resistance. World Dev. 2018, 111, 148–160. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.X.; Zheng, H.R.; Wang, Y.Y.; Bai, L.L.; Du, X.L.; Wu, Y.; Lu, J.X. Molecular Characteristics and Phylogenetic Analysis of Clostridium perfringens from Different Regions in China, from 2013 to 2021. Front. Microbiol. 2023, 14, 1195083. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.; Thomson, L.; Mahmoudzadeh, N.; Khaled, A. Estimating Deaths from Foodborne Disease in the UK for 11 Key Pathogens. BMJ Open Gastroenterol. 2020, 7, e000377. [Google Scholar] [CrossRef] [PubMed]
- Dolan, G.P.; Foster, K.; Lawler, J.; Amar, C.; Swift, C.; Aird, H.; Gorton, R. An Epidemiological Review of Gastrointestinal Outbreaks Associated with Clostridium perfringens, North East of England, 2012–2014. Epidemiol. Infect. 2016, 144, 1386–1393. [Google Scholar] [CrossRef]
- Rajkovic, A.; Jovanovic, J.; Monteiro, S.; Decleer, M.; Andjelkovic, M.; Foubert, A.; Beloglazova, N.; Tsilla, V.; Sas, B.; Madder, A.; et al. Detection of Toxins Involved in Foodborne Diseases Caused by Gram-Positive Bacteria. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1605–1657. [Google Scholar] [CrossRef]
- Thomas, M.; Murray, R. Estimating the Burden of Food-Borne Illness in Canada. Can. Commun. Dis. Rep. 2014, 40, 299–302. [Google Scholar] [CrossRef]
- Camargo, A.; Rámirez, J.D.; Kiu, R.; Hall, L.J.; Muñoz, M. Unveiling the Pathogenic Mechanisms of Clostridium perfringens Toxins and Virulence Factors. Emerg. Microbes Infect. 2024, 13, 2341968. [Google Scholar] [CrossRef] [PubMed]
- Asha, N.J.; Tompkins, D.; Wilcox, M.H. Comparative Analysis of Prevalence, Risk Factors, and Molecular Epidemiology of Antibiotic-Associated Diarrhea Due to Clostridium Difficile, Clostridium perfringens, and Staphylococcus Aureus. J. Clin. Microbiol. 2006, 44, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Dong, W.; Ma, L.; Dong, Y.; Wang, S.; Yuan, Y.; Ma, Q.; Xu, J.; Yan, W.; Nan, J.; et al. Prevalence and Genetic Diversity of Clostridium Perfringen Isolates in Hospitalized Diarrheal Patients from Central Chin. Infect. Drug Resist. 2021, 14, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- Sarasoja, M.; Nilson, B.; Wide, D.; Lindberg, Å.; Torisson, G.; Holm, K. Epidemiology, Aetiology and Clinical Characteristics of Clostridial Bacteraemia: A 6-Year Population-Based Observational Study of 386 Patients. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, M.-L.; Thibodeau, A.; Fravalo, P.; Archambault, M.; Arsenault, J.; Fournaise, S.; Letellier, A.; Quessy, S. Broiler Chicken Carcasses and Their Associated Abattoirs as a Source of Enterotoxigenic Clostridium perfringens: Prevalence and Critical Steps for Contamination. AIMS Microbiol. 2018, 4, 439–454. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, Y.; Liu, Q.; Li, T.; Li, Y.; Guo, K.; Zhang, Y. Tracing Clostridium perfringens Strains from Beef Processing of Slaughter House by Pulsed-Field Gel Electrophoresis, and the Distribution and Toxinotype of Isolates in Shaanxi Province, China. Food Microbiol. 2022, 101, 103887. [Google Scholar] [CrossRef] [PubMed]
