Advances in Research on Bacterial Oxidation of Mn(II): A Visualized Bibliometric Analysis Based on CiteSpace
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Retrieval Strategy
2.2. Data Analysis and Visualization
3. Results
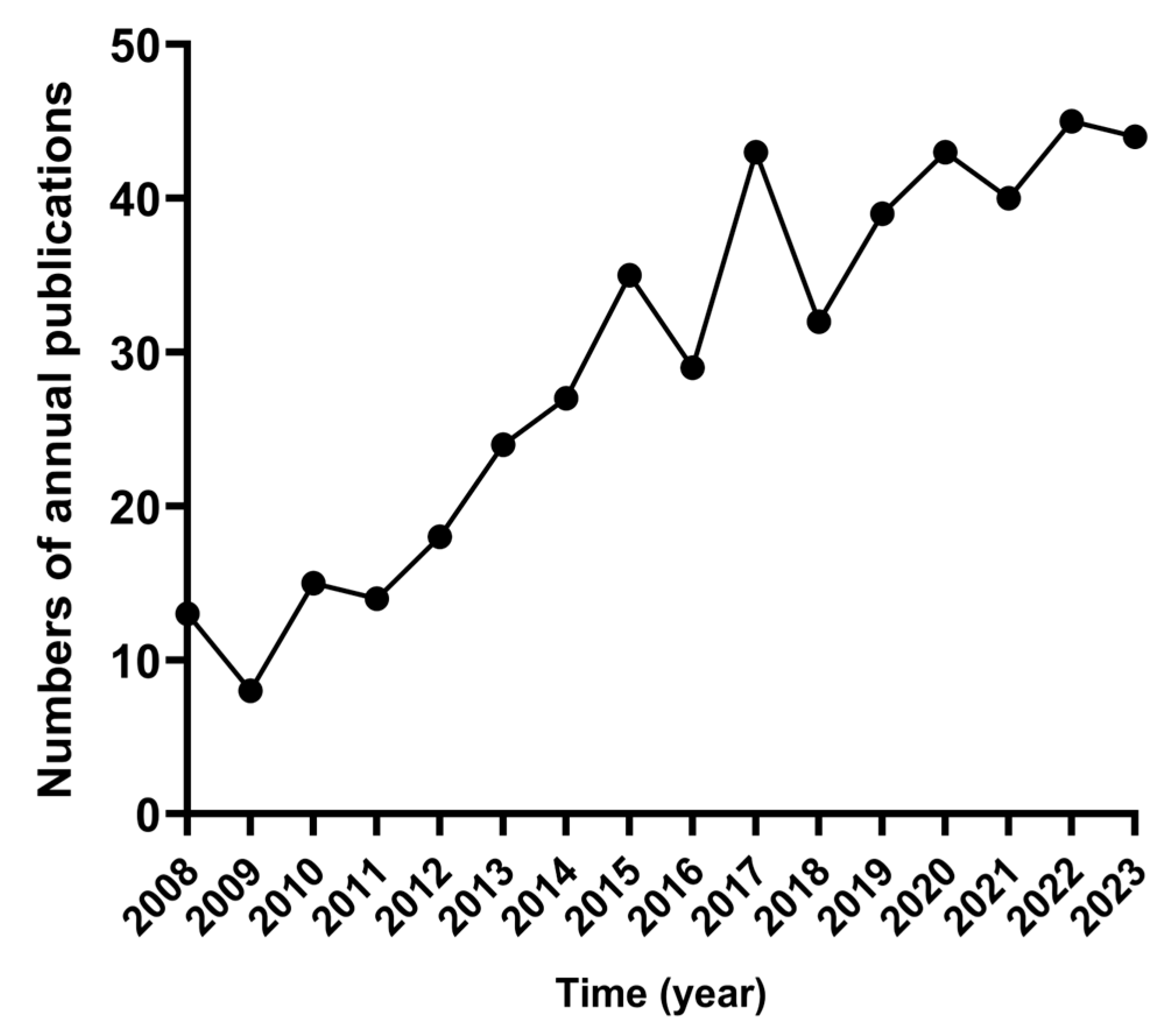
3.1. Trend Analysis of Annual Publications
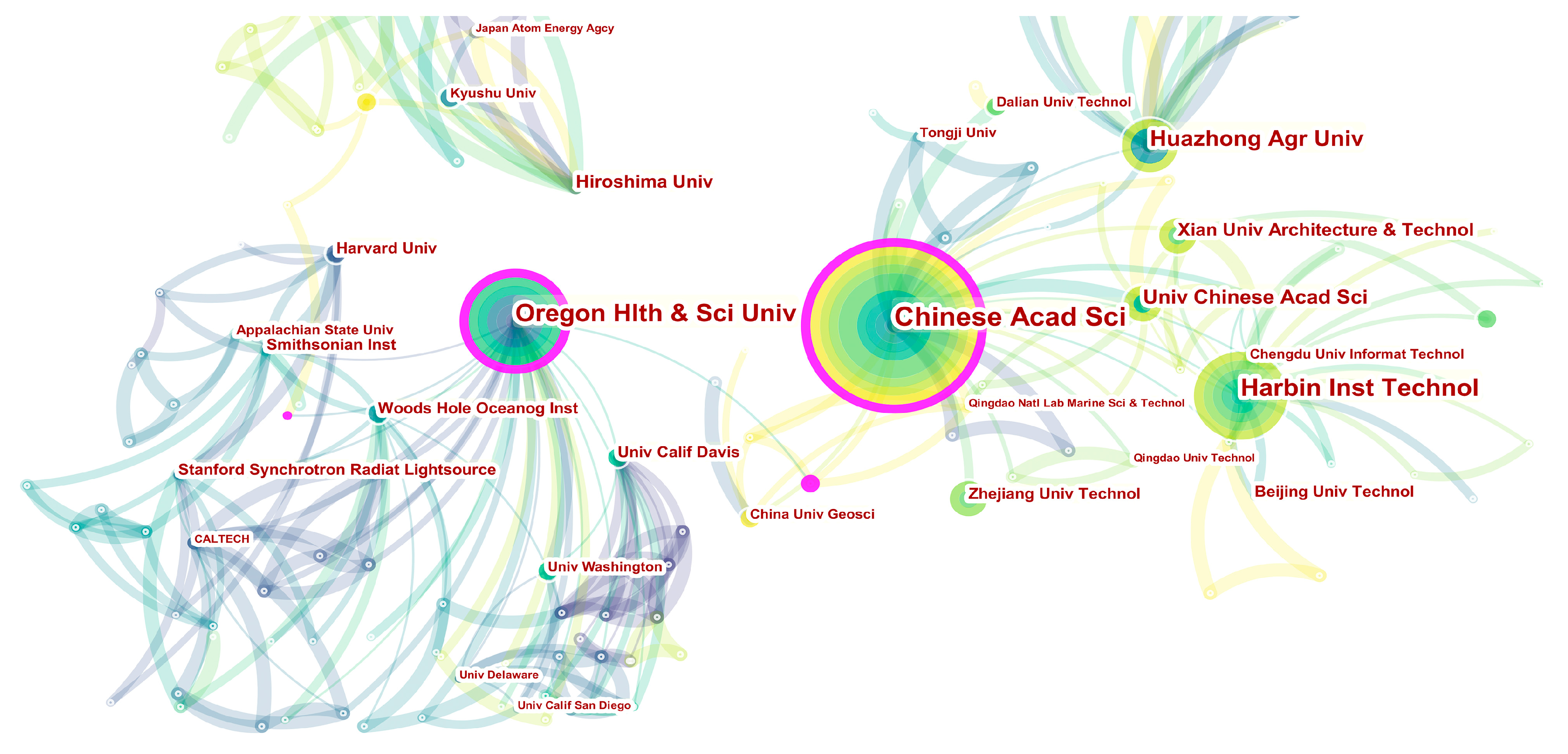
3.2. Analysis of Countries and Institutions
3.3. Analysis of Disciplinary Classifications
3.4. Analysis of Authors of Co-Occurrence and Co-Citation
3.5. Analysis of Journal Citations
3.6. Analysis of Co-Cited References
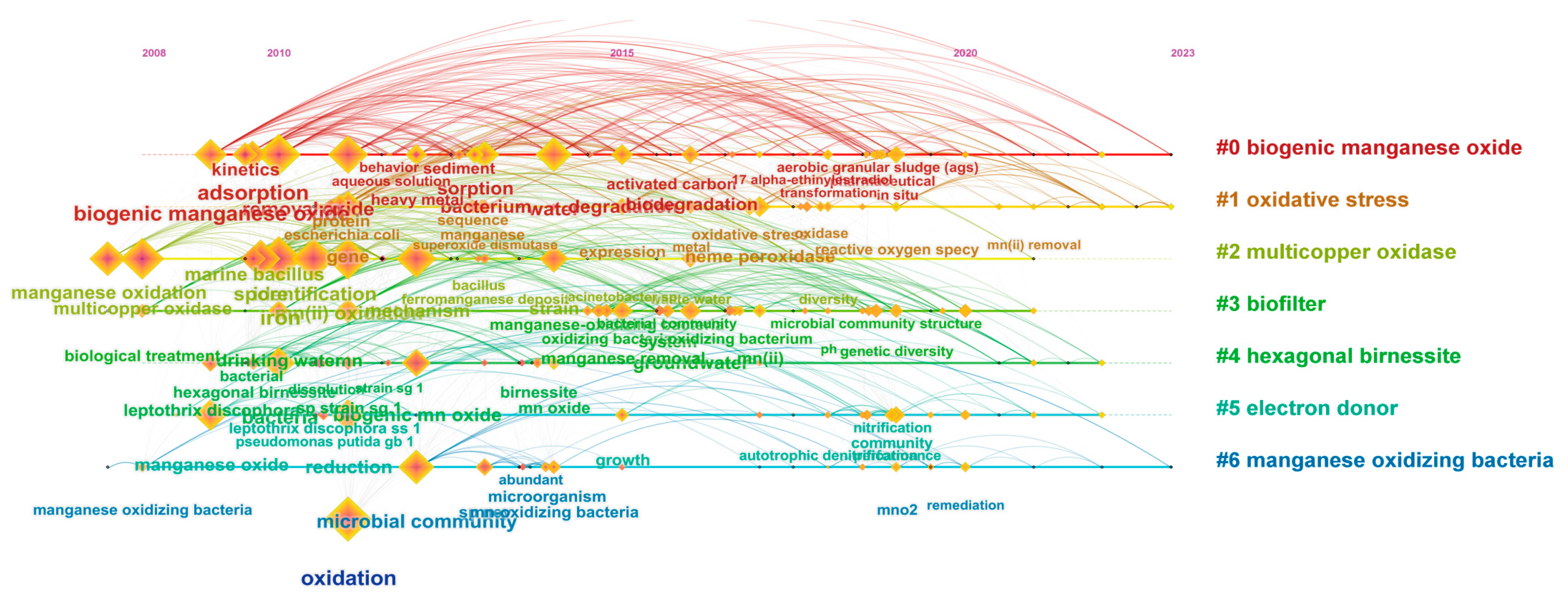
3.7. Analysis of Temporal and Burst of Keywords
3.8. Analysis of Keyword Clusters
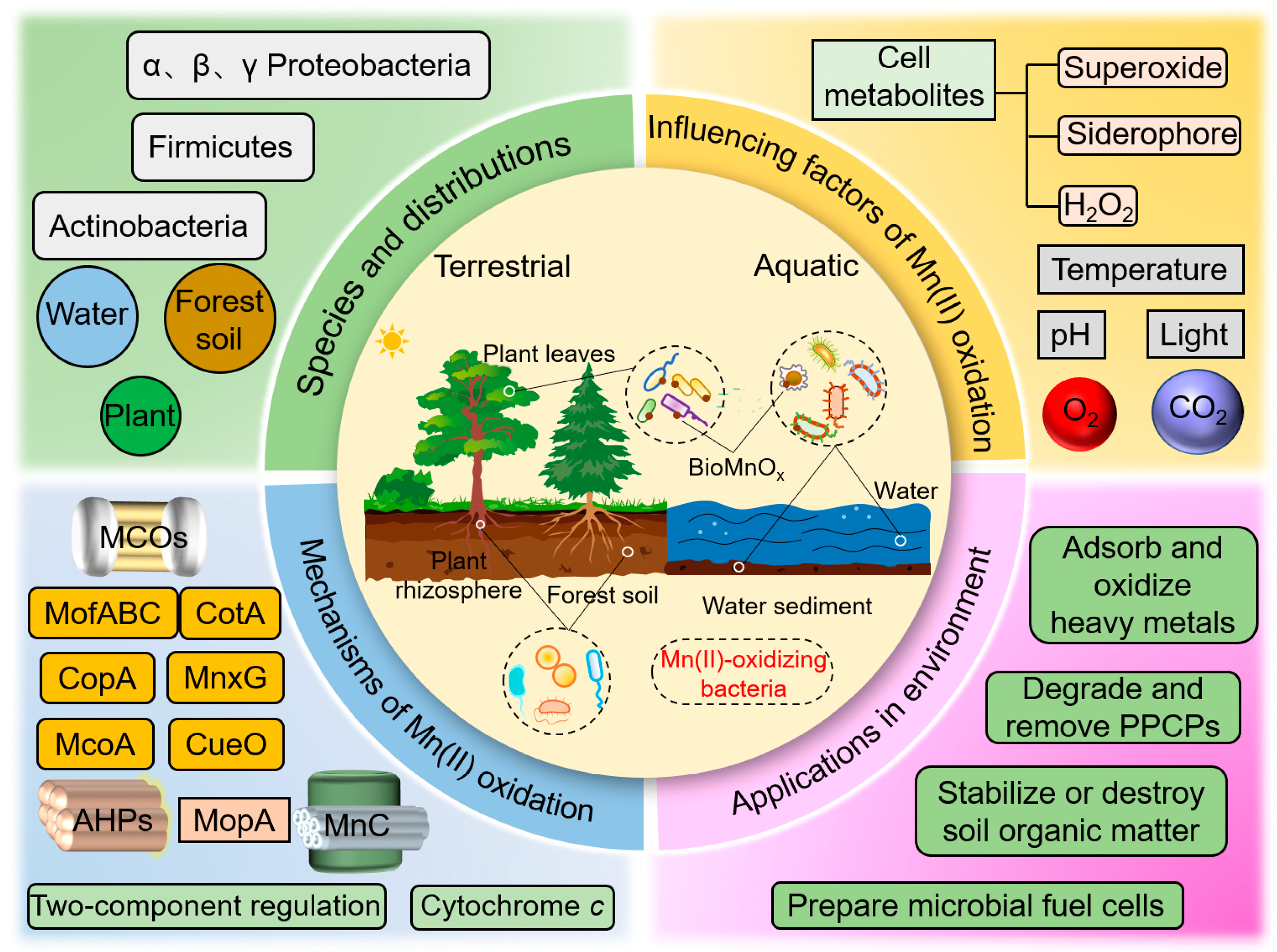
4. Discussion
4.1. Research Hotspots and Trends
4.1.1. Species and Ecological Distribution
4.1.2. Factors Influencing Bacterial Mn(II) Oxidation
4.1.3. Mechanisms of Mn(II) Oxidation in Bacteria
4.1.4. Environmental Applications
4.2. Outlook
- (1)
- Isolating and screening Mn(II)-oxidizing bacteria from more diverse habitats. Mn(II)-oxidizing strains are predominantly sourced from aquatic ecosystems, such as marine and freshwater environments. Some research also focuses on soils and plants in mining areas. Exploring more Mn(II)-oxidizing bacteria from terrestrial habitats, extreme environments, or internal biological environments, such as plant leaves and rhizosphere and animal or insect intestines, is imperative. In addition, it is necessary to explore more co-cultured Mn(II) oxidation systems among microbial communities, especially chemolithoautotrophic bacteria. This interspecific cooperation and collaboration between dual or multiple bacteria from different sources can reveal more specific Mn cycling processes. The isolation and identification of these bacteria contribute to revealing the role of Mn(II)-oxidizing bacteria across the entire ecosystem;
- (2)
- Utilizing multi-omics approaches to elucidate the Mn(II) oxidation mechanism and using genomics, transcriptomics, proteomics, metabolomics, and other technologies to collaboratively uncover novel genes involved in the Mn(II) oxidation mechanism. Then, exploring the regulation of Mn(II) oxidation-related metabolic networks by investigating the interactions among different genes;
- (3)
- In situ monitoring of the process of Mn(II)-oxidizing bacteria producing BioMnOx. Through in situ monitoring, dynamic environmental monitoring data, and meteorological data, bacterial growth status and redox potentials can be obtained, thereby exploring the interactions between different compounds (nutrients, heavy metals, organic compounds, antibiotics, etc.) and Mn compounds in a regional environment and explaining the transformation rule of Mn valence state during the formation of BioMnOx. Such monitoring contributes to further understanding the role of Mn(II)-oxidizing bacteria in the ecological balance and biogeochemical cycling under global environmental changes;
- (4)
- Transforming Mn(II)-oxidizing engineering bacteria to increase their applicability. Using synthetic biology methods, Mn(II) oxidation-related genes from different bacterial strains are fused, co-expressed, and modified to create novel and efficient Mn(II)-oxidizing engineering bacteria more suitable for rapidly degrading multiple substrates in complex environments. Subsequently, these engineered bacteria can be applied in producing bio Mn-based biological batteries, biomineralization, and metallurgy fields and are expected to play a significant role in developing renewable energy, mineral recovery, and environmental remediation.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Learman, D.R.; Hansel, C.M. Comparative Proteomics of Mn(II)-Oxidizing and Non-Oxidizing Roseobacter Clade Bacteria Reveal an Operative Manganese Transport System but Minimal Mn(II)-Induced Expression of Manganese Oxidation and Antioxidant Enzymes. Environ. Microbiol. Rep. 2014, 6, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Beukes, L.S.; Schmidt, S. Isolation and Characterization of a Manganese-Oxidizing Bacterium from a Biofiltration System for the Treatment of Borehole Water in KwaZulu-Natal (South Africa). Eng. Life Sci. 2012, 12, 544–552. [Google Scholar] [CrossRef]
