Antibacterial and Antibiofilm Effects of L-Carnitine-Fumarate on Oral Streptococcal Strains Streptococcus mutans and Streptococcus sobrinus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds, Bacterial Strains, and Culture Conditions
2.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Studies
2.3. Kinetic Study of LC on Planktonic Form
2.4. Minimum Biofilm Inhibitory Concentration (MBIC) and Minimum Biofilm Biocidal Concentration (MBBC) Studies
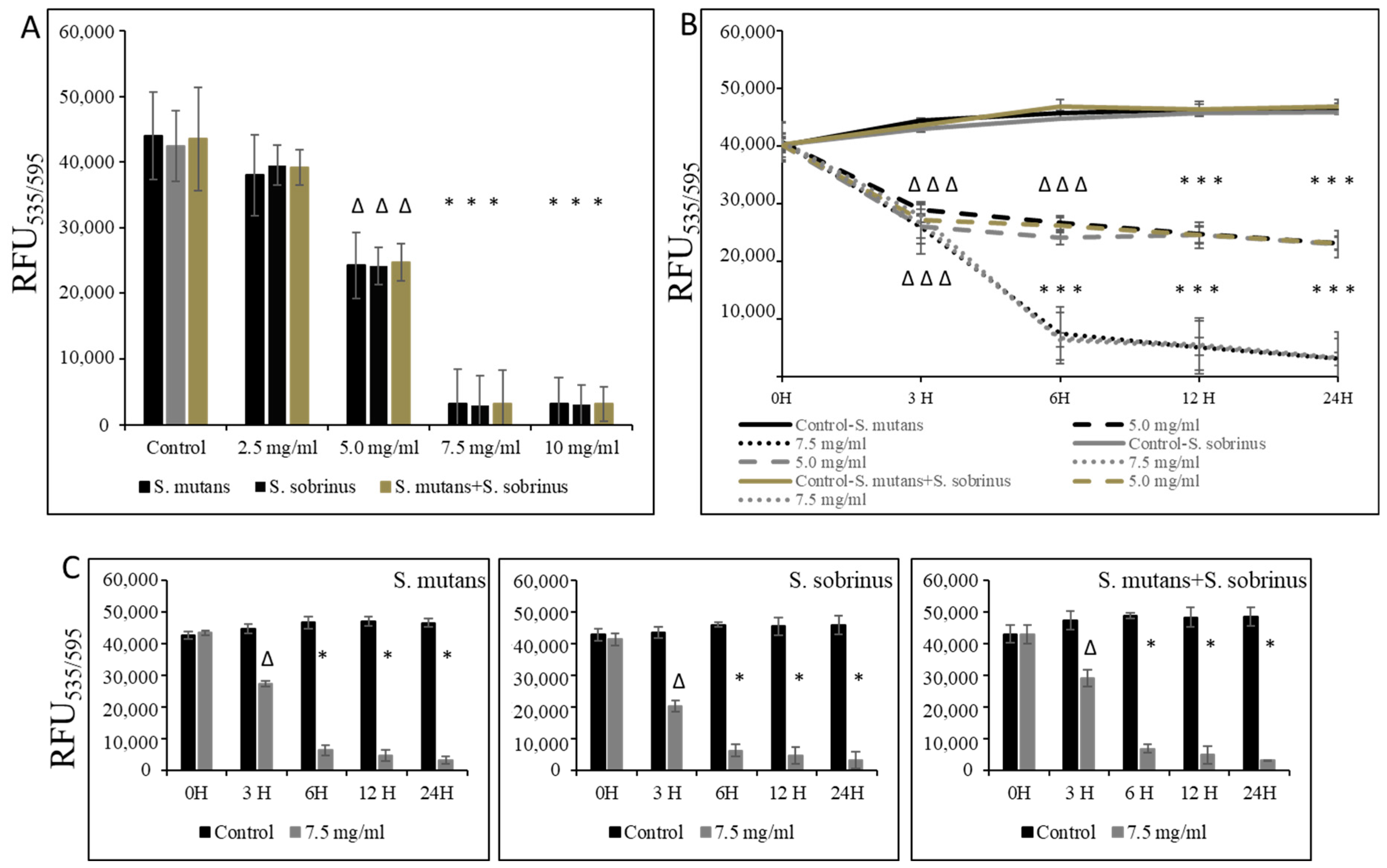
2.5. Kinetic Study of LC on Biofilm Formation
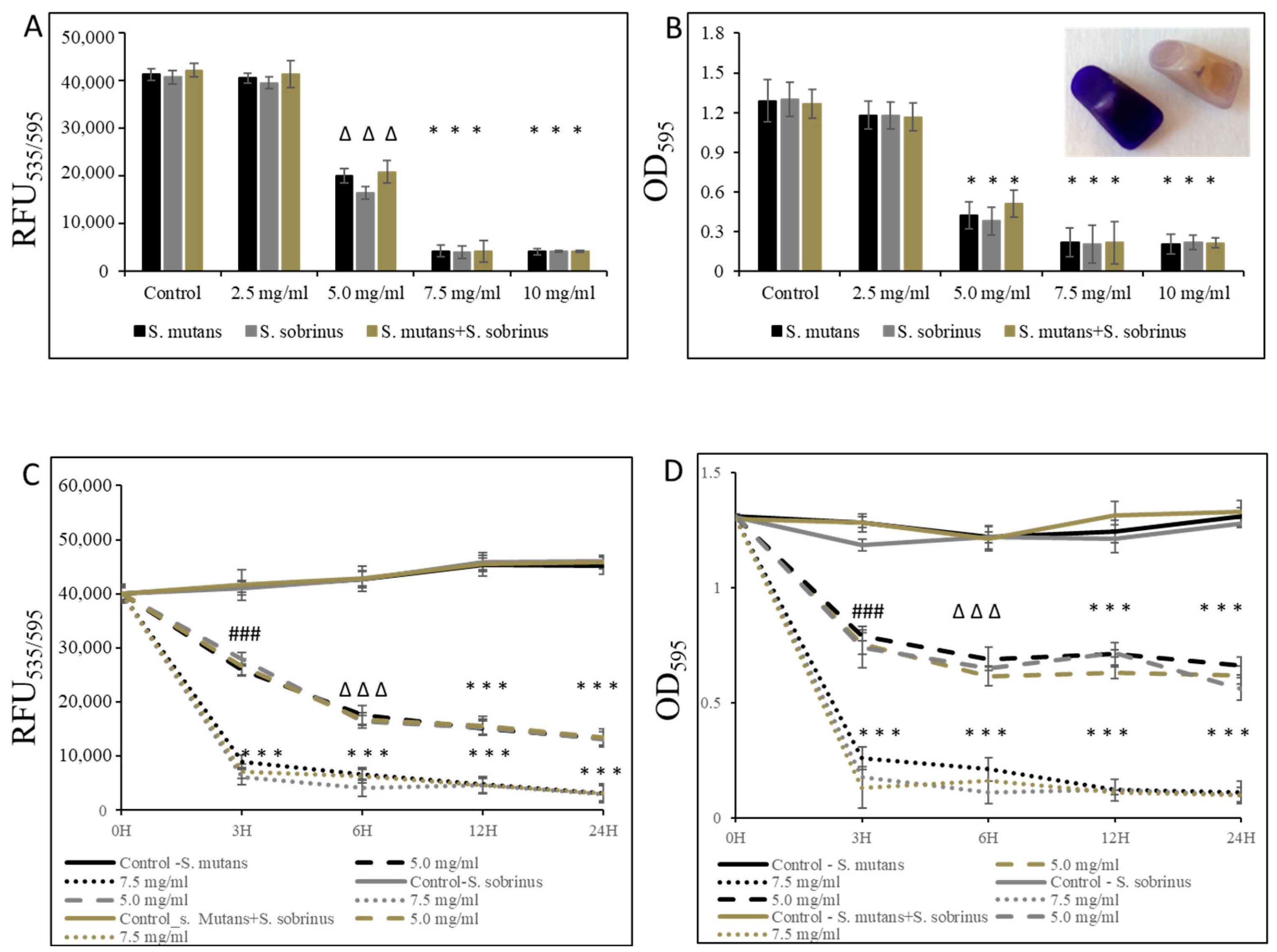
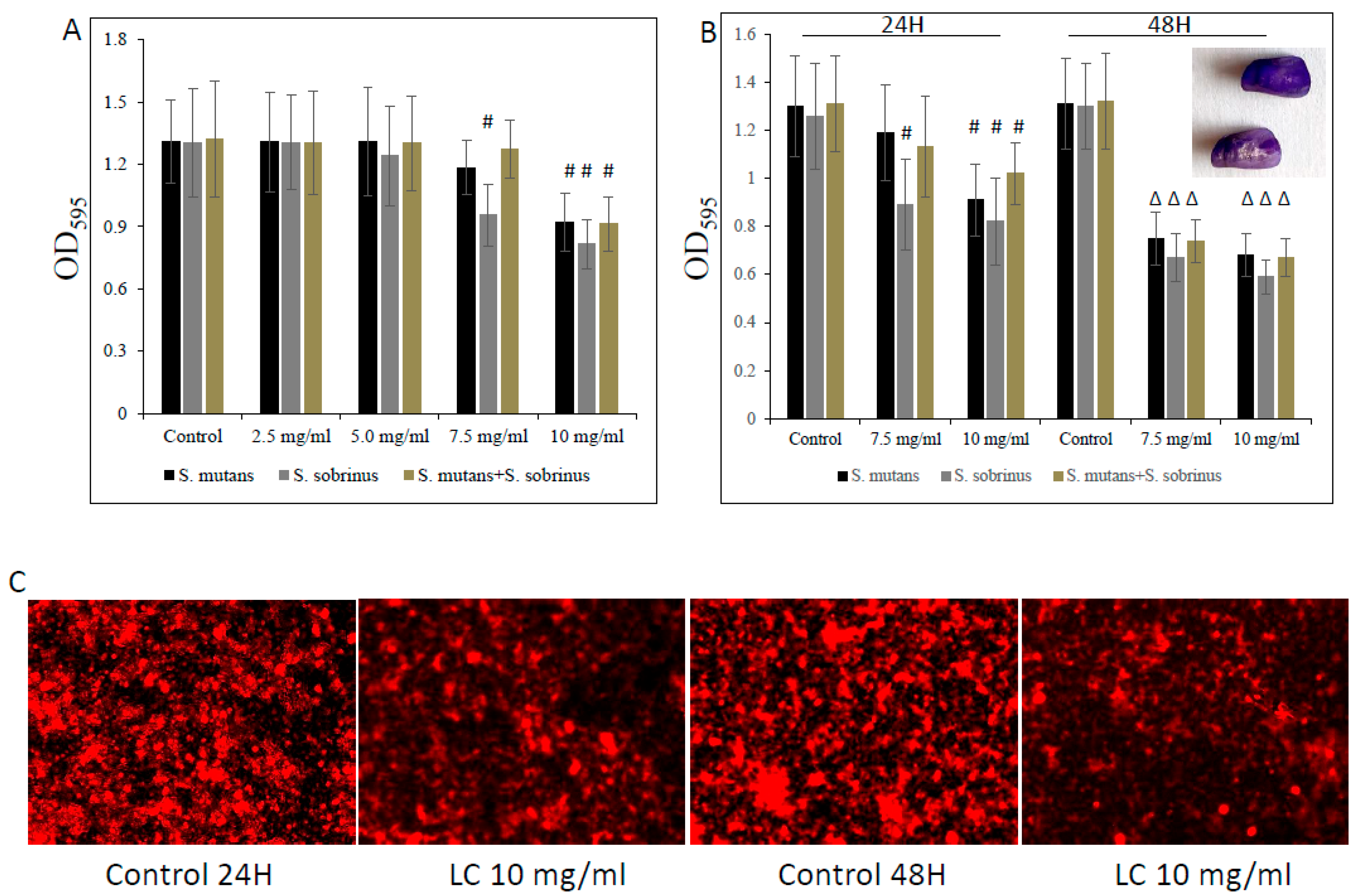
2.6. Biofilm Viability Assays
2.7. Crystal Violet Staining
2.8. The Phosphoenolpyruvate (PEP): Carbohydrate Phosphotransferase System (PTS) Assay
2.9. Qualitative Determination of EPS
2.10. Aciduricity Assay
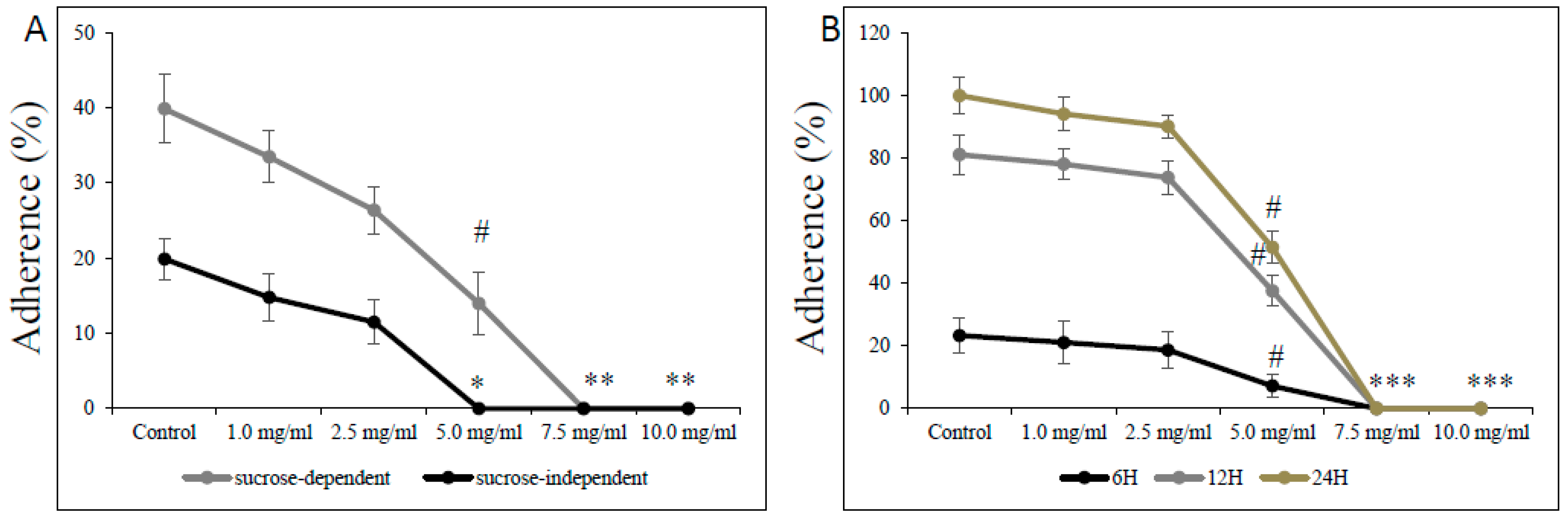
2.11. Adherence Assay
2.12. Statistical Analysis
3. Results
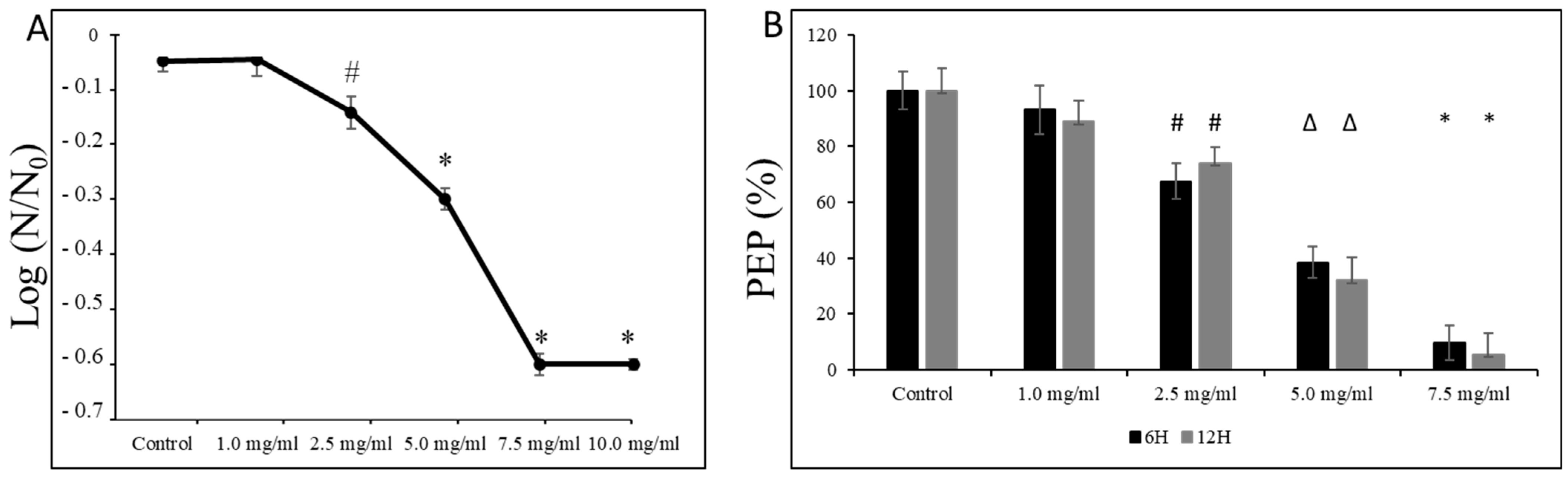
3.1. Bacteriostatic and Bactericidal Effect of L-Carnitine Forms on the Planktonic Form of Oral Streptococci
3.2. Bacteriostatic and Bactericidal Effect of L-Carnitine Forms on the Biofilm Form of Oral Streptococci
3.3. Effect of L-Carnitine-Fumarate on Biochemical and Physiological Processes of S. mutans UA159 Strain
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef]