- Issimov, A.; Baibatyrov, T.; Tayeva, A.; Kenenbay, S.; Abzhanova, S.; Shambulova, G.; Kuzembayeva, G.; Kozhakhiyeva, M.; Brel-Kisseleva, I.; Safronova, O.; et al. Prevalence of Clostridium perfringens and Detection of Its Toxins in Meat Products in Selected Areas of West Kazakhstan. Agriculture 2022, 12, 1357. [Google Scholar] [CrossRef]
- Jiang, Y.; Pan, Y.; Yin, J. Prevalence, Toxin-Genotype Distribution, and Transmission of Clostridium perfringens from the Breeding and Milking Process of Dairy Farms. Food Microbiol. 2024, 120, 104485. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Labbé, R. Distribution of Enterotoxin- and Epsilon-Positive Clostridium perfringens Spores in U.S. Retail Spices. J. Food Prot. 2018, 81, 394–399. [Google Scholar] [CrossRef]
- Azimirad, M.; Gholami, F.; Yadegar, A.; Knight, D.R.; Shamloei, S.; Aghdaei, H.A.; Zali, M.R. Prevalence and Characterization of Clostridium perfringens Toxinotypes among Patients with Antibiotic-Associated Diarrhea in Iran. Sci. Rep. 2019, 9, 7792. [Google Scholar] [CrossRef]
- Santana, J.A.; Ferreira, A.C.D.A.; Souza, M.D.C.C.D.; Moreira, M.A.S.; Lima, M.C.; Cruz, D.S.G.; Lobato, F.C.F.; Silva, R.O.S. Isolation and Genotyping of Clostridium perfringens from Goats in Minas Gerais, Brazil. Ciênc. Rural 2018, 48, e20180101. [Google Scholar] [CrossRef]
- Hayati, M.; Tahamtan, Y. Toxin Typing of Clostridium perfringens Associated with Enterotoxaemia in Sheep in Fars Province. Arch. Razi Inst. 2021, 76, 691–697. [Google Scholar] [CrossRef]
- Khan, M.U.Z.; Humza, M.; Yang, S.; Alvi, M.A.; Iqbal, M.Z.; Zain-ul-Fatima, H.; Khalid, S.; Munir, T.; Cai, J. Occurrence and Toxicogenetic Profiling of Clostridium perfringens in Buffalo and Cattle: An Update from Pakistan. Toxins 2021, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ning, C.; Ji, C.; Li, Y.; Li, J.; Meng, Q.; Qiao, J.; Wang, L.; Cai, K.; Zhang, J.; et al. Antimicrobial Resistance Profiling and Molecular Typing of Ruminant-Borne Isolates of Clostridium perfringens from Xinjiang, China. J. Glob. Antimicrob. Resist. 2021, 27, 41–45. [Google Scholar] [CrossRef]
- Schoster, A.; Kunz, T.; Lauper, M.; Graubner, C.; Schmitt, S.; Weese, J.S. Prevalence of Clostridium difficile and Clostridium perfringens in Swiss Horses with and without Gastrointestinal Disease and Microbiota Composition in Relation to Clostridium Difficile Shedding. Vet. Microbiol. 2019, 239, 108433. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, L.; Miao, Z.; Huo, Y.; Zhong, Z. Prevalence and characterization of Clostridium perfringens isolated from different chicken farms in China. Anaerobe 2021, 72, 102467. [Google Scholar] [CrossRef]
- Moustafa, S.; Zakaria, I.; Moustafa, A.; AboSakaya, R.; Selim, A. Bacteriological and Serological Investigation of Clostridium perfringens in Lambs. Sci. Rep. 2022, 12, 19715. [Google Scholar] [CrossRef]
- Yadav, J.P.; Kaur, S.; Dhaka, P.; Vijay, D.; Bedi, J.S. Prevalence, Molecular Characterization, and Antimicrobial Resistance Profile of Clostridium perfringens from India: A Scoping Review. Anaerobe 2022, 77, 102639. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Feng, H.; Ma, J.; Wang, B.; Feng, J.; Zhang, H.; Jiang, Y.; Li, R.; Wang, J.; Yang, Z. Prevalence, Toxin-Typing and Antimicrobial Susceptibility of Clostridium perfringens in Sheep with Different Feeding Modes from Gansu and Qinghai Provinces, China. Anaerobe 2022, 73, 102516. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Song, Z.; Liao, S.; Qi, N.; Li, J.; Lv, M.; Lin, X.; Cai, H.; Hu, J.; Liu, S.; et al. Animal Model Studies, Antibiotic Resistance and Toxin Gene Profile of NE Reproducing Clostridium perfringens Type A and Type G Strains Isolated from Commercial Poultry Farms in China. Microorganisms 2023, 11, 622. [Google Scholar] [CrossRef]