- Guo, X.; Tang, Y.; Yin, J.; Li, R.; Qin, B.; Jiang, L.; Chen, X.; Huang, Z. Long-Term Manganese Exposure-Mediated Benthic Diatom Assemblage in a Subtropical Stream: Distribution, Substrate Preferences and Mn-Tolerance. J. Environ. Manag. 2022, 322, 116153. [Google Scholar] [CrossRef]
- Dobson, A.W.; Erikson, K.M.; Aschner, M. Manganese Neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1012, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.J.C.; Sarkar, S.K.; Rakshit, D.; Venkatachalam, P.; Prasad, M.N.V. Chapter 17—Acid Mine Drainages From Abandoned Mines: Hydrochemistry, Environmental Impact, Resource Recovery, and Prevention of Pollution. In Environmental Materials and Waste; Prasad, M.N.V., Shih, K., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 413–462. ISBN 978-0-12-803837-6. [Google Scholar]
- Zeng, L.; Liu, X.; Ma, J.; Yang, J.; Yang, J.; Zhou, Y. Current Progress on Manganese in Constructed Wetlands: Bibliometrics, Effects on Wastewater Treatment, and Plant Uptake. Environ. Res. 2024, 249, 118382. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, E.J. Reductive Transformation of Birnessite by Aqueous Mn(II). Environ. Sci. Technol. 2011, 45, 6366–6372. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.-H.; Ren, N.-Q.; Show, P.L. Biological Remediation of Acid Mine Drainage: Review of Past Trends and Current Outlook. Environ. Sci. Ecotechnology 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Sun, X.; Huang, H.; Bai, Y.; Wang, Y.; Luo, H.; Yao, B.; Zhang, X.; Su, X. Oxidation of a Non-Phenolic Lignin Model Compound by Two Irpex Lacteus Manganese Peroxidases: Evidence for Implication of Carboxylate and Radicals. Biotechnol. Biofuels. 2017, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Lanson, B.; Zhang, S.; Liu, L.; Peacock, C.L.; Post, J.E.; Zhu, M.; Li, W.; Wang, Q.; Zhang, J.; et al. Effect and Fate of Ni during Aging and Thermal-Induced Phyllomanganate-to-Tectomanganate Transformation. Geochim. Cosmochim. Acta. 2022, 333, 200–215. [Google Scholar] [CrossRef]
- Berg, B.; Lönn, M. Long-Term Effects of Climate and Litter Chemistry on Rates and Stable Fractions of Decomposing Scots Pine and Norway Spruce Needle Litter—A Synthesis. Forests 2022, 13, 125. [Google Scholar] [CrossRef]
- Remucal, C.K.; Ginder-Vogel, M. A Critical Review of the Reactivity of Manganese Oxides with Organic Contaminants. Environ. Sci. Process. Impacts. 2014, 16, 1247–1266. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, H.; Jiang, X.; Shen, J.; Faheem, M.; Sun, X.; Li, J.; Han, W.; Wang, L.; Liu, X. The Key Role of Biogenic Manganese Oxides in Enhanced Removal of Highly Recalcitrant 1,2,4-Triazole from Bio-Treated Chemical Industrial Wastewater. Environ. Sci. Pollut. Res. 2017, 24, 10570–10583. [Google Scholar] [CrossRef] [PubMed]
- Zerfass, C.; Christie-Oleza, J.A.; Soyer, O.S. Manganese Oxide Biomineralization Provides Protection against Nitrite Toxicity in a Cell-Density-Dependent Manner. Appl. Environ. Microbiol. 2019, 85, e02129-18. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Ikegaya, H.; Fujibayashi, M.; Hashimoto, H.; Suzuki, S.; Okano, K.; Ichise, S.; Miyata, N. Effects of Algal Extracellular Polysaccharides on the Formation of Filamentous Manganese Oxide Particles in the Near-Bottom Layer of Lake Biwa. Microorganisms 2023, 11, 1814. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.; Casciotti, K.; Tebo, B. Localization of Mn(II)-Oxidizing Activity and the Putative Multicopper Oxidase, MnxG, to the Exosporium of the Marine Bacillus sp. Strain SG-1. Arch. Microbiol. 