Exploring Oral and Vaginal Probiotic Solutions for Women’s Health from Puberty to Menopause: A Narrative Review
Abstract
:1. Introduction
2. Methods
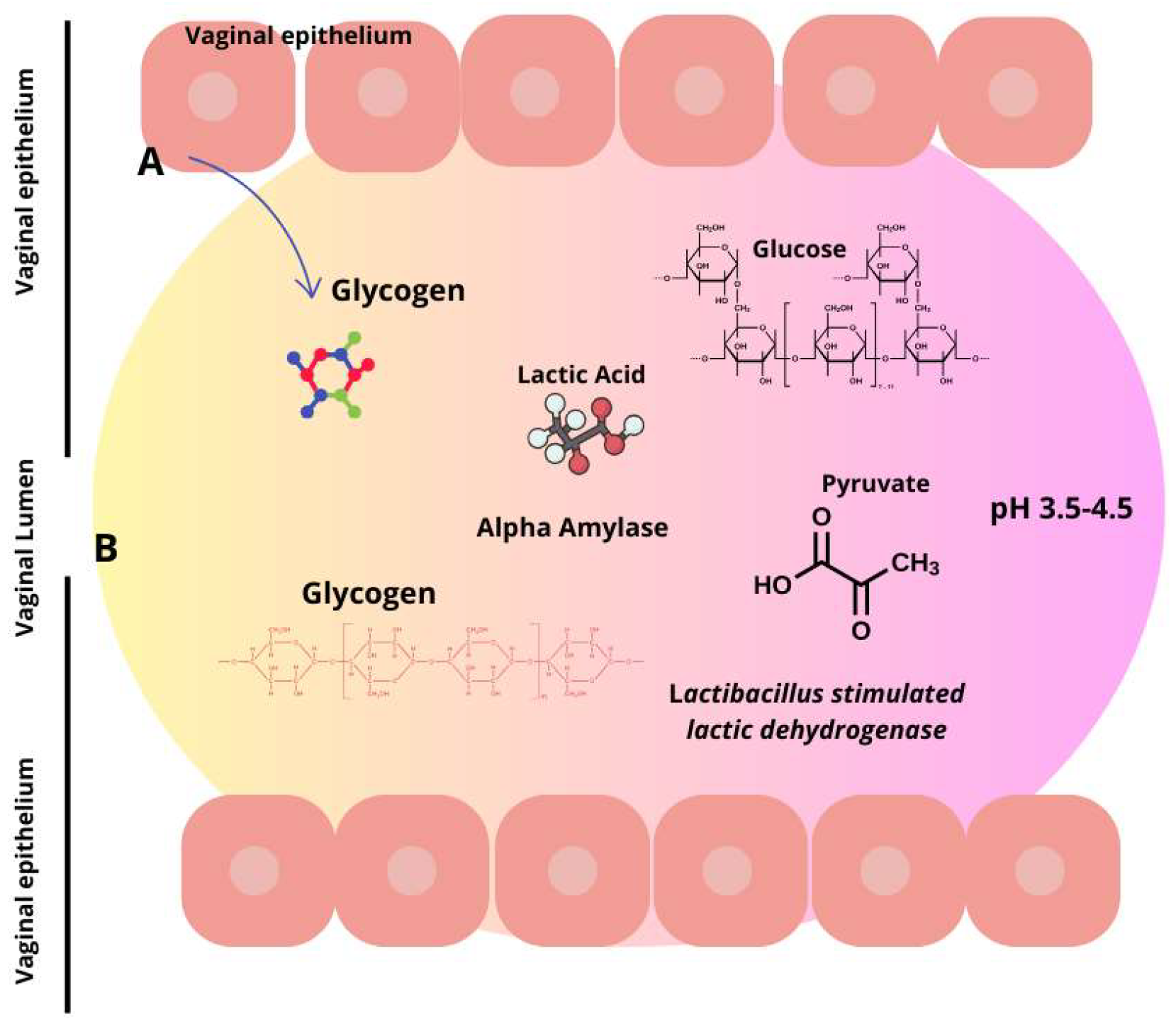
3. Understanding the Vaginal Microbiota and the Factors That Alter Its Composition
3.1. Fungi and Their Role in Vaginal Microbiota
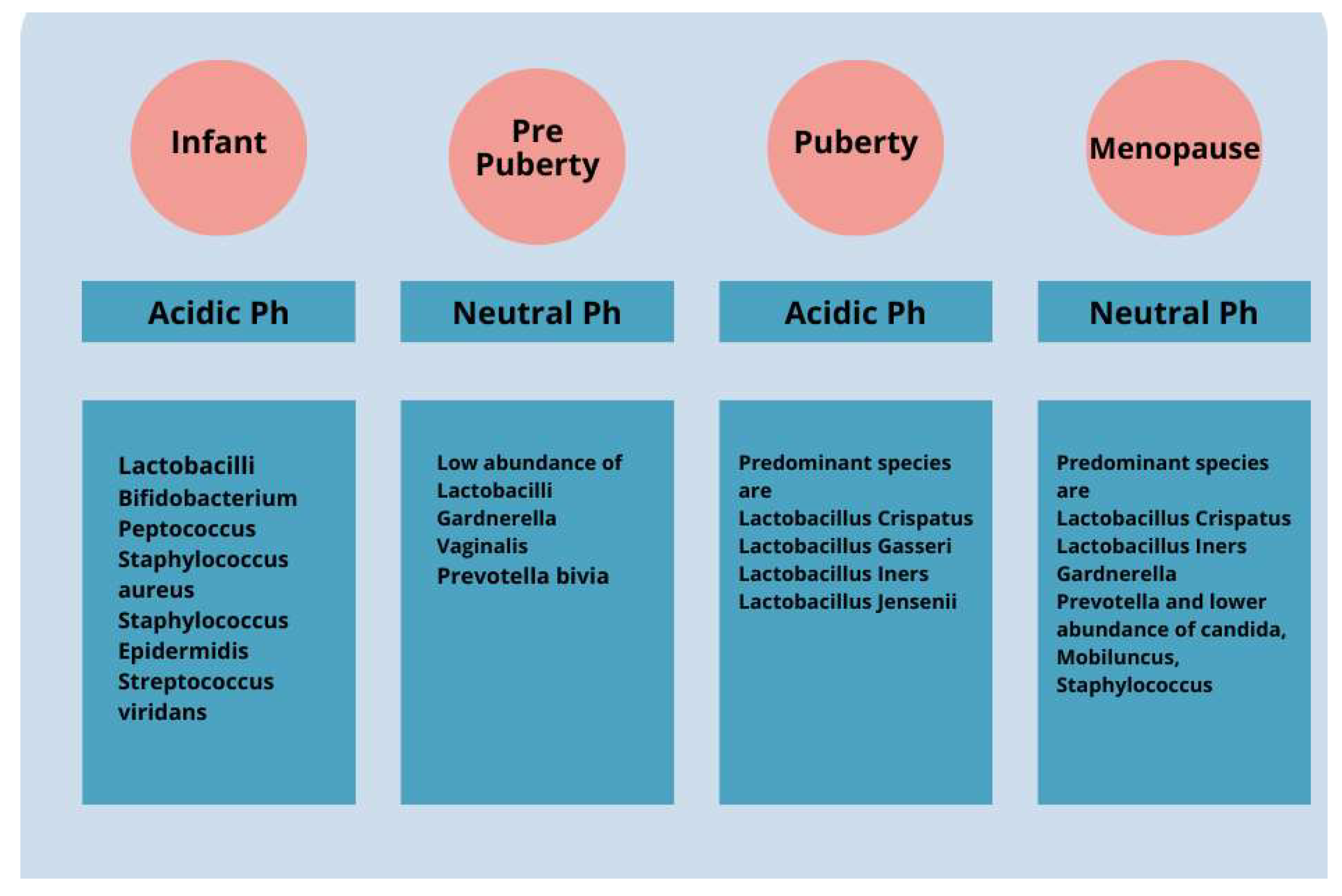
3.2. Changes in VMB Over a Woman’s Life
3.2.1. Childhood, Puberty, and Adolescence
3.2.2. Importance of Estrogen
3.2.3. Effects of Menopause on the Vaginal Microbiota
3.3. Effects of Hormonal Contraceptives on the Vaginal Microbiota
3.4. Effects of Diet and Socioeconomic Factors on Vaginal Microbiota
3.5. Impact of Urbanization on the Vaginal Microbiota
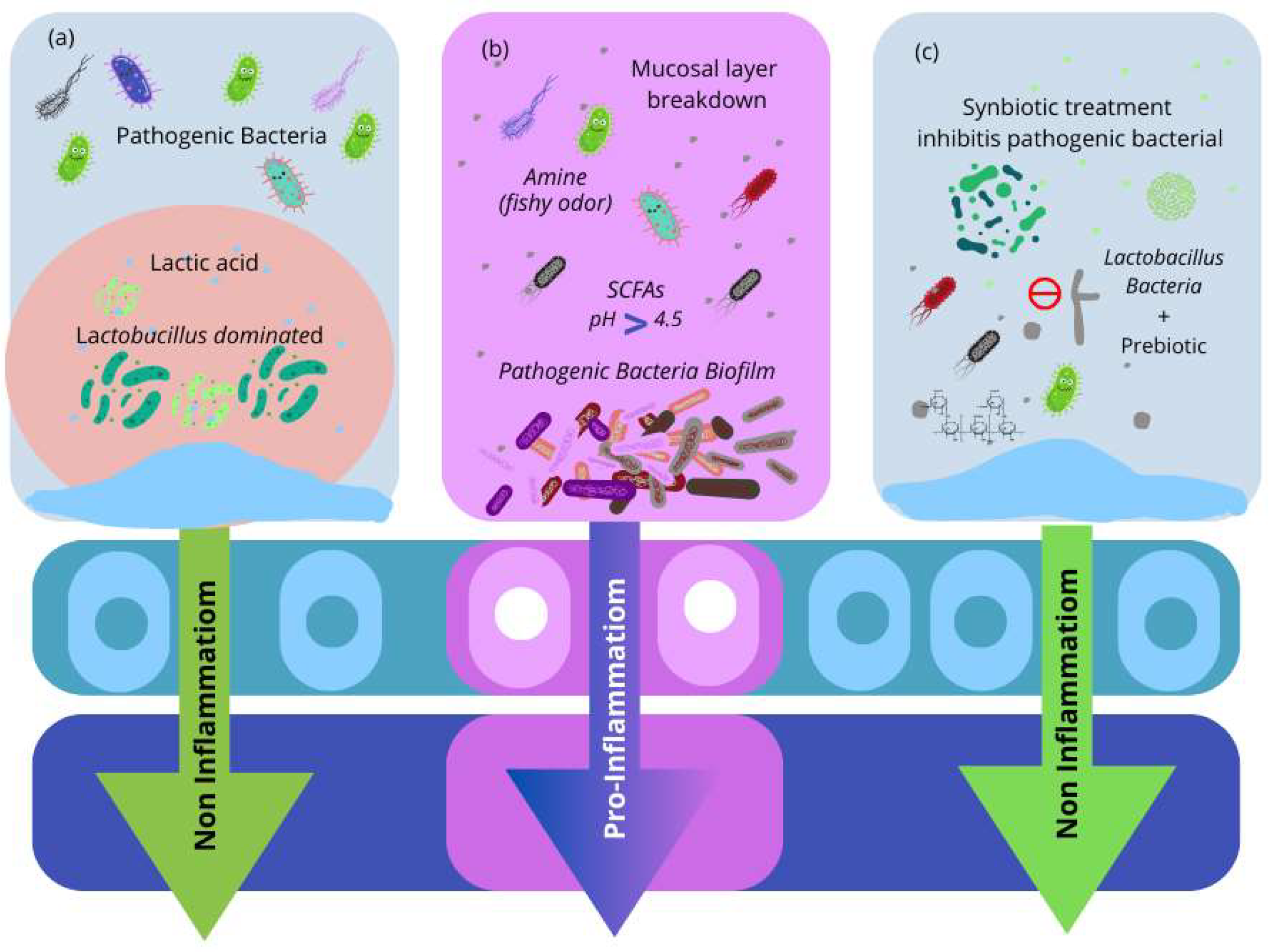
3.6. Vaginal Infections and Antimicrobial Therapy
4. Probiotics
4.1. Forms of Probiotic Delivery
Advantages of Nanosystems in Probiotic Delivery
4.2. L. Crispatus: Role in Vaginal Health
4.3. Oral and Vaginal Probiotic Effects on VMB Composition
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human microbiome: An academic update on human body site specific surveillance and its possible role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef] [PubMed]
- Diop, K.; Cadoret, F.; Nguyen, T.T.; Baudoin, J.-P.; Armstrong, N.; Raoult, D.; Bretelle, F.; Fournier, P.-E.; Fenollar, F. Vaginimicrobium propionicum gen. nov., sp. nov., a novel propionic acid bacterium derived from human vaginal discharge. Int. J. Syst. Evol. Microbiol. 2020, 70, 4091–4097. [Google Scholar] [CrossRef] [PubMed]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Salliss, M.E.; Farland, L.V.; Mahnert, N.D.; Herbst-Kralovetz, M.M. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod. Update 2021, 28, 92–131. [Google Scholar] [CrossRef] [PubMed]
- Venneri, M.A.; Franceschini, E.; Sciarra, F.; Rosato, E.; D’Ettorre, G.; Lenzi, A. Human genital tracts microbiota: Dysbiosis crucial for infertility. J. Endocrinol. Investig. 2022, 45, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Punzón-Jiménez, P.; Labarta, E. The impact of the female genital tract microbiome in women health and reproduction: A review. J. Assist. Reprod. Genet. 2021, 38, 2519–2541. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef] [PubMed]
- Fransson, E.; Gudnadottir, U.; Hugerth, L.W.; Itzel, E.W.; Hamsten, M.; Boulund, F.; Pennhag, A.; Du, J.; Schuppe-Koistinen, I.; Brusselaers, N.; et al. Cohort profile: The Swedish Maternal Microbiome project (SweMaMi)—Assessing the dynamic associations between the microbiome and maternal and neonatal adverse events. BMJ Open 2022, 12, e065825. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, K.; Kozioł, M.M.; Kimber-Trojnar, Ż.; Kępa, J.; Satora, M.; Rekowska, A.K.; Leszczyńska-Gorzelak, B. Premature rupture of membranes and changes in the vaginal microbiome—Probiotics. Reprod. Biol. 2024, 24, 100899. [Google Scholar] [CrossRef] [PubMed]