- Li, J.; Pradhan, A.; McClane, B.A. NanJ Is the Major Sialidase for Clostridium perfringens Type F Food Poisoning Strain 01E809. Infect. Immun. 2023, 91, e00053-23. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hosomi, K.; Nasu, A.; Kondoh, M.; Kunisawa, J. Development of Adjuvant-Free Bivalent Food Poisoning Vaccine by Augmenting the Antigenicity of Clostridium perfringens Enterotoxin. Front. Immunol. 2018, 9, 403835. [Google Scholar] [CrossRef]
- Li, J.; Uzal, F.A.; McClane, B.A. Clostridium perfringens Sialidases: Potential Contributors to Intestinal Pathogenesis and Therapeutic Targets. Toxins 2016, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lei, Y.-X.; Li, J.-W.; Ma, Y.-Z.; Wang, X.-Y.; Meng, F.-H.; Wu, Y.-J.; Wang, N.; Liang, J.; Zhao, C.-Q.; et al. G Protein-Coupled Receptor 120 Mediates Host Defense against Clostridium perfringens Infection through Regulating NOD-like Receptor Family Pyrin Domain-Containing 3 Inflammasome Activation. J. Agric. Food Chem. 2023, 71, 7119–7130. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Johnson, C.N.; Byrd, J.A.; Ricke, S.C.; Piva, A.; Grilli, E.; Swaggerty, C.L. Impact of a Blend of Microencapsulated Organic Acids and Botanicals on the Microbiome of Commercial Broiler Breeders under Clinical Necrotic Enteritis. Animals 2023, 13, 1627. [Google Scholar] [CrossRef]
- Low, K.E.; Smith, S.P.; Abbott, D.W.; Boraston, A.B. The Glycoconjugate-Degrading Enzymes of Clostridium perfringens: Tailored Catalysts for Breaching the Intestinal Mucus Barrier. Glycobiology 2021, 31, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, L.; Callens, C.; Dargatz, M.; Flügel, M.; Hark, S.; Thiemann, F.; Pelzer, S.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. NanI Sialidase Contributes to Toxin Expression and Host Cell Binding of Clostridium perfringens Type G Strain CP56 in Vitro. Vet. Microbiol. 2022, 266, 109371. [Google Scholar] [CrossRef]
- Guo, S.; Liu, D.; Zhang, B.; Li, Z.; Li, Y.; Ding, B.; Guo, Y. Two Lactobacillus Species Inhibit the Growth and α-Toxin Production of Clostridium perfringens and Induced Proinflammatory Factors in Chicken Intestinal Epithelial Cells in Vitro. Front. Microbiol. 2017, 8, 2081. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Chi, H.; Shi, J.; Sun, R.; Du, K.; Song, Y.; Zhu, M.; Zhang, L.; Huang, J. Visual Detection of Clostridium perfringens Alpha Toxin by Combining Nanometer Microspheres with Smart Phones. Microorganisms 2020, 8, 1865. [Google Scholar] [CrossRef]
- Chinen, K. Sudden Death Caused by Clostridium perfringens Sepsis Presenting as Massive Intravascular Hemolysis. Autops. Case Rep. 2020, 10, e2020185. [Google Scholar] [CrossRef]
