The Drug Susceptibility of Non-Tuberculous Mycobacteria (NTM) in a Referral Hospital in Rome from 2018 to 2023
Abstract
:1. Introduction
2. Methods
2.1. Study Setting
2.2. Molecular Identification and Molecular Resistance of NTM Strains (mDST)
2.3. Phenotypic Drug Susceptibility Tests (pDSTs)
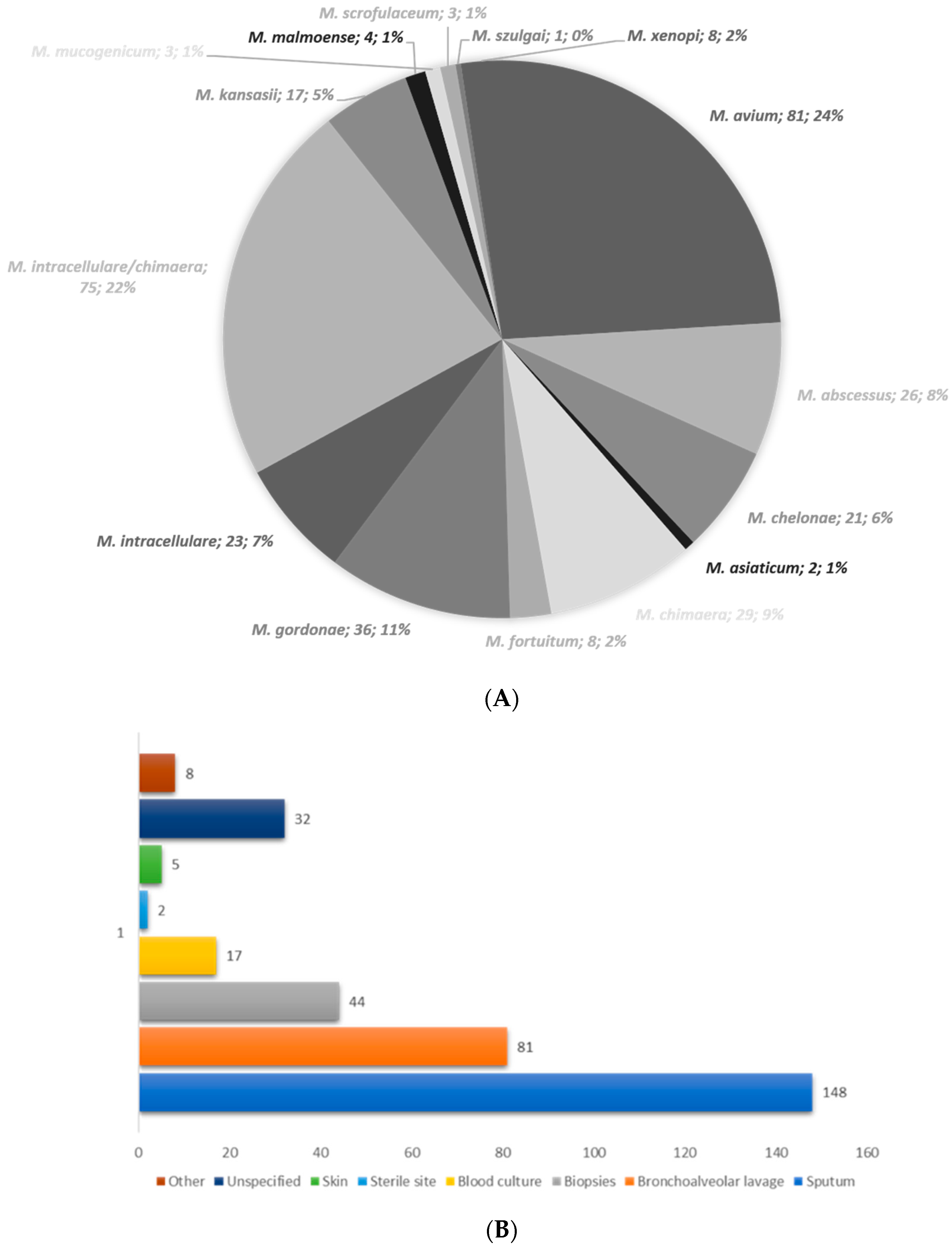
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wi, Y.M. Treatment of extrapulmonary nontuberculous mycobacterial diseases. Infect. Chemother. 2019, 51, 245–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General overview of nontuberculous mycobacteria opportunistic pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loizos, A.; Soteriades, E.S.; Pieridou, D.; Koliou, M.G. Lymphadenitis by non-tuberculous mycobacteria in children. Pediatr. Int. 2018, 60, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.N.; Mølhave, M.; Fløe, A.; van Ingen, J.; Schön, T.; Lillebaek, T.; Andersen, A.B.; Wejse, C. Global trends of pulmonary infections with nontuberculous mycobacteria: A systematic review. Int. J. Infect. Dis. 2022, 125, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mu, W.; Zhang, J.; Wen, S.W.; Pakhale, S. Global prevalence of non-tuberculous mycobacteria in adults with non-cystic fibrosis bronchiectasis 2006–2021: A systematic review and meta-analysis. BMJ Open 2022, 12, e055672. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, G.; Bukowski, J.; Kowalska, W.; Pilaczyńska-Cemel, M.; Krawiecka, D. Trends from the last decade with nontuberculous mycobacteria lung disease (NTM-LD): Clinicians’ perspectives in regional center of Pulmonology in Bydgoszcz, Poland. Pathogens 2023, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- NTM Info and Research Inc. Available online: https://ntminfo.org/ (accessed on 29 July 2024).