- Mancilla, V.; Jimenez, N.R.; Bishop, N.S.; Flores, M.; Herbst-Kralovetz, M.M. The Vaginal Microbiota, Human Papillomavirus Infection, and Cervical Carcinogenesis: A Systematic Review in the Latina Population. J. Epidemiol. Glob. Health 2024, 2, 480–497. [Google Scholar] [CrossRef] [PubMed]
- Pagar, R.; Deshkar, S.; Mahore, J.; Patole, V.; Deshpande, H.; Gandham, N.; Mirza, S.; Junnarkar, M.; Nawani, N. The microbial revolution: Unveiling the benefits of vaginal probiotics and prebiotics. Microbiol. Res. 2024, 286, 127787. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.; Chew, S.Y.; Than, L.T. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Coudray, M.S.; Madhivanan, P. Bacterial vaginosis-A brief synopsis of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Hibberd, A.A.; Reimari, J.; Junnila, J.; Yeung, N.; Maukonen, J.; Crawford, G.; Ouwehand, A.C. Recovery of vaginal microbiota after standard treatment for bacterial vaginosis infection: An observational study. Microorganisms 2020, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Tidbury, F.D.; Langhart, A.; Weidlinger, S.; Stute, P. Non-antibiotic treatment of bacterial vaginosis—A systematic review. Arch. Gynecol. Obstet. 2021, 303, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, T.; Li, Y.; Zhang, T.; Wang, Q.; He, J.; Wang, L.; Li, L.; Yang, N.; Fang, Y. Probiotics for the treatment of women with bacterial vaginosis: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Pharmacol. 2019, 864, 172660. [Google Scholar] [CrossRef] [PubMed]
- Basavaprabhu, H.N.; Sonu, K.S.; Prabha, R. Mechanistic insights into the action of probiotics against bacterial vaginosis and its mediated preterm birth: An overview. Microb. Pathog. 2020, 141, 104029. [Google Scholar] [CrossRef] [PubMed]
- Tomás, M.; Palmeira-de-Oliveira, A.; Simões, S.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Bacterial vaginosis: Standard treatments and alternative strategies. Int. J. Pharm. 2020, 587, 119659. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Li, D. The role of probiotics in vaginal health. Front. Cell Infect Microbiol. 2022, 12, 963868. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy Vaginal Microbiota and Influence of Probiotics Across the Female Life Span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef] [PubMed]
- Lepargneur, J.P. Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann. Biol. Clin. 2016, 74, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Cardile, V.; Petronio, G.; Petronio, G.; Pino, P.; Furneri, P.M. Biological properties and production of bacteriocins like inhibitory substances (BLISs) from Lactobacillus crispatus and Lactobacillus gasseri. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8171–8179. [Google Scholar]
- Pramanick, R.; Mayadeo, N.; Warke, H.; Begum, S.; Aich, P.; Aranha, C. Vaginal microbiota of asymptomatic bacterial vaginosis, diagnosed by Nugent score, associated with specific host immune response. Indian J. Med. Microbiol. 2019, 37, 392–399. [Google Scholar]