- Liu, S.; Yang, X.; Zhang, H.; Zhang, J.; Zhou, Y.; Wang, T.; Hu, N.; Deng, X.; Bai, X.; Wang, J. Amentoflavone Attenuates Clostridium perfringens Gas Gangrene by Targeting Alpha-Toxin and Perfringolysin O. Front. Pharmacol. 2020, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Takagishi, T.; Takehara, M.; Seike, S.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Clostridium perfringens α-Toxin Impairs Erythropoiesis by Inhibition of Erythroid Differentiation. Sci. Rep. 2017, 7, 5217. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, A.; Ohtani, K.; Komine-Aizawa, S.; Matsumoto, A.; Kamiya, S.; Hayakawa, S. Pathogenic Characterization of Clostridium perfringens Strains Isolated From Patients With Massive Intravascular Hemolysis. Front. Microbiol. 2021, 12, 713509. [Google Scholar] [CrossRef]
- Takehara, M.; Seike, S.; Sonobe, Y.; Bandou, H.; Yokoyama, S.; Takagishi, T.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Clostridium perfringens α-Toxin Impairs Granulocyte Colony-Stimulating Factor Receptor-Mediated Granulocyte Production While Triggering Septic Shock. Commun. Biol. 2019, 2, 45. [Google Scholar] [CrossRef]
- Bruggisser, J.; Iacovache, I.; Musson, S.C.; Degiacomi, M.T.; Posthaus, H.; Zuber, B. Cryo-EM Structure of the Octameric Pore of Clostridium perfringens β-Toxin. EMBO Rep. 2022, 23, e54856. [Google Scholar] [CrossRef] [PubMed]
- Lencer, W.I. Everything Illuminated—Clostridium perfringens β-Toxin. Cell Host Microbe 2020, 28, 5–6. [Google Scholar] [CrossRef]
- Tarek, B.; Bruggisser, J.; Cattalani, F.; Posthaus, H. Platelet Endothelial Cell Adhesion Molecule 1 (CD31) Is Essential for Clostridium perfringens Beta-Toxin Mediated Cytotoxicity in Human Endothelial and Monocytic Cells. Toxins 2021, 13, 893. [Google Scholar] [CrossRef]
- Bruggisser, J.; Tarek, B.; Wyder, M.; Müller, P.; von Ballmoos, C.; Witz, G.; Enzmann, G.; Deutsch, U.; Engelhardt, B.; Posthaus, H. CD31 (PECAM-1) Serves as the Endothelial Cell-Specific Receptor of Clostridium perfringens β-Toxin. Cell Host Microbe 2020, 28, 69–78.e6. [Google Scholar] [CrossRef]
- Navarro, M.A.; McClane, B.A.; Uzal, F.A. Mechanisms of Action and Cell Death Associated with Clostridium perfringens Toxins. Toxins 2018, 10, 212. [Google Scholar] [CrossRef]
- Thiel, A.; Mogel, H.; Bruggisser, J.; Baumann, A.; Wyder, M.; Stoffel, M.H.; Summerfield, A.; Posthaus, H. Effect of Clostridium perfringens β-Toxin on Platelets. Toxins 2017, 9, 336. [Google Scholar] [CrossRef]
- Finnie, J.W.; Uzal, F.A. Pathology and Pathogenesis of Brain Lesions Produced by Clostridium perfringens Type D Epsilon Toxin. Int. J. Mol. Sci. 2022, 23, 9050. [Google Scholar] [CrossRef] [PubMed]
- Savva, C.G.; Clark, A.R.; Naylor, C.E.; Popoff, M.R.; Moss, D.S.; Basak, A.K.; Titball, R.W.; Bokori-Brown, M. The Pore Structure of Clostridium perfringens Epsilon Toxin. Nat. Commun. 2019, 10, 2641. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sannino, D.; Linden, J.R.; Haigh, S.; Zhao, B.; Grigg, J.B.; Zumbo, P.; Dündar, F.; Butler, D.; Profaci, C.P.; et al. Epsilon Toxin-Producing Clostridium perfringens Colonize the Multiple Sclerosis Gut Microbiome Overcoming CNS Immune Privilege. J. Clin. Investig. 2023, 133, e163239. [Google Scholar] [CrossRef]
- Shetty, S.V.; Mazzucco, M.R.; Winokur, P.; Haigh, S.V.; Rumah, K.R.; Fischetti, V.A.; Vartanian, T.; Linden, J.R. Clostridium perfringens Epsilon Toxin Binds to and Kills Primary Human Lymphocytes. Toxins 2023, 15, 423. [Google Scholar] [CrossRef] [PubMed]