2002, 178, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.J.; Tebo, B.M. Cr(III) Is Indirectly Oxidized by the Mn(II)-Oxidizing Bacterium Bacillus sp. Strain SG-1. Environ. Sci. Technol. 2007, 41, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, M.; Liu, Y.; Tang, W. Manganese(II) Oxidation by the Multi-Copper Oxidase CopA from Brevibacillus Panacihumi MK-8. Enzyme Microb. Technol. 2018, 117, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, X.; Liu, B.; Xie, G.; Xing, D. Characterization of Manganese Oxidation by Brevibacillus at Different Ecological Conditions. Chemosphere 2018, 205, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, Z.; Zhang, Z.; Chen, H.; Liu, J.; Ali, M.; Liu, F.; Li, L. Population Structure of Manganese-Oxidizing Bacteria in Stratified Soils and Properties of Manganese Oxide Aggregates under Manganese-Complex Medium Enrichment. PLoS ONE 2013, 8, e73778. [Google Scholar] [CrossRef]
- Liang, J.; Bai, Y.; Men, Y.; Qu, J. Microbe-Microbe Interactions Trigger Mn(II)-Oxidizing Gene Expression. ISME J. 2017, 11, 67–77. [Google Scholar] [CrossRef]
- Bai, Y.; Su, J.; Wen, Q.; Huang, T.; Chang, Q.; Ali, A. Characterization and Mechanism of Mn(II)-Based Mixotrophic Denitrifying Bacterium (Cupriavidus sp. HY129) in Remediation of Nitrate (NO3−-N) and Manganese (Mn(II)) Contaminated Groundwater. J. Hazard. Mater. 2021, 408, 124414. [Google Scholar] [CrossRef]
- Ridge, J.P.; Lin, M.; Larsen, E.I.; Fegan, M.; McEwan, A.G.; Sly, L.I. A Multicopper Oxidase Is Essential for Manganese Oxidation and Laccase-like Activity in Pedomicrobium sp. ACM 3067. Environ. Microbiol. 2007, 9, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Caputo, N.; Davis, R.; Torpey, J.; Tebo, B. Mn(II) Oxidation Is Catalyzed by Heme Peroxidases in “Aurantimonas Manganoxydans” Strain SI85-9A1 and Erythrobacter sp. Strain SD-21. Appl. Environ. Microbiol. 2009, 75, 4130–4138. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Paulley, J.; Gaines, J.; Valderas, M.; Martin, D.; Menscher, E.; Brown, T.; Burns, C.; Roop, R. The Manganese Transporter MntH Is a Critical Virulence Determinant for Brucella. Infect. Immun. 2009, 77, 3466–3474. [Google Scholar] [CrossRef] [PubMed]
- Andeer, P.F.; Learman, D.R.; McIlvin, M.; Dunn, J.A.; Hansel, C.M. Extracellular Haem Peroxidases Mediate Mn(II) Oxidation in a Marine Roseobacter Bacterium via Superoxide Production. Environ. Microbiol. 2015, 17, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Learman, D.R.; Voelker, B.M.; Vazquez-Rodriguez, A.I.; Hansel, C.M. Formation of Manganese Oxides by Bacterially Generated Superoxide. Nat. Geosci. 2011, 4, 95–98. [Google Scholar] [CrossRef]
- Bocioaga, D.; El Gheriany, I.A.; Lion, L.W.; Ghiorse, W.C.; Shuler, M.L.; Hay, A.G. Development of a Genetic System for a Model Manganese-Oxidizing Proteobacterium, Leptothrix Discophora SS1. Microbiology. 2014, 160, 2396–2405. [Google Scholar] [CrossRef] [PubMed]
- Corstjens, P.L.A.M.; de Vrind, J.P.M.; Goosen, T.; Jong, E.W.d.V. Identification and Molecular Analysis of the Leptothrix Discophora SS-1 mofA Gene, a Gene Putatively Encoding a Manganese-oxidizing Protein with Copper Domains. Geomicrobiol. J. 1997, 14, 91–108. [Google Scholar] [CrossRef]
- Eggerichs, T.; Otte, T.; Opel, O.; Ruck, W.K.L. Direct and Mn-Controlled Indirect Iron Oxidation by Leptothrix Discophora SS-1 and Leptothrix Cholodnii. Geomicrobiol. J. 2015, 32, 934–943. [Google Scholar] [CrossRef]
- El Gheriany, I.A.; Bocioaga, D.; Hay, A.G.; Ghiorse, W.C.; Shuler, M.L.; Lion, L.W. Iron Requirement for Mn(II) Oxidation by Leptothrix Discophora SS-1. Appl. Environ. Microbiol. 2009, 75, 1229–1235. [Google Scholar] [CrossRef]