- Han, Y.; Ren, Q.L. Does probiotics work for bacterial vaginosis and vulvovaginal candidiasis. Curr. Opin. Pharmacol. 2021, 61, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.X.; Li, T.; Zhang, X.; Wang, S.X.; Liu, Z.H. Lactobacillus crispatus modulates vaginal epithelial cell innate response to candida albicans. Chin. Med. J. 2017, 130, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.S.; Gaydos, C.A. Molecular diagnosis of bacterial vaginosis: An update. J. Clin. Microbiol. 2018, 56, e00342-18. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Brooks, J.P.; Serrano, M.G.; Sheth, N.U.; Girerd, P.H.; Edwards, D.J.; Strauss, J.F., III; Vaginal Microbiome Consortium; Jefferson, K.K.; Buck, G. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014, 160, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Van Der Veer, C.; Van Houdt, R.; Alberts, C.J.; De Vries, H.J.; Bruisten, S.M.; Snijder, M.B.; Prins, M.; Geerlings, S.E.; van der Loeff, M.F.S.; et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, The Netherlands. PLoS ONE 2017, 12, e0181135. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, Y.; Miller, D.; Rehman, N.O.; Cheng, X.; Yeo, J.-Y.; Joe, B.; Hill, J.W. Microbial reconstitution reverses early female puberty induced by maternal high-fat diet during lactation. Endocrinology 2020, 161, bqz041. [Google Scholar] [CrossRef]
- Huang, C.; Liu, H.; Yang, W.; Li, Y.; Wu, B.; Chen, J.; Yang, Z.; Liao, C.; Liu, L.; Zhang, X. Distinct gut microbiota structure and function of children with idiopathic central and peripheral precocious puberty. Int. J. Endocrinol. 2022, 2022, 7175250. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Tan, B.; Yi, Q.; Liu, H.; Deng, H.; Chen, Y.; Wang, R.; Tian, J.; Zhu, J. Gut microbiota and its derived SCFAs regulate the HPGA to reverse obesity-induced precocious puberty in female rats. Front. Endocrinol. 2022, 13, 1051797. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, J.; Yang, Z.; Feng, X.; Li, J.; Li, D.; Huang, M.; Li, Y.; Qiu, M.; Lu, X. The association of gut microbiota with idiopathic central precocious puberty in girls. Front. Endocrinol. 2020, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Bo, T.; Liu, M.; Tang, L.; Lv, J.; Wen, J.; Wang, D. Effects of high-fat diet during childhood on precocious puberty and gut microbiota in mice. Front. Microbiol. 2022, 13, 930747. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L.; Lau, R.J. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin. Infect. Dis. 1997, 25 (Suppl. S2), S123–S126. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Driussi, S.; De Santo, D.; Penacchioni, P.; Iannicelli, T.; Lanzafame, P.; De Seta, F.; Quadrifoglio, F.; de Aloysio, D.; Guaschino, S. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J. Clin. Microbiol. 2002, 40, 2147–2152. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Seo, H.; Rahim, M.A.; Lee, S.; Kim, Y.S.; Song, H.Y. Changes in the microbiome of vaginal fluid after menopause in Korean women. J. Microbiol. Biotechnol. 2021, 31, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Caretto, M.; Giannini, A.; Russo, E.; Simoncini, T. Preventing urinary tract infections after menopause without antibiotics. Maturitas 2017, 99, 43–46. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Auriemma, R.S.; Vetrani, C.; Cataldi, M.; Frias-Toral, E.; Pugliese, G.; Camajani, E.; Savastano, S.; Colao, A.; et al. Probiotics and Prebiotics: Any Role in Menopause-Related Diseases? Curr. Nutr. Rep. 2023, 12, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, H.; Wang, G.; Zhao, J.; Chen, H.; Lu, X.; Chen, W. Lactic Acid Bacteria: A Promising Tool for Menopausal Health Management in Women. Nutrients 2022, 14, 4466. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Ahannach, S.; Gehrmann, T.; Wittouck, S.; Eilers, T.; Oerlemans, E.; Condori, S.; Dillen, J.; Spacova, I.; Vander Donck, L.; et al. A citizen-science-enabled catalogue of the vaginal microbiome and associated factors. Nat. Microbiol. 2023, 8, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.S.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. mSphere 2020, 5, e00823-20. [Google Scholar] [CrossRef] [PubMed]
- Krog, M.C.; Hugerth, L.W.; Fransson, E.; Bashir, Z.; Nyboe Andersen, A.; Edfeldt, G.; Engstrand, L.; Schuppe-Koistinen, I.; Nielsen, H.S. The healthy female microbiome across body sites: Effect of hormonal contraceptives and the menstrual cycle. Hum. Reprod. 2022, 37, 1525–1543. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.; Rantsi, T.; Virtanen, A.; Kervinen, K.; Nieminen, P.; Kalliala, I.; Salonen, A. Vaginal Microbiota Composition Correlates between Pap Smear Microscopy and Next Generation Sequencing and Associates to Socioeconomic Status. Sci. Rep. 2019, 9, 7750. [Google Scholar] [CrossRef] [PubMed]
- Dabee, S.; Tanko, R.F.; Brown, B.P.; Bunjun, R.; Balle, C.; Feng, C.; Konstantinus, I.N.; Jaumdally, S.Z.; Onono, M.; Nair, G.; et al. Comparison of Female Genital Tract Cytokine and Microbiota Signatures Induced by Initiation of Intramuscular DMPA and NET-EN Hormonal Contraceptives—A Prospective Cohort Analysis. Front. Immunol. 2021, 12, 760504. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; Łaniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. eBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Taghinezhad, S.S.; Keyvani, H.; Bermúdez-Humarán, L.G.; Donders, G.G.G.; Fu, X.; Mohseni, A.H. Twenty years of research on HPV vaccines based on genetically modified lactic acid bacteria: An overview on the gut-vagina axis. Cell Mol. Life Sci. 2021, 78, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Borgogna, J.C.; Shardell, M.D.; Santori, E.K.; Nelson, T.M.; Rath, J.M.; Glover, E.D.; Ravel, J.; Gravitt, P.E.; Yeoman, C.J.; Brotman, R.M. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: A cross-sectional analysis. BJOG 2020, 127, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.P.; Sun, T.T.; Wang, S.; Fan, Q.B.; Shi, H.H.; Zhu, L.; Lang, J.H. Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Int. J. Gynecol. Cancer 2019, 29, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Criscuolo, A.A.; Dei Giudici, A.; Senatori, R.; Sesti, F.; Ciotti, M.; Piccione, E. Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: Results of a preliminary, uncontrolled, open trial. Minerva Obstet. Gynecol. 2021, 73, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, Y.; Li, R.; Chen, X.; Wan, L.; Zhao, W. Associations of cervicovaginal lactobacilli with high-risk human papillomavirus infection, cervical intraepithelial neoplasia, and cancer: A systematic review and meta-analysis. J. Infect. Dis. 2019, 220, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.T.; Chen, H.L.; Wang, C.F.; Yang, G.L.; Han, S.M.; Zhang, S.L. Depiction of vaginal microbiota in women with high-risk human papillomavirus infection. Front. Public Health 2020, 8, 587298. [Google Scholar] [CrossRef] [PubMed]
- Abdolalipour, E.; Mahooti, M.; Salehzadeh, A.; Torabi, A.; Mohebbi, S.R.; Gorji, A.; Ghaemi, A. Evaluation of the antitumor immune responses of probiotic Bifidobacterium bifidum in human papillomavirus-induced tumor model. Microb. Pathog. 2020, 145, 104207. [Google Scholar] [CrossRef] [PubMed]
- Macklaim, J.M.; Clemente, J.C.; Knight, R.; Gloor, G.B.; Reid, G. Changes in vaginal microbiota flowing antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 2015, 26, 27799. [Google Scholar] [PubMed]
- Selis, N.N.; Oliveira, H.B.M.; Souza, C.L.S.; Almeida, J.B.; Andrade, Y.; Silva, L.S.C.; Romano, C.C.; Rezende, R.P.; Yatsuda, R.; Uetanabaro, A.P.; et al. Lactobacillus plantarum Lp62 exerts probiotic effects against Gardnerella vaginalis ATCC 49154 in bacterial vaginosis. Lett. Appl. Microbiol. 2021, 73, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Yi, N. A systematic review and meta-analysis on the efficacy of probiotics for bacterial vaginosis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 90–98. [Google Scholar] [PubMed]
- Mastromarino, P.; Macchia, S.; Meggiorini, L.; Trinchieri, V.; Mosca, L.; Perluigi, M.; Midulla, C. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009, 15, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, H.; Henyk, N.; Maliuk, V.; Khyzhnyak, T.; Tynna, Y.; Filipiuk, I.; Veresniuk, N.; Zubrytska, L.; Quintens, J.; Richir, K.; et al. Oral intake of lactobacilli can be helpful in symptomatic bacterial vaginosis: A randomized clinical study. J. Low Genit Tract Dis. 2020, 24, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, X.; Chen, W.; Luo, Y.; Yuan, L.; Xia, Y.; Nelson, K.E.; Huang, S.; Zhang, S.; Wang, Y.; et al. The restoration of the vaginal microbiota after treatment for bacterial vaginosis with metronidazole or probiotics. Microb. Ecol. 2013, 65, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kakkar, V.; Bhushan, I. Crosstalk between vaginal microbiome and female health: A review. Microb. Pathog. 