- Gopalaswamy, R.; Shanmugam, S.; Mondal, R.; Subbian, S. Of tuberculosis and non-tuberculous mycobacterial infections—A comparative analysis of epidemiology, diagnosis and treatment. J. Biomed. Sci. 2020, 27, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cowman, S.; van Ingen, J.; Griffith, D.E.; Loebinger, M.R. Non-tuberculous mycobacterial pulmonary disease. Eur. Respir. J. 2019, 54, 1900250. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kwak, S.H.; Yong, S.H.; Lee, S.H.; Leem, A.Y.; Kim, S.Y.; Lee, S.H.; Chung, K.; Kim, E.Y.; Jung, J.Y.; et al. The association between behavioral risk factors and nontuberculous mycobacterial pulmonary disease. Yonsei Med. J. 2021, 62, 702–707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- CLSI M24; Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, 3rd Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Calado Nogueira de Moura, V.; Nguyen, M.H.; Hunkins, J.J.; Daley, C.L.; Khare, R. In vitro susceptibility patterns for slowly growing non-tuberculous mycobacteria in the USA from 2018 to 2022. J. Antimicrob. Chemother. 2023, 8, 2849–2858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Philley, J.V.; DeGroote, M.A.; Honda, J.R.; Chan, M.M.; Kasperbauer, S.; Walter, N.D.; Chan, E.D. Treatment of non-tuberculous mycobacterial lung disease. Curr. Treat. Options Infect. Dis. 2016, 8, 275–296. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saxena, S.; Spaink, H.P.; Forn-Cuní, G. Drug resistance in nontuberculous mycobacteria: Mechanisms and models. Biology 2021, 10, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huh, H.J.; Kim, S.Y.; Shim, H.J.; Kim, D.H.; Yoo, I.Y.; Kang, O.K.; Ki, C.S.; Shin, S.Y.; Jhun, B.W.; Shin, S.J.; et al. GenoType NTM-DR performance evaluation for identification of Mycobacterium avium complex and Mycobacterium abscessus and determination of clarithromycin and amikacin resistance. J. Clin. Microbiol. 2019, 57, e00516-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ratnatunga, C.N.; Lutzky, V.P.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Field, M.; Bell, S.C.; Thomson, R.M.; Miles, J.J. The rise of non-tuberculosis mycobacterial lung disease. Front. Immunol. 2020, 11, 303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Q.; Yan, J.; Liao, X.; Wang, C.; Wang, C.; Jiang, G.; Dong, L.; Wang, F.; Huang, H.; Wang, G.; et al. Trends and species diversity of non-tuberculous mycobacteria isolated from respiratory samples in northern China, 2014–2021. Front. Public Health 2022, 10, 923968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, Y.M.; Kim, M.J.; Kim, Y.J. Increasing trend of nontuberculous mycobacteria isolation in a referral clinical laboratory in South Korea. Medicina 2021, 57, 720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fröberg, G.; Maurer, F.P.; Chryssanthou, E.; Fernström, L.; Benmansour, H.; Boarbi, S.; Mengshoel, A.T.; Keller, P.M.; Viveiros, M.; Machado, D.; et al. Towards clinical breakpoints for non-tuberculous mycobacteria—Determination of epidemiological cut off values for the Mycobacterium avium complex and Mycobacterium abscessus using broth microdilution. Clin. Microbiol. Infect. 2023, 29, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Calcagno, A.; Coppola, N.; Sarmati, L.; Tadolini, M.; Parrella, R.; Matteelli, A.; Riccardi, N.; Trezzi, M.; Di Biagio, A.; Pirriatore, V.; et al. Drugs for treating infections caused by non-tubercular mycobacteria: A narrative review from the study group on mycobacteria of the Italian Society of Infectious Diseases and Tropical Medicine. Infection 2024, 52, 737–765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, D.; Liu, H.; Wan, K.; Wang, R.; Yang, Z. Trends in the prevalence and antibiotic resistance of non-tuberculous mycobacteria in