- Morcrette, H.; Bokori-Brown, M.; Ong, S.; Bennett, L.; Wren, B.W.; Lewis, N.; Titball, R.W. Clostridium perfringens Epsilon Toxin Vaccine Candidate Lacking Toxicity to Cells Expressing Myelin and Lymphocyte Protein. NPJ Vaccines 2019, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Huang, J.; Kang, L.; Gao, S.; Yuan, Y.; Li, Y.; Wang, J.; Xin, W.; Wang, J. Clostridium perfringens Epsilon Toxin Binds to Erythrocyte MAL Receptors and Triggers Phosphatidylserine Exposure. J. Cell. Mol. Med. 2020, 24, 7341–7352. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xin, W.; Huang, J.; Ji, B.; Gao, S.; Chen, L.; Kang, L.; Yang, H.; Shen, X.; Zhao, B.; et al. Hemolysis in Human Erythrocytes by Clostridium perfringens Epsilon Toxin Requires Activation of P2 Receptors. Virulence 2018, 9, 1601–1614. [Google Scholar] [CrossRef]
- Ji, B.; Huang, J.; Zou, K.; Liu, M.; Pei, Y.; Huang, J.; Wang, Y.; Wang, J.; Zhou, R.; Xin, W.; et al. Direct Visualization of the Dynamic Process of Epsilon Toxin on Hemolysis. Small Methods 2023, 7, e2300028. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Kobayashi, K.; Takehara, M. Cathepsin Release from Lysosomes Promotes Endocytosis of Clostridium perfringens Iota-Toxin. Toxins 2021, 13, 721. [Google Scholar] [CrossRef]
- Takehara, M.; Takagishi, T.; Seike, S.; Oda, M.; Sakaguchi, Y.; Hisatsune, J.; Ochi, S.; Kobayashi, K.; Nagahama, M. Cellular Entry of Clostridium perfringens Iota-Toxin and Clostridium Botulinum C2 Toxin. Toxins 2017, 9, 247. [Google Scholar] [CrossRef]
- Nagahama, M.; Takehara, M.; Kobayashi, K. Interaction of Clostridium perfringens Iota Toxin and Lipolysis-Stimulated Lipoprotein Receptor (LSR). Toxins 2018, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, M.; Takehara, M.; Miyamoto, K.; Ishidoh, K.; Kobayashi, K. Acid Sphingomyelinase Promotes Cellular Internalization of Clostridium perfringens Iota-Toxin. Toxins 2018, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh Gohari, I.; Li, J.; Navarro, M.A.; Mendonça, F.S.; Uzal, F.A.; McClane, B.A. Identification of Orphan Histidine Kinases That Impact Sporulation and Enterotoxin Production by Clostridium perfringens Type F Strain SM101 in a Pathophysiologically-Relevant Ex Vivo Mouse Intestinal Contents Model. PLoS Pathog. 2023, 19, e1011429. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.R.; Odiase, P.; Rachakonda, K.; Garg, A.P.; Adunyah, S.E.; Rachakonda, G. Application of C-Terminal Clostridium perfringens Enterotoxin in Treatment of Brain Metastasis from Breast Cancer. Cancers 2022, 14, 4309. [Google Scholar] [CrossRef] [PubMed]
- Ogbu, C.P.; Roy, S.; Vecchio, A.J. Disruption of Claudin-Made Tight Junction Barriers by Clostridium perfringens Enterotoxin: Insights from Structural Biology. Cells 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, A.J.; Rathnayake, S.S.; Stroud, R.M. Structural Basis for Clostridium perfringens Enterotoxin Targeting of Claudins at Tight Junctions in Mammalian Gut. Proc. Natl. Acad. Sci. USA 2021, 118, e2024651118. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Suzuki, H.; Tani, K.; Nishikawa, K.; Irie, K.; Ogura, Y.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015, 347, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Dominik, P.K.; Roy, S.; Ogbu, C.P.; Erramilli, S.K.; Kossiakoff, A.A.; Vecchio, A.J. Development, Structure, and Mechanism of Synthetic Antibodies That Target Claudin and Clostridium perfringens Enterotoxin Complexes. J. Biol. Chem. 2022, 298, 102357. [Google Scholar] [CrossRef] [PubMed]