2019, 136, 103696. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L.; Zariffard, M.R.; Cohen, M.H.; Spear, G.T. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Scapaticci, M.; Da Rin, G.; Bartolini, A. Comparison between a novel molecular tool and conventional methods for diagnostic classification of bacterial vaginosis: Is integration of the two approaches necessary for a better evaluation? New Microbiol. 2020, 43, 121–126. [Google Scholar] [PubMed]
- Muscariello, L.; De Siena, B.; Marasco, R. Lactobacillus Cell Surface Proteins Involved in Interaction with Mucus and Extracellular Matrix Components. Curr. Microbiol. 2020, 77, 3831–3841. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.S.; Yan, T.R.; Chen, J.Y. Treating vaginitis with probiotics in non-pregnant females: A systematic review and meta-analysis. Exp. Ther. Med. 2020, 20, 3749–3765. [Google Scholar] [CrossRef] [PubMed]
- Lyra, A.; Ala-Jaakkola, R.; Yeung, N.; Datta, N.; Evans, K.; Hibberd, A.; Lehtinen, M.J.; Forssten, S.D.; Ibarra, A.; Pesonen, T.; et al. A Healthy Vaginal Microbiota Remains Stable during Oral Probiotic Supplementation: A Randomised Controlled Trial. Microorganisms 2023, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Mändar, R.; Sõerunurk, G.; Štšepetova, J.; Smidt, I.; Rööp, T.; Kõljalg, S.; Saare, M.; Ausmees, K.; Le, D.D.; Jaagura, M.; et al. Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: A randomised double-blind placebo controlled clinical trial. Benef. Microbes 2023, 14, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Strus, M.; Kucharska, A.; Kukla, G.; Brzychczy-Włoch, M.; Maresz, K.; Heczko, P.B. The In Vitro Activity of Vaginal Lactobacillus with Probiotic Properties against Candida. Infect. Dis. Obstet. Gynecol. 2005, 13, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Vujic, G.; Jajac Knez, A.; Despot Stefanovic, V.; Kuzmic Vrbanovic, V. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: A double-blind, randomized, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ya, W.; Reifer, C.; Miller, L.E. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: A double-blind, randomized, placebo-controlled study. Am. J. Obstet. Gynecol. 2010, 203, 120.e1–120.e6. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.; Ruiz, F.; Giordano, W.; Barberis, I.L. Vaginal colonization and activity of the probiotic bacterium Lactobacillus fermentum L23 in a murine model of vaginal tract infection. J. Med. Microbiol. 2010, 59, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Chang, Y.-Y.; Chang, W.-C.; Lin, H.-C.; Wang, M.-H.; Lin, W.-C.; Chiu, T.-H. Oral Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 to reduce Group B Streptococcus colonization in pregnant women: A randomized controlled trial Taiwan. J. Obstet. Gynecol. 