- Zhang, D.-C.; Liu, Y.-X.; Li, X.-Z. Characterization of Bacterial Diversity Associated with Deep Sea Ferromanganese Nodules from the South China Sea. J. Microbiol. 2015, 53, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Kaur, J.; Srivastava, S.; Bist, V.; Dharmesh, V.; Kriti, K.; Bisht, S.; Srivastava, P.K.; Srivastava, S. Potential of Methyltransferase Containing Pseudomonas Oleovorans for Abatement of Arsenic Toxicity in Rice. Sci. Total Environ. 2023, 856, 158944. [Google Scholar] [CrossRef] [PubMed]
- Geszvain, K.; McCarthy, J.K.; Tebo, B.M. Elimination of Manganese(II,III) Oxidation in Pseudomonas Putida GB-1 by a Double Knockout of Two Putative Multicopper Oxidase Genes. Appl. Environ. Microbiol. 2013, 79, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Webb, S.M.; Estes, E.R.; Hansel, C.M. Chromium(III) Oxidation by Biogenic Manganese Oxides with Varying Structural Ripening. Environ. Sci.-Process. IMPACTS 2014, 16, 2127–2136. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Li, W.; Zhang, W.; Xu, Z.; Dai, M.; Zhao, G. Combination of the Endophytic Manganese-Oxidizing Bacterium Pantoea Eucrina SS01 and Biogenic Mn Oxides: An Efficient and Sustainable Complex in Degradation and Detoxification of Malachite Green. Chemospher 2021, 280, 130785. [Google Scholar] [CrossRef] [PubMed]
- Djoko, K.Y.; Chong, L.X.; Wedd, A.G.; Xiao, Z. Reaction Mechanisms of the Multicopper Oxidase CueO from Escherichia Coli Support Its Functional Role as a Cuprous Oxidase. J. Am. Chem. Soc. 2010, 132, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Yao, P.; Yin, H.; Liang, Z.; Li, G.; An, T. BPA Degradation Using Biogenic Manganese Oxides Produced by an Engineered Escherichia Coli with a Non-Blue Laccase from Bacillus sp. GZB. Chemosphere 2023, 326, 138407. [Google Scholar] [CrossRef]
- Geszvain, K.; Smesrud, L.; Tebo, B.M. Identification of a Third Mn(II) Oxidase Enzyme in Pseudomonas Putida GB-1. Appl. Environ. Microbiol. 2016, 82, 3774–3782. [Google Scholar] [CrossRef]
- Bramness, J.G.; Henriksen, B.; Person, O.; Mann, K. A Bibliometric Analysis of European versus USA Research in the Field of Addiction. Research on Alcohol, Narcotics, Prescription Drug Abuse, Tobacco and Steroids 2001–2011. Eur. Addict. Res. 2014, 20, 16–22. [Google Scholar] [CrossRef]
- Chen, C. Searching for Intellectual Turning Points: Progressive Knowledge Domain Visualization. Proc. Natl. Acad. Sci. USA 2004, 101, 5303–5310. [Google Scholar] [CrossRef]
- Chen, C.; Ibekwe-SanJuan, F.; Hou, J. The Structure and Dynamics of Cocitation Clusters: A Multiple-Perspective Cocitation Analysis. J. Am. Soc. Inf. Sci. Technol. 2010, 61, 1386–1409. [Google Scholar] [CrossRef]
- Jones, M.R.; Luther, G.W., 3rd; Mucci, A.; Tebo, B.M. Concentrations of Reactive Mn(III)-L and MnO2 in Estuarine and Marine Waters Determined Using Spectrophotometry and the Leuco Base, Leucoberbelin Blue. Talanta. 2019, 200, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Oldham, V.E.; Jones, M.R.; Tebo, B.M.; Luther, G.W. Oxidative and Reductive Processes Contributing to Manganese Cycling at Oxic-Anoxic Interfaces. SI Honor. Mar. Chem. 2017, 195, 122–128. [Google Scholar] [CrossRef]
- Toyoda, K.; Tebo, B.M. The Effect of Ca2+ Ions and Ionic Strength on Mn(II) Oxidation by Spores of the Marine Bacillus sp. SG-1. Geochim. Cosmochim. Acta. 2013, 101, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.M.; Tebo, B.M.; Barger, J.R. Structural Influences of Sodium and Calcium Ions on the Biogenic Manganese Oxides Produced by the Marine Bacillus sp. Strain SG-1. Geomicrobiol. J. 2005, 22, 181–193. [Google Scholar] [CrossRef]
- Huang, X.; Shin, J.H.; Pinochet-Barros, A.; Su, T.T.; Helmann, J.D. Bacillus Subtilis MntR Coordinates the Transcriptional Regulation of Manganese Uptake and Efflux Systems. Mol. Microbiol. 2017, 103, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Geszvain, K.; Yamaguchi, A.; Maybee, J.; Tebo, B.M. Mn(II) Oxidation in Pseudomonas Putida GB-1 Is Influenced by Flagella Synthesis and Surface Substrate. Arch. Microbiol. 2011, 193, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Geszvain, K.; Butterfield, C.; Davis, R.E.; Madison, A.S.; Lee, S.W.; Parker, D.L.; Soldatova, A.; Spiro, T.G.; Luther, G.W.; Tebo, B.M. The Molecular Biogeochemistry of Manganese(II) Oxidation. Biochem. Soc. Trans. 2012, 40, 1244–1248. [Google Scholar] [CrossRef]