- Nobre dos Santos, M.; Melo dos Santos, L.; Francisco, S.B.; Cury, J.A. Relationship among dental plaque composition, daily sugar exposure and caries in the primary dentition. Caries Res. 2002, 36, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Slade, H.D. Biology, immunology, and carcinogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Hamada, S. Studying biofilm formation of mutans streptococci. Methods Enzymol. 1999, 310, 513–523. [Google Scholar] [PubMed]
- Hamada, S.; Koga, T.; Ooshima, T. Virulence factors of Streptococcus mutans and dental caries prevention. J. Dent. Res. 1984, 63, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.H.; Hamilton, I.R. Survival of oral bacteria. Crit. Rev. Oral Biol. Med. 1998, 9, 54–85. [Google Scholar] [CrossRef] [PubMed]
- Burne, R.A. Oral streptococci… products of their environment. J. Dent. Res. 1998, 77, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Kuramitsu, H.K. Molecular genetic analysis of the virulence of oral bacterial pathogens: An historical perspective. Crit. Rev. Oral Biol. Med. 2003, 14, 331–344. [Google Scholar] [CrossRef]
- Yamashita, Y.; Bowen, W.H.; Burne, R.A.; Kuramitsu, H.K. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 1993, 61, 3811–3817. [Google Scholar] [CrossRef] [PubMed]
- Banas, J.A.; Vickerman, M.M. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 2003, 14, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Okahashi, N.; Takahashi, I.; Kanamoto, T.; Asakawa, H.; Iwaki, M. Surface hydrophobicity, adherence, and aggregation of cell surface protein antigen mutants of Streptococcus mutans serotype c. Infect. Immun. 