2016, 55, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Vicariotto, F.; Del Piano, M.; Mogna, L.; Mogna, G. Effectiveness of the association of 2 probiotic strains formulated in a slow release vaginal product, in women affected by vulvovaginal candidiasis: A pilot study. J. Clin. Gastroenterol. 2012, 46, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Vicariotto, F.; Mogna, L.; Del Piano, M. Effectiveness of the Two Microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, Formulated in Slow-release Vaginal Tablets, in Women Affected by Bacterial Vaginosis: A Pilot Study. J. Clin. Gastroenterol. 2014, 48, S106–S112. [Google Scholar] [CrossRef]
- Gille, C.; Böer, B.; Marschal, M.; Urschitz, M.S.; Heinecke, V.; Hund, V.; Speidel, S.; Tarnow, I.; Mylonas, I.; Franz, A.; et al. Effect of probiotics on vaginal health in pregnancy. EFFPRO, a randomized controlled trial. Am. J. Obstet. Gynecol. 2016, 215, e1–e608. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, R.; Mastromarino, P.; Ramalaxmi, B.A.; Balakrishna, N.V.; Sesikeran, B. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: A randomized, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3097–3105. [Google Scholar] [CrossRef]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.M.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.; et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG 2020, 127, 275–284. [Google Scholar] [CrossRef]
- Stojanovic, N.; Plećaš, D.; Plešinac, S. Normal vaginal flora, disorders and application of probiotics in pregnancy. Arch. Gynecol. Obstet. 2012, 286, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Farr, A.; Sustr, V.; Kiss, H.; Rosicky, I.; Graf, A.; Makristathis, A.; Foessleitner, P.; Petricevic, L. Oral probiotics to reduce vaginal group B streptococcal colonization in late pregnancy. Sci. Rep. 2020, 10, 19745. [Google Scholar] [CrossRef] [PubMed]
| Probiotic Strains | Diseases | Key Characteristics | Ref | |
|---|---|---|---|---|
| L. crispatus (DSM32717 and DSM32720) | BV | Increased vaginal lactobacilli and reduced BV-associated bacteria and VVC symptoms | Age = 18–50 years; BV (n = 89) and VVC (n = 93) patients | [70] |
| L. crispatus (DSM32720, DSM32718 and DSM32716 | VVC | |||
| Lactobacillus strains | VD | Defensive role in stabilizing and modulating vaginal microbiota | Age: 18–40 years; pre-menopausal women | [71] |
| L. rhamnosus GR-1, L. reuteri RC-14 | BV | Restored balance of vaginal microbiota in 61.5% of patients without side effects | Age: women older than 18 years and detected with vaginal infections (n = 544) | [72] |
| L. rhamnosus, L. acidophilus, Streptococcus thermophilus | BV | Lowered incidence of BV and G. vaginalis recurrence after treatment | Age: 18–55 years old with recurrent history of BV (n = 120) | [73] |