- Benz, R.; Popoff, M.R. Clostridium perfringens Enterotoxin: The Toxin Forms Highly Cation-Selective Channels in Lipid Bilayers. Toxins 2018, 10, 341. [Google Scholar] [CrossRef]
- Mehdizadeh Gohari, I.; Li, J.; Navarro, M.; Uzal, F.; McClane, B. Effects of Claudin-1 on the Action of Clostridium perfringens Enterotoxin in Caco-2 Cells. Toxins 2019, 11, 582. [Google Scholar] [CrossRef]
- Lee, K.-W.; Lillehoj, H.S. Role of Clostridium perfringens Necrotic Enteritis B-like Toxin in Disease Pathogenesis. Vaccines 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, Z.S.; Hajizade, A.; Razmyar, J.; Ahmadian, G.; Arpanaei, A. Mesoporous Silica Nanoparticles-Based Formulations of a Chimeric Proteinous Vaccine Candidate against Necrotic Enteritis Disease. Mater. Sci. Eng. C 2021, 128, 112316. [Google Scholar] [CrossRef] [PubMed]
- Freitas, N.F.Q.R.; Barbosa, J.D.; Otaka, D.Y.; Ferreira, M.R.A.; Rodrigues, R.R.; Moreira Jr, C.; Conceição, F.R.; Salvarani, F.M. Clostridium perfringens α and β Recombinant Toxoids in Equine Immunization. Pesqui. Veterinária Bras. 2020, 40, 776–780. [Google Scholar] [CrossRef]
- Bai, J.; Qiao, X.; Ma, Y.; Han, M.; Jia, S.; Huang, X.; Han, B.; Wang, L.; Li, Y.; Xu, Y. Protection Efficacy of Oral Bait Probiotic Vaccine Constitutively Expressing Tetravalent Toxoids against Clostridium perfringens Exotoxins in Livestock (Rabbits). Vaccines 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as Alternatives to Antibiotics for Food Producing Animals. Part 2: New Approaches and Potential Solutions. Vet. Res. 2018, 49, 70. [Google Scholar] [CrossRef] [PubMed]
- Soto, L.F.; Romaní, A.C.; Jiménez-Avalos, G.; Silva, Y.; Ordinola-Ramirez, C.M.; Lopez Lapa, R.M.; Requena, D. Immunoinformatic Analysis of the Whole Proteome for Vaccine Design: An Application to Clostridium perfringens. Front. Immunol. 2022, 13, 942907. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Abrar, A.; Munir, S.; Aslam, S.; Allemailem, K.S.; Khurshid, M.; Ashfaq, U.A. Proteome-Wide Mapping and Reverse Vaccinology Approaches to Design a Multi-Epitope Vaccine against Clostridium perfringens. Vaccines 2021, 9, 1079. [Google Scholar] [CrossRef]
- Mak, P.H.W.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production Systems and Important Antimicrobial Resistant-Pathogenic Bacteria in Poultry: A Review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef]
- Gambino, D.; Vicari, D.; Vitale, M.; Schirò, G.; Mira, F.; Giglia, M.L.; Riccardi, A.; Gentile, A.; Giardina, S.; Carrozzo, A.; et al. Study on Bacteria Isolates and Antimicrobial Resistance in Wildlife in Sicily, Southern Italy. Microorganisms 2021, 9, 203. [Google Scholar] [CrossRef]