1990, 58, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ooshima, T.; Matsumura, M.; Hoshino, T.; Kawabata, S.; Sobue, S.; Fujiwara, T. Contribution of three glucosyltransferases to sucrose-dependent adherence of Streptococcus mutans. J. Dent. Res. 2001, 80, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Tamasada, M.; Kawabata, S.; Fujiwara, T.; Hamada, S. Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. J. Dent. Res. 2004, 83, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Xiao, J.; Klein, M.I.; Jeon, J.G. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Koo, H. Structural organization and dynamics of exopolysaccharide matrix and microcolonies formation by Streptococcus mutans in biofilms. J. Appl. Microbiol. 2010, 108, 2103–2113. [Google Scholar] [PubMed]
- Lynch, D.J.; Fountain, T.L.; Mazurkiewicz, J.E.; Banas, J.A. Glucan-binding proteins are essential for shaping Streptococcus mutans biofilm architecture. FEMS Microbiol. Lett. 2007, 268, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Klein, M.I.; Heim, K.P.; Fan, Y.; Bitoun, J.P.; Ahn, S.-J.; Burne, R.A.; Koo, H.; Brady, J.L.; Wen, Z.T. Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J. Bacteriol. 2014, 196, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Meadows, J.M.; Wargo, M.J. Carnitine in bacterial physiology and metabolism. Microbiology 2015, 161, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Peluso, G.; Barbarisi, A.; Savica, V.; Reda, E.; Nicolai, R.; Benatti, P.; Calvani, M. Carnitine: An osmolyte that plays a metabolic role. J. Cell. Biochem. 