| Genital infections | Complete inhibition of pathogen proliferation as a natural alternative treatment | Age: 18–40 years; healthy, non-pregnant, pre-menopausal women | [74] | |
| L. rhamnosus GR-1 L. reuteri RC-14 | GBR infection | Dramatically reduced GBS colonization rates during pregnancy | Pregnant women at 35–37 weeks of gestation with a positive GBS screening (n = 110) | [75] |
| L. fermentum LF15 L. plantarum LP01 | BV | Its primary function was to establish a mechanical barrier on the surface of the vaginal mucosa to prevent Gardnerella from developing. Long-term physiological defense | Age: 18–50 years; pre-menopausal, non-pregnant women (n = 34) | [76] |
| L. fermentum LF10, L. fermentum LF11, L. acidophilus LA02 | VVC | Long-term physiological resistance by colonizing vaginal mucosa against pathogens | Age: 23–64 years; menopausal women (n = 30) | [77] |
| (a) L. rhamnosus, GR-1 (b) L. reuteri, RC-14 | Pre-term delivery | Supported vaginal microbiome during pregnancy | Women with less than 12 completed weeks of pregnancy (n = 320) | [78] |
| L. rhamnosus GR-1 L. reuteri RC-14 | BV | The vaginal microbiome was unaffected by oral probiotics administered in the early stages of pregnancy | Women between 9 and 14 weeks of pregnancy; verified by an ultrasound scan (n = 366) | [79] |
| L. brevis CD2, L. salivarius subsp. salicinius, L. plantarum | BV | Lactobacilli therapy resulted in a substantial decrease in IL-1 and IL-6 vaginal cytokines | Age: 20–40 years; with history of BV Non-HIV, non-pregnant (n = 67) | [80] |
| Lactobacillus crispatus | BV and VVC | Significantly increased lactobacilli count in the vagina while the mean proportion of some BV-related bacteria decreased. Oral and vaginal capsules lowered the amount of discharge and itching/irritation | Age: 18–50 years; BV (n = 89) and VVC (n = 93) patients | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, M.; D’Urso, F.; Ciccarese, G.; Di Gaudio, F.; Broccolo, F. Exploring Oral and Vaginal Probiotic Solutions for Women’s Health from Puberty to Menopause: A Narrative Review. Microorganisms 2024, 12, 1614. https://doi.org/10.3390/microorganisms12081614
Romeo M, D’Urso F, Ciccarese G, Di Gaudio F, Broccolo F. Exploring Oral and Vaginal Probiotic Solutions for Women’s Health from Puberty to Menopause: A Narrative Review. Microorganisms. 2024; 12(8):1614. https://doi.org/10.3390/microorganisms12081614
Chicago/Turabian StyleRomeo, Marcello, Fabiana D’Urso, Giulia Ciccarese, Francesca Di Gaudio, and Francesco Broccolo. 2024. "Exploring Oral and Vaginal Probiotic Solutions for Women’s Health from Puberty to Menopause: A Narrative Review" Microorganisms 12, no. 8: 1614. https://doi.org/10.3390/microorganisms12081614
APA StyleRomeo, M., D’Urso, F., Ciccarese, G., Di Gaudio, F., & Broccolo, F. (2024). Exploring Oral and Vaginal Probiotic Solutions for Women’s Health from Puberty to Menopause: A Narrative Review. Microorganisms, 12(8), 1614. https://doi.org/10.3390/microorganisms12081614