- García-Vela, S.; Martínez-Sancho, A.; Said, L.B.; Torres, C.; Fliss, I. Pathogenicity and Antibiotic Resistance Diversity in Clostridium perfringens Isolates from Poultry Affected by Necrotic Enteritis in Canada. Pathogens 2023, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Duc, H.M.; Hoa, T.T.K.; Ha, C.T.T.; Van Hung, L.; Van Thang, N.; Minh Son, H.; Flory, G.A. Prevalence and Antibiotic Resistance Profile of Clostridium perfringens Isolated from Pork and Chicken Meat in Vietnam. Pathogens 2024, 13, 400. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Padgett, C.; Bailey, C.; Gancel, F.; Drider, D. Protective Effects of Novel Lactobacillaceae Strains Isolated from Chicken Caeca against Necrotic Enteritis Infection: In Vitro and In Vivo Evidences. Microorganisms 2022, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, B.; Zhou, Y.; Tang, L.; Zeng, Z.; Zhang, H.; Li, W. Protective Effects of Lactobacillus Plantarum 16 and Paenibacillus Polymyxa 10 Against Clostridium perfringens Infection in Broilers. Front. Immunol. 2021, 11, 628374. [Google Scholar] [CrossRef] [PubMed]
- Hyun, W.B.; Kang, H.S.; Lee, J.W.; Abraha, H.B.; Kim, K.P. A Newly-Isolated Bacillus Subtilis BSC35 Produces Bacteriocin-like Inhibitory Substance with High Potential to Control Clostridium perfringens in Food. LWT 2021, 138, 110625. [Google Scholar] [CrossRef]
- Kadekar, D.; Udrea, A.C.; Bak, S.Y.; Christensen, N.; Gibbs, K.; Shen, C.; Bernardeau, M. Cell-Free Culture Supernatant of Lactobacillus Acidophilus AG01 and Bifidobacterium Animalis Subsp. Lactis AG02 Reduces the Pathogenicity of NetB-Positive Clostridium perfringens in a Chicken Intestinal Epithelial Cell Line. Microorganisms 2024, 12, 839. [Google Scholar] [CrossRef]
- Zhu La, A.L.T.; Wen, Q.; Xiao, Y.; Hu, D.; Liu, D.; Guo, Y.; Hu, Y. A New Bacillus Velezensis Strain CML532 Improves Chicken Growth Performance and Reduces Intestinal Clostridium perfringens Colonization. Microorganisms 2024, 12, 771. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.R.A.V.; do Carmo, M.S.; Melo, B.O.; Alves, M.S.; dos Santos, C.I.; Monteiro, S.G.; Bomfim, M.R.Q.; Fernandes, E.S.; Monteiro-Neto, V. In Vitro Antimicrobial Activity and Probiotic Potential of Bifidobacterium and Lactobacillus against Species of Clostridium. Nutrients 2019, 11, 448. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, W.; Su, W.; Wen, C.; Gong, T.; Zhang, Y.; Wang, Y.; Jin, M.; Lu, Z. Protective Effects of Bacillus Amyloliquefaciens 40 Against Clostridium perfringens Infection in Mice. Front. Nutr. 2021, 8, 733591. [Google Scholar] [CrossRef]
- Hernández, S.; Vives, M.J. Phages in Anaerobic Systems. Viruses 2020, 12, 1091. [Google Scholar] [CrossRef]
- Bloot, A.P.M.; Kalschne, D.L.; Nogues, D.R.N.; Amaral, J.S.; Flores, E.L.M.; Colla, E.; Habu, S.; Baraldi, I.J.; Canan, C. Phytic Acid against Clostridium perfringens Type A: A Food Matrix Study. Foods 2022, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, T.; Li, W.; Wang, H.; Yan, L.; Zhang, X.; Zhao, L.; Wang, N.; Zhang, B. Arginine Alleviates Clostridium perfringens α Toxin-Induced Intestinal Injury in Vivo and in Vitro via the SLC38A9/mTORC1 Pathway. Front. Immunol. 2024, 15, 1357072. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Toghyani, M.; Kheravii, S.K.; Pineda, L.; Han, Y.; Swick, R.A.; Wu, S.-B. Organic Acid Blends Improve Intestinal Integrity, Modulate Short-Chain Fatty Acids Profiles and Alter Microbiota of Broilers under Necrotic Enteritis Challenge. Anim. Nutr. 2022, 8, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Liu, B.; Wu, L.; Bao, H.; García, P.; Wang, Y.; Zhou, Y.; Zhang, H. A Broad-Spectrum Phage Endolysin (LysCP28) Able to Remove Biofilms and Inactivate Clostridium perfringens Strains. Foods 2023, 12, 411. [Google Scholar] [CrossRef]