- Geszvain, K.; Tebo, B.M. Identification of a Two-Component Regulatory Pathway Essential for Mn(II) Oxidation in Pseudomonas Putida GB-1. Appl. Environ. Microbiol. 2010, 76, 1224–1231. [Google Scholar] [CrossRef]
- Su, J.; Bao, P.; Bai, T.; Deng, L.; Wu, H.; Liu, F.; He, J. CotA, a Multicopper Oxidase from Bacillus Pumilus WH4, Exhibits Manganese-Oxidase Activity. PLoS ONE 2013, 8, e60573. [Google Scholar] [CrossRef]
- Cai, Y.; Li, D.; Liang, Y.; Luo, Y.; Zeng, H.; Zhang, J. Effective Start-up Biofiltration Method for Fe, Mn, and Ammonia Removal and Bacterial Community Analysis. Bioresour. Technol. 2015, 176, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Kim, D.-G.; Ko, S.-O. Synergistic Effects of Biogenic Manganese Oxide and Mn(II)-Oxidizing Bacterium Pseudomonas Putida Strain MnB1 on the Degradation of 17 α-Ethinylestradiol. J. Hazard. Mater. 2018, 344, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, Z.; Du, X.; Bai, L.; Yu, H.; Ding, A.; Li, G.; Liang, H.; Aminabhavi, T.M. Removal of Manganese from Groundwater in the Ripened Sand Filtration: Biological Oxidation versus Chemical Auto-Catalytic Oxidation. Chem. Eng. J. 2020, 382, 123033. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Qin, Z.; Luo, P.; Ma, Z.; Tan, M.; Kang, H.; Huang, Z. A Novel Manganese Oxidizing Bacterium-Aeromonas Hydrophila Strain DS02: Mn(II) Oxidization and Biogenic Mn Oxides Generation. J. Hazard. Mater. 2019, 367, 539–545. [Google Scholar] [CrossRef]
- Butterfield, C.N.; Soldatova, A.V.; Lee, S.W.; Spiro, T.G.; Tebo, B.M. Mn(II,III) Oxidation and MnO2 Mineralization by an Expressed Bacterial Multicopper Oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, 11731–11735. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.N.; Pinto, A.; Anantharaman, K.; Ruberg, S.A.; Kramer, E.L.; Raskin, L.; Dick, G.J. Diverse Manganese(II)-Oxidizing Bacteria Are Prevalent in Drinking Water Systems. Environ. Microbiol. Rep. 2017, 9, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. CiteSpace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Su, J.; Deng, L.; Huang, L.; Guo, S.; Liu, F.; He, J. Catalytic Oxidation of Manganese(II) by Multicopper Oxidase CueO and Characterization of the Biogenic Mn Oxide. Water. Res. 2014, 56, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, Z.; Liao, B.; Zhu, Y.; Zhang, X.; Wang, C.; He, J. Microcalorimetric Study of the Effect of Manganese on the Growth and Metabolism in a Heterogeneously Expressing Manganese-Dependent Superoxide Dismutase (Mn-SOD) Strain. J. Therm. Anal. Calorim. 2017, 130, 1407–1416. [Google Scholar] [CrossRef]
- Rajasabapathy, R.; Mohandass, C.; Dastager, S.G.; Liu, Q.; Li, W.J.; Colaco, A. Citreicella Manganoxidans sp. nov., a Novel Manganese Oxidizing Bacterium Isolated from a Shallow Water Hydrothermal Vent in Espalamaca (Azores). Antonie Van Leeuwenhoek 2015, 108, 1433–1439. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Wang, Y.; Dai, X.; Zhang, X.H. Celeribacter Manganoxidans sp. nov., a Manganese-Oxidizing Bacterium Isolated from Deep-Sea Sediment of a Polymetallic Nodule Province. Int. J. Syst. Evol. Microbiol. 2015, 65, 4180–4185. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, X.; Rensing, C.; Yuan, Y.; Zhou, S.; Nealson, K.H. Light-Independent Anaerobic Microbial Oxidation of Manganese Driven by an Electrosyntrophic Coculture. ISME J. 2023, 17, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Leadbetter, J.R. Bacterial Chemolithoautotrophy via Manganese Oxidation. Nature 2020, 583, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Bargar, J.R.; Tebo, B.M.; Bergmann, U.; Webb, S.M.; Glatzel, P.; Chiu, V.Q.; Villalobos, M. Biotic and Abiotic Products of Mn(II) Oxidation by Spores of the Marine Bacillus sp. Strain SG-1. Am. Mineral. 2005, 90, 143–154. [Google Scholar] [CrossRef]