2000, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. L-Carnitine: Therapeutic applications of a conditionally-essential amino acid. Altern. Med. Rev. 1998, 3, 345–360. [Google Scholar] [PubMed]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Bremer, J. Carnitine—Metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480. [Google Scholar] [CrossRef] [PubMed]
- Kleber, H.P. Bacterial carnitine metabolism. FEMS Microbiol. Lett. 1997, 147, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J.; Seim, H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 1998, 18, 39–61. [Google Scholar] [CrossRef] [PubMed]
- Olgun, A.; Kisa, O.; Yildiran, S.T.; Tezcan, S.; Akman, S.; Erbi, M.K. Antimicrobial efficacy of L-carnitine. Ann. Microbiol. 2004, 54, 95–101. [Google Scholar]
- Calvani, M.; Critelli, L.; Gallo, G.; Giorgi, F.; Gramiccioli, G.; Santaniello, M.; Scafetta, N.; Tinti, M.O.; De Angelis, F. L-carnitine esters as “soft”, broad-spectrum antimicrobial amphiphiles. J. Med. Chem. 1998, 41, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Savle, P.S.; Doncel, G.F.; Bryant, S.D.; Hubieki, M.P.; Robinette, R.G.; Gandour, R.D. Acyl-carnitine analogues as topical, microbicidal spermicides. Bioorg. Med. Chem. Lett. 1999, 9, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P.; Lewis, J.S.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J.; Limbago, B.; et al. M100. Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; Fifteenth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Sumera, W.; Niedzwiecki, A.; Rath, M. 10-undecynoic acid is a new anti-adherent agent killing biofilm of oral Streptococcus spp. PLoS ONE 2019, 14, e0214763. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.-M.; Klein, C.; Schwindt, D.; von Ohle, C. Metabolic activity of Streptococcus mutans biofilms and gene expression during exposure to xylitol and sucrose. Int. J. Oral Sci. 2014, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Vacca-Smith, A.M.; Bowen, W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hwang, G.; Liu, Y.; Gao, L.; Kilpatrick-Liverman, L.; Santarpia, P.; Zhou, X.; Koo, H. L-Arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J. Bacteriol. 2016, 198, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, S.-K.; Chang, K.-W.; Han, S.-K.; Yi, H.-K.; Jeon, J.-G. In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and virulence factors of Streptococcus mutans and Streptococcus sobrinus. Arch. Oral Biol. 2006, 51, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Svensäter, G.; Larsson, U.B.; Greif, E.C.; Cvitkovitch, D.G.; Hamilton, I.R. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 1997, 12, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Hamada, S.; Torii, M.; Kotani, S.; Tsuchitani, Y. Adherence of Streptococcus sanguis clinical isolates to smooth surfaces and interactions of the isolates with Streptococcus mutans glucosyltransferase. Infect. Immun. 1981, 32, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Asakawa, H.; Okahashi, N.; Hamada, S. Sucrose-dependent cell adherence and cariogenicity of serotype c Streptococcus mutans. J. Gen. Microbiol. 1986, 132, 2873–2883. [Google Scholar] [CrossRef] [PubMed]