- Johnson, S.; Gerding, D.N. Fecal Fixation: Fecal Microbiota Transplantation for Clostridium difficile Infection. Clin. Infect. Dis. 2017, 64, 272–274. [Google Scholar] [CrossRef]



| Site | Disease | Prevalence Rate (%) or Number of Infections | Reference |
|---|---|---|---|
| USA | Foodborne illness | 107 | [19] |
| Canada | Foodborne infection | 1.6 × 105 | [20] |
| England | Foodborne illness | 9 × 106 | [21] |
| Iran | Antibiotic-associated diarrhea | 15% | [30] |
| — | Antibiotic-associated diarrhea | 5%–20% | [16] |
| Year | Site | Disease | Species | Prevalence Rate (%) or Number of Infections | Reference |
|---|---|---|---|---|---|
| 2013–2016 | Switzerland | — | Horse | 8% | [35] |
| 2014–2015 | Samsun (Turkey) | Septicemia | Sheep | 45.2% | [36] |
| 2021–2022 | Kalubiya and Menofia (Egypt) | — | Lamb | 17.5% | [37] |
| 2000–2021 | India | — | Pig | 60% | [38] |
| Sheep | 56.3% | [38] | |||
| Goat | 38.7% | [38] | |||
| Cattle | 35% | [38] | |||
| — | Weifang, Guangdong, Taian, Pingying (China) | — | Chicken | 38.42% | [36] |
| 2019–2020 | Gansu (China) | — | Sheep | 14.7% | [39] |
| 2020 | Anhui, Guangdong, Guangxi, Fujian (China) | — | Ruminant | 44.173% | [40] |
| Year | Nation | Type of Vaccine | Species | Advantages | Patent Number |
|---|---|---|---|---|---|
| 2018 | China | Subunit vaccine | — | Simple preparation process, low immunizing dose, higher vaccine efficacy | CN107753940A |
| 2017 | China | Toxin vaccine | Cattle and Sheep | Strong immunity, and no toxic or side effects | CN107875377A |
| 2013 | South Korea | Genetically engineered vaccine | Poultry | Safe and efficient | KP101293667B1 |
| 2008 | Canada | Peptide vaccine | Poultry | Safe and cost-effective | CA2685533A1 |
| 2008 | China | Attenuated live vaccine | — | Safe and strong immunity | EP2007420A2 |
| 1995 | UK | Genetically engineered vaccine | — | Safe and easy to produce | EP0642581A1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ba, X.; Jin, Y.; Ning, X.; Gao, Y.; Li, W.; Li, Y.; Wang, Y.; Zhou, J. Clostridium perfringens in the Intestine: Innocent Bystander or Serious Threat? Microorganisms 2024, 12, 1610. https://doi.org/10.3390/microorganisms12081610
Ba X, Jin Y, Ning X, Gao Y, Li W, Li Y, Wang Y, Zhou J. Clostridium perfringens in the Intestine: Innocent Bystander or Serious Threat? Microorganisms. 2024; 12(8):1610. https://doi.org/10.3390/microorganisms12081610
Chicago/Turabian StyleBa, Xuli, Youshun Jin, Xuan Ning, Yidan Gao, Wei Li, Yunhui Li, Yihan Wang, and Jizhang Zhou. 2024. "Clostridium perfringens in the Intestine: Innocent Bystander or Serious Threat?" Microorganisms 12, no. 8: 1610. https://doi.org/10.3390/microorganisms12081610
APA StyleBa, X., Jin, Y., Ning, X., Gao, Y., Li, W., Li, Y., Wang, Y., & Zhou, J. (2024). Clostridium perfringens in the Intestine: Innocent Bystander or Serious Threat? Microorganisms, 12(8), 1610. https://doi.org/10.3390/microorganisms12081610