- Estes, E.R.; Andeer, P.F.; Nordlund, D.; Wankel, S.D.; Hansel, C.M. Biogenic Manganese Oxides as Reservoirs of Organic Carbon and Proteins in Terrestrial and Marine Environments. Geobiology 2017, 15, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of Manganese(II) Oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Vasilatos, C.; Economou-Eliopoulos, M. Fossilized Bacteria in Fe-Mn-Mineralization: Evidence from the Legrena Valley, W. Lavrion Mine (Greece). Minerals 2018, 8, 107. [Google Scholar] [CrossRef]
- Okano, K.; Furuta, S.; Ichise, S.; Miyata, N. Whole-Genome Sequences of Two Manganese(II)-Oxidizing Bacteria, Bosea sp. Strain BIWAKO-01 and Alphaproteobacterium Strain U9-1i. Genome Announc. 2016, 4, e01309-16. [Google Scholar] [CrossRef] [PubMed]
- Hansel, C.M.; Francis, C.A. Coupled Photochemical and Enzymatic Mn(II) Oxidation Pathways of a Planktonic Roseobacter-Like Bacterium. Appl. Environ. Microbiol. 2006, 72, 3543–3549. [Google Scholar] [CrossRef]
- Jiang, S.; Kim, D.-G.; Kim, J.; Ko, S.-O. Characterization of the Biogenic Manganese Oxides Produced by Pseudomonas Putida Strain MnB1. Environ. Eng. Res. 2010, 15, 183–190. [Google Scholar] [CrossRef]
- Learman, D.R.; Wankel, S.D.; Webb, S.M.; Martinez, N.; Madden, A.S.; Hansel, C.M. Coupled Biotic–Abiotic Mn(II) Oxidation Pathway Mediates the Formation and Structural Evolution of Biogenic Mn Oxides. Geochim. Cosmochim. Acta 2011, 75, 6048–6063. [Google Scholar] [CrossRef]
- Villalobos, M.; Toner, B.; Bargar, J.; Sposito, G. Characterization of the Manganese Oxide Produced by Pseudomonas Putida Strain MnB1. Geochim. Cosmochim. Acta 2003, 67, 2649–2662. [Google Scholar] [CrossRef]
- Okazaki, M.; Sugita, T.; Shimizu, M.; Ohode, Y.; Iwamoto, K.; de Vrind-de Jong, E.W.; de Vrind, J.P.; Corstjens, P.L. Partial Purification and Characterization of Manganese-Oxidizing Factors of Pseudomonas Fluorescens GB-1. Appl. Environ. Microbiol. 1997, 63, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Shao, Z.; Liao, S.; Johnstone, L.; Rensing, C.; Wang, G. Genome Sequence of Deep-Sea Manganese-Oxidizing Bacterium Marinobacter Manganoxydans MnI7-9. J. Bacteriol. 2012, 194, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Nakama, K.; Medina, M.; Lien, A.; Ruggieri, J.; Collins, K.; Johnson, H.A. Heterologous Expression and Characterization of the Manganese-Oxidizing Protein from Erythrobacter sp. Strain SD21. Appl. Environ. Microbiol. 2014, 80, 6837–6842. [Google Scholar] [CrossRef]
- Brouwers, G.J.; Vijgenboom, E.; Corstjens, P.L.A.M.; De Vrind, J.P.M.; De Vrind-De Jong, E.W. Bacterial Mn2+ Oxidizing Systems and Multicopper Oxidases: An Overview of Mechanisms and Functions. Geomicrobiol. J. 2000, 17, 1–24. [Google Scholar] [CrossRef]
- Dick, G.J.; Torpey, J.W.; Beveridge, T.J.; Tebo, B.M. Direct Identification of a Bacterial Manganese(II) Oxidase, the Multicopper Oxidase MnxG, from Spores of Several Different Marine Bacillus Species. Appl. Environ. Microbiol. 2008, 74, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Barynin, V.V.; Whittaker, M.M.; Antonyuk, S.V.; Lamzin, V.S.; Harrison, P.M.; Artymiuk, P.J.; Whittaker, J.W. Crystal Structure of Manganese Catalase from Lactobacillus Plantarum. Struct. Lond. Engl. 1993 2001, 9, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chadwick, G.L.; Lingappa, U.F.; Leadbetter, J.R. Comparative Genomics on Cultivated and Uncultivated Freshwater and Marine “Candidatus Manganitrophaceae” Species Implies Their Worldwide Reach in Manganese Chemolithoautotrophy. mBio 2022, 13, e0342121. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Ding, Z.; Ruan, X.; Luo, J.; Chen, J.; Zheng, J.; Tang, J. Discovery of a Novel Native Bacterium of Providencia sp. with High Biosorption and Oxidation Ability of Manganese for Bioleaching of Heavy Metal Contaminated Soils. Chemosphere 2020, 241, 125039. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Yang, M.; Ning, X.; Nan, Z. Experimental Study on Treatment of Heavy Metal–Contaminated Soil by Manganese-Oxidizing Bacteria. Environ. Sci. Pollut. Res. 2022, 29, 5526–5540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Pan, H.; Xu, J.; Xu, W.; Liu, L. Acclimation of a Marine Microbial Consortium for Efficient Mn(II) Oxidation and Manganese Containing Particle Production. J. Hazard. Mater. 2016, 304, 434–440. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wei, Z.; Zhang, Q.; Zou, J.; Pan, X. Metal Oxyanion Removal from Wastewater Using Manganese-Oxidizing Aerobic Granular Sludge. Chemosphere. 2019, 236, 124353. [Google Scholar] [CrossRef] [PubMed]
- Forrez, I.; Carballa, M.; Verbeken, K.; Vanhaecke, L.; Schluesener, M.; Ternes, T.; Boon, N.; Verstraete, W. Diclofenac Oxidation by Biogenic Manganese Oxides. Environ. Sci. Technol. 2010, 44, 3449–3454. [Google Scholar] [CrossRef]
- Kim, S.S.; Bargar, J.R.; Nealson, K.H.; Flood, B.E.; Kirschvink, J.L.; Raub, T.D.; Tebo, B.M.; Villalobos, M. Searching for Biosignatures Using Electron Paramagnetic Resonance (EPR) Analysis of Manganese Oxides. Astrobiology. 2011, 11, 775–786. [Google Scholar] [CrossRef]

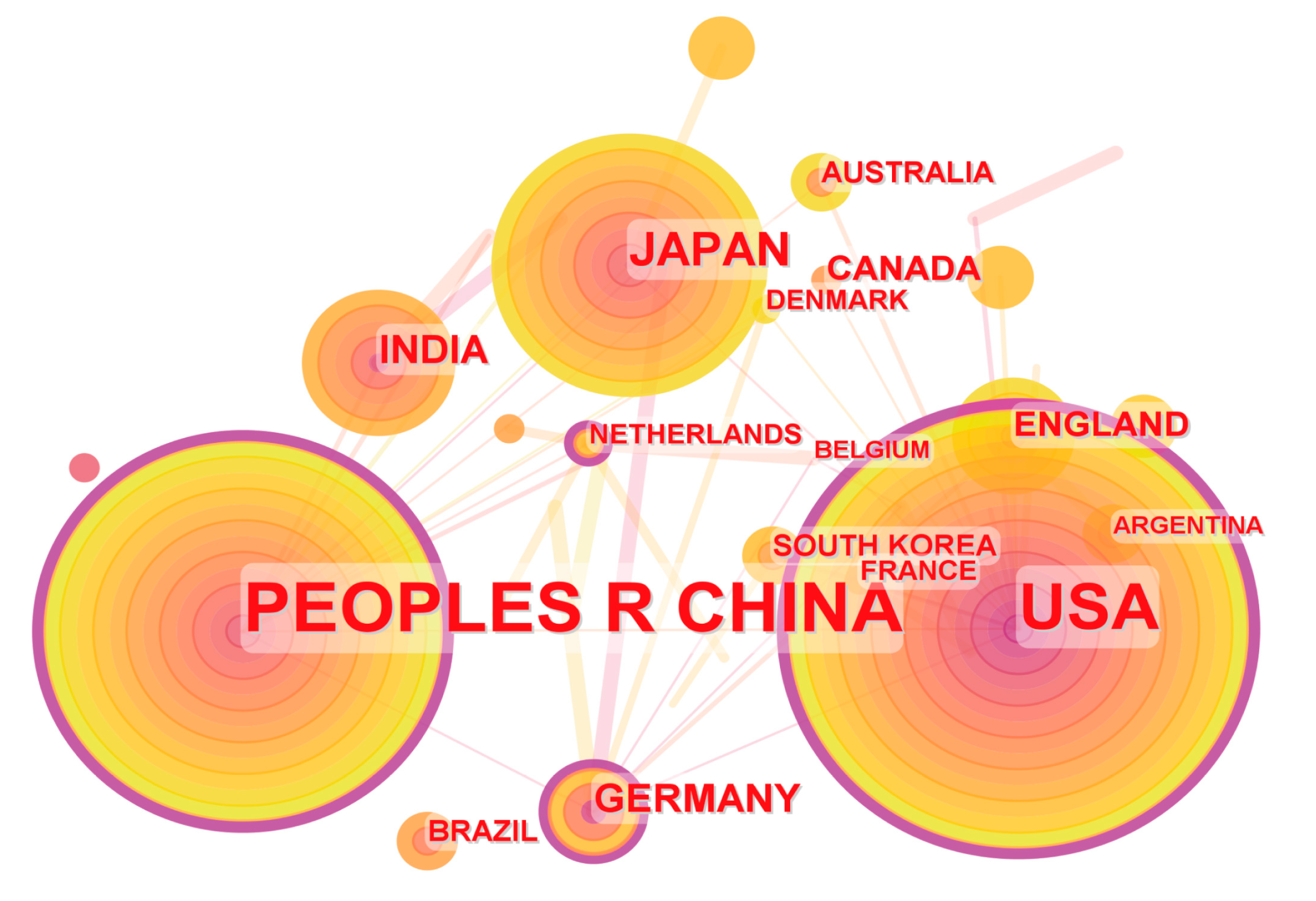



| Rank | Country | Count | Rank | Country | Centrality |
|---|---|---|---|---|---|
| 1 | China | 197 | 1 | USA | 0.38 |
| 2 | USA | 128 | 2 | China | 0.34 |
| 3 | Japan | 33 | 3 | Germany | 0.15 |
| 4 | Germany | 20 | 4 | Netherlands | 0.14 |
| 5 | India | 18 | 5 | England | 0.09 |
| 6 | Canada | 11 | 6 | Japan | 0.04 |
| 7 | England | 10 | 7 | South Korea | 0.04 |
| 8 | France | 9 | 8 | Pakistan | 0.04 |
| 9 | Australia | 8 | 9 | Mexico | 0.04 |
| 10 | South Korea | 9 | 10 | France | 0.03 |
| Rank | Count | Centrality | Institution | Country |
|---|---|---|---|---|
| 1 | 41 | 0.19 | Chinese Academy of Science | China |
| 2 | 28 | 0.06 | Harbin Institute of Technology | China |
| 3 | 27 | 0.23 | Oregon Health and Science University | USA |
| 4 | 20 | 0.07 | Huazhong Agricultural University | China |
| 5 | 14 | 0.00 | University of Chinese Academy of Sciences | China |
| 6 | 12 | 0.00 | Xi’an University of Architecture and Technology | China |
| 7 | 9 | 0.05 | Hiroshima University | Japan |
| 8 | 8 | 0.04 | Woods Hole Oceanographic Institution | USA |
| 9 | 8 | 0.03 | Smithsonian Institution | USA |
| 10 | 8 | 0.00 | Beijing University of Technology | China |
| Rank | Category | Count | Rank | Category | Centrality |
|---|---|---|---|---|---|
| 1 | Environmental Sciences & Ecology | 151 | 1 | Environmental Sciences & Ecology | 0.41 |
| 2 | Environmental Sciences | 122 | 2 | Chemistry | 0.41 |
| 3 | Engineering | 87 | 3 | Biotechnology & Applied Microbiology | 0.39 |
| 4 | Microbiology | 73 | 4 | Biochemistry & Molecular Biology | 0.34 |
| 5 | Engineering, Environmental | 72 | 5 | Environmental Sciences | 0.32 |
| 6 | Biotechnology & Applied Microbiology | 52 | 6 | Engineering | 0.18 |
| 7 | Water Resources | 39 | 7 | Chemistry | 0.18 |
| 8 | Geology | 32 | 8 | Microbiology | 0.15 |
| 9 | Geosciences | 28 | 9 | Agriculture | 0.14 |
| 10 | Biochemistry & Molecular Biology | 25 | 10 | Toxicology | 0.09 |
| Rank | Top Ten Productive Author | Count | Rank | Top Ten Co-Cited Author | Citation |
|---|---|---|---|---|---|
| 1 | Tebo BM | 22 | 1 | Tebo BM | 212 |
| 2 | Bai YH | 13 | 2 | Francis CA | 104 |
| 3 | Qu JH | 12 | 3 | Learman DR | 94 |
| 4 | Zhang J | 10 | 4 | Villalobos M | 89 |
| 5 | Pan XL | 9 | 5 | Geszvain K | 87 |
| 6 | Hansel CM | 8 | 6 | Dick GJ | 80 |
| 7 | Liu F | 8 | 7 | Webb SM | 79 |
| 8 | He ZF | 7 | 8 | Miyata N | 74 |
| 9 | Santelli CM | 7 | 9 | Anderson CR | 71 |
| 10 | Wei Z | 7 | 10 | Brouwers GJ | 69 |
| Rank | Citation | Cited Journal | IF2022–2023 | JCR | Country |
|---|---|---|---|---|---|
| 1 | 301 | Applied and Environmental Microbiology | 4.32 | Q2 | USA |