- Rukayadi, Y.; Hwang, J.K. In vitro activity of xanthorrhizol against Streptococcus mutans biofilms. Lett. Appl. Microbiol. 2006, 42, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Vicari, E.; Calogero, A.E. Effects of treatment with carnitines in infertile patients with prostato-vesiculo-epididymitis. Hum. Reprod. 2001, 16, 2338–2342. [Google Scholar] [CrossRef] [PubMed]
- Vicari, E.; Rubino, C.; De Palma, A.; Longo, G.; Lauretta, M.; Consoli, S.; Arancio, A. Antioxidant therapeutic efficiency after the use of carnitine in infertile patients with bacterial or non-bacterial prostato-vesiculo-epididymitis. Arch. Ital. Urol. Androl. 2001, 73, 15–25. [Google Scholar]
- Winter, S.C.; Szabo-Aczel, S.; Curry, C.J.; Hutchinson, H.T.; Hogue, R.; Shug, A. Plasma carnitine deficiency. Clinical observations in 51 pediatric patients. Am. J. Dis. Child. 1987, 141, 660–665. [Google Scholar] [CrossRef]
- Rubin, M.R.; Volek, J.S.; Gómez, A.L.; Ratamess, N.A.; French, D.N.; Sharman, M.J.; Kraemer, W.J. Safety measures of L-carnitine L-tartrate supplementation in healthy men. J. Strength Cond. Res. 2001, 15, 486–490. [Google Scholar]
- Bellamine, A.; Durkee, S. Genotoxicity and subchronic oral toxicity studies of L-carnitine and L-carnitine L-tartrate. J. Drug Metab. Toxicol. 2021, 12, 253. [Google Scholar]
- Cao, Y.; Wang, Y.; Liu, C.; Wang, L.; Han, Z.; Wang, C. Comparison of pharmacokinetics of L-carnitine, acetyl-L-carnitine and propionyl-L-carnitine after single oral administration of L-carnitine in healthy volunteers. Clin. Investig. Med. 2009, 32, E13–E19. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Yang, X. High L-carnitine ingestion impairs liver function by disordering gut bacteria composition in mice. J. Agric. Food Chem. 2020, 68, 5707–5714. [Google Scholar] [CrossRef]
- Rajakovich, L.; Fu, B.; Bollenbach, M.; Balkus, E. Elucidation of an anaerobic pathway for metabolism of L-carnitine-derived γ-butyrobetaine to trimethylamine in human gut bacteria. Proc. Natl. Acad. Sci. USA 2021, 118, e2101498118. [Google Scholar] [CrossRef]
- Koeth, R.; Lam-Galvez, B.; Kirsop, J.; Wang, Z.; Levison, B.; Gu, X.; Copeland, M.; Bartlett, D.; Cody, D.; Dai, H.; et al. L-carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019, 129, 373–387. [Google Scholar] [CrossRef]
- Strathmann, M.; Wingender, J.; Flemming, H.C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods 2002, 50, 237–248. [Google Scholar] [CrossRef]

| Test Parameters | L-Carnitine-Fumarate (mg/mL) | ||||
|---|---|---|---|---|---|
| MIC90 | MBC90 | MBIC | MBBC | MBEC | |
| S. mutans UA159 | 6.0 | 7.0 | 7.5 | 10.0 | ns |
| S. sobrinus SL1 | 5.5 | 6.5 | 7.5 | 10.0 | ns |
| S. mutans + S. sobrinus UA159 + SL1 | 6.0 | 7.0 | 7.5 | 10.0 | ns |
| L-carnitine HCl (mg/mL) | |||||
| MIC90 | MBC90 | MBIC | MBBC | MBEC | |
| S. mutans UA159 | 6.0 | 7.0 | 7.5 | 10.0 | ns |
| S. sobrinus SL1 | 5.5 | 6.5 | 7.5 | 10.0 | ns |
| S. mutans + S. sobrinus UA159 + SL1 | 6.0 | 7.0 | 7.5 | 10.0 | ns |
| Test Parameters | Control | 2.5 mg/mL | 5.0 mg/mL | 7.5 mg/mL | 10.0 mg/mL |
|---|---|---|---|---|---|