| 2 | 232 | Environmental Science & Technology | 11.09 | Q1 | USA |
| 3 | 223 | Water Research | 12.75 | Q1 | England |
| 4 | 214 | Geochimica et Cosmochimica Acta | 4.97 | Q1 | USA |
| 5 | 205 | Geomicrobiology Journal | 2.30 | Q3 | USA |
| 6 | 188 | Journal of Bacteriology | 3.06 | Q3 | USA |
| 7 | 180 | Proceedings of the National Academy of Science of the United States of America | 10.71 | Q1 | USA |
| 8 | 168 | Annual Review of Earth and Planetary Sciences | 14.29 | Q1 | USA |
| 9 | 158 | Chemosphere | 8.80 | Q1 | England |
| 10 | 153 | PLoS One | 3.64 | Q2 | USA |
| Title | Authors | Year | Citation Frequency |
|---|---|---|---|
| Synergistic effects of biogenic manganese oxide and Mn(II)-oxidizing bacterium Pseudomonas putida strain MnB1 on the degradation of 17 α-ethinylestradiol | Tran TN et al. [53] | 2018 | 30 |
| A novel manganese oxidizing bacterium-Aeromonas hydrophila strain DS02: Mn(II) oxidization and biogenic Mn oxides generation | Zhang Y et al. [55] | 2019 | 29 |
| Elimination of Manganese(II,III) Oxidation in Pseudomonas Putida GB-1 by a Double Knockout of Two Putative Multicopper Oxidase Genes | Geszvain K et al. [34] | 2013 | 27 |
| Mn(II, III) oxidation and MnO2 mineralization by an expressed bacterial multicopper oxidase | Butterfield CN et al. [56] | 2013 | 23 |
| Diverse manganese(II)-oxidizing bacteria are prevalent in drinking water systems | Marcus DN et al. [57] | 2017 | 22 |
| Effective start-up biofiltration method for Fe, Mn, and ammonia removal and bacterial community analysis | Cai YN et al. [52] | 2015 | 22 |
| Extracellular haem peroxidases mediate Mn(II) oxidation in a marine Roseobacter bacterium via superoxide production | Andeer PF et al. [26] | 2015 | 21 |
| CotA, a multicopper oxidase from Bacillus pumilus WH4, exhibits manganese-oxidase activity | Su JM et al. [51] | 2013 | 21 |
| Formation of manganese oxides by bacterially generated superoxide | Learman DR et al. [27] | 2011 | 20 |
| Identification of a third Mn(II) oxidase enzyme in Pseudomonas putida GB-1 | Geszvain K et al. [39] | 2016 | 20 |
| Rank | Keyword | Frequency | Centrality |
|---|---|---|---|
| 1 | Mn(II) oxidation | 104 | 0.08 |
| 2 | oxidation | 92 | 0.16 |
| 3 | iron | 81 | 0.19 |
| 4 | identification | 65 | 0.10 |
| 5 | removal | 62 | 0.09 |
| 6 | multicopper oxidase | 61 | 0.04 |
| 7 | water | 42 | 0.06 |
| 8 | oxides | 42 | 0.07 |
| 9 | spores | 38 | 0.07 |
| 10 | mechanisms | 38 | 0.06 |
| 11 | manganese oxidation | 38 | 0.05 |
| 12 | biogenic manganese oxides | 34 | 0.09 |
| 13 | microbial community | 32 | 0.11 |
| 14 | bacteria | 32 | 0.08 |
| 15 | adsorption | 32 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, W.; Wang, H.; Wang, J.; Wang, Y.; Liu, Y.; Luo, Y.; He, M.; Cheng, S.; Mei, H.; He, J.; et al. Advances in Research on Bacterial Oxidation of Mn(II): A Visualized Bibliometric Analysis Based on CiteSpace. Microorganisms 2024, 12, 1611. https://doi.org/10.3390/microorganisms12081611
Mo W, Wang H, Wang J, Wang Y, Liu Y, Luo Y, He M, Cheng S, Mei H, He J, et al. Advances in Research on Bacterial Oxidation of Mn(II): A Visualized Bibliometric Analysis Based on CiteSpace. Microorganisms. 2024; 12(8):1611. https://doi.org/10.3390/microorganisms12081611
Chicago/Turabian StyleMo, Wentao, Hang Wang, Jianghan Wang, Yue Wang, Yunfei Liu, Yi Luo, Minghui He, Shuang Cheng, Huiting Mei, Jin He, and et al. 2024. "Advances in Research on Bacterial Oxidation of Mn(II): A Visualized Bibliometric Analysis Based on CiteSpace" Microorganisms 12, no. 8: 1611. https://doi.org/10.3390/microorganisms12081611
APA StyleMo, W., Wang, H., Wang, J., Wang, Y., Liu, Y., Luo, Y., He, M., Cheng, S., Mei, H., He, J., & Su, J. (2024). Advances in Research on Bacterial Oxidation of Mn(II): A Visualized Bibliometric Analysis Based on CiteSpace. Microorganisms, 12(8), 1611. https://doi.org/10.3390/microorganisms12081611