| Dry weight (mg/biofilm) | 4.41 ± 0.38 | 4.35 ± 0.31 | 3.95 ± 0.34 | 3.62 ± 0.26 * | 3.51 ± 0.29 * |
| Total protein (mg/biofilm) | 2.69 ± 0.29 | 2.48 ± 0.31 | 2.17 ± 0.28 * | 1.93 ± 0.29 * | 1.84 ± 0.34 * |
| Soluble polysaccharides (mg/biofilm) | 0.37 ± 0.19 | 0.36 ± 0.18 | 0.33 ± 0.04 | 0.26 ± 0.08 * | 0.24 ± 0.03 * |
| Insoluble polysaccharides (mg/biofilm) | 1.19 ± 0.15 | 1.18 ± 0.16 | 0.12 ± 0.17 | 0.89 ± 0.11 * | 0.81 ± 0.12 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Antibacterial and Antibiofilm Effects of L-Carnitine-Fumarate on Oral Streptococcal Strains Streptococcus mutans and Streptococcus sobrinus. Microorganisms 2024, 12, 1613. https://doi.org/10.3390/microorganisms12081613
Goc A, Sumera W, Rath M, Niedzwiecki A. Antibacterial and Antibiofilm Effects of L-Carnitine-Fumarate on Oral Streptococcal Strains Streptococcus mutans and Streptococcus sobrinus. Microorganisms. 2024; 12(8):1613. https://doi.org/10.3390/microorganisms12081613
Chicago/Turabian StyleGoc, Anna, Waldemar Sumera, Matthias Rath, and Aleksandra Niedzwiecki. 2024. "Antibacterial and Antibiofilm Effects of L-Carnitine-Fumarate on Oral Streptococcal Strains Streptococcus mutans and Streptococcus sobrinus" Microorganisms 12, no. 8: 1613. https://doi.org/10.3390/microorganisms12081613
APA StyleGoc, A., Sumera, W., Rath, M., & Niedzwiecki, A. (2024). Antibacterial and Antibiofilm Effects of L-Carnitine-Fumarate on Oral Streptococcal Strains Streptococcus mutans and Streptococcus sobrinus. Microorganisms, 12(8), 1613. https://doi.org/10.3390/microorganisms12081613