mainland China, 2000–2019: Systematic review and meta-analysis. Front. Public Health 2020, 8, 295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416, Erratum in Am. J. Respir. Crit. Care Med. 2007, 175, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Duan, H.; Huang, H.; Lu, Y.; Chu, N. Species identification and clarithromycin susceptibility testing of 278 clinical nontuberculosis mycobacteria isolates. Biomed. Res. Int. 2015, 2015, 506598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwon, B.S.; Kim, M.N.; Sung, H.; Koh, Y.; Kim, W.S.; Song, J.W.; Oh, Y.M.; Lee, S.D.; Lee, S.W.; Lee, J.S.; et al. In Vitro MIC values of rifampin and ethambutol and treatment outcome in Mycobacterium avium complex lung disease. Antimicrob. Agents Chemother. 2018, 62, e00491-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- In, X.; Oh, J.; Cho, J.Y.; Lee, S.; Rhee, S.J. Population pharmacokinetic analysis of amikacin for optimal pharmacotherapy in Korean patients with nontuberculous mycobacterial pulmonary disease. Antibiotics 2020, 9, 784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raaijmakers, J.; Schildkraut, J.A.; Hoefsloot, W.; van Ingen, J. The role of amikacin in the treatment of nontuberculous mycobacterial disease. Expert. Opin. Pharmacother. 2021, 22, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Victoria, L.; Gupta, A.; Gómez, J.L.; Robledo, J. Mycobacterium abscessus complex: A review of recent developments in an emerging pathogen. Front. Cell Infect. Microbiol. 2021, 11, 659997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, E.H.; Huh, H.J.; Song, D.J.; Lee, S.H.; Kim, C.K.; Shin, S.Y.; Ki, C.S.; Jhun, B.W.; Moon, S.M.; Kwon, O.J.; et al. Drug susceptibility patterns of Mycobacterium abscessus and Mycobacterium massiliense isolated from respiratory specimens. Diagn. Microbiol. Infect. Dis. 2019, 93, 107–111. [Google Scholar] [CrossRef] [PubMed]
- CLSI M62; Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes, 1st Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Pfister, P.; Jenni, S.; Poehlsgaard, J.; Thomas, A.; Douthwaite, S.; Ban, N.; Bottger, E.C. The structural basis of macrolide-ribosome binding assessed using mutagenesis of 23S rRNA positions 2058 and 2059. J. Mol. Biol. 2004, 342, 1569–1581. [Google Scholar] [CrossRef]
- Christianson, S.; Grierson, W.; Wolfe, J.; Sharma, M.K. Rapid molecular detection of macrolide resistance in the Mycobacterium avium complex: Are we there yet? J. Clin. Microbiol. 2013, 51, 2425–2426. [Google Scholar] [CrossRef]
- Huh, H.J.; Kim, S.Y.; Jhun, B.W.; Shin, S.J.; Koh, W.J. Recent advances in molecular diagnostics and understanding mechanisms of drug resistance in nontuberculous mycobacterial diseases. Infect. Genet. Evol. 2019, 72, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Bastian, S.; Veziris, N.; Roux, A.L.; Brossier, F.; Gaillard, J.L.; Jarlier, V.; Cambau, E. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob. Agents Chemother. 2011, 55, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Nessar, R.; Cambau, E.; Reyrat, J.M.; Murray, A.; Gicquel, B. Mycobacterium abscessus: A new antibiotic nightmare. J. Antimicrob. Chemother. 2012, 67, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Mougari, F.; Loiseau, J.; Veziris, N.; Bernard, C.; Bercot, B.; Sougakoff, W.; Jarlier, V.; Raskine, L.; Cambau, E. Evaluation of the new GenoType NTM-DR kit for the molecular detection of antimicrobial resistance in non-tuberculous mycobacteria. J. Antimicrob. Chemother. 2017, 72, 1669–1677. [Google Scholar] [CrossRef]
- Zimenkov, D. Variability of Mycobacterium avium complex isolates drug susceptibility testing by broth microdilution. Antibiotics 2022, 11, 1756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| N° | Female | N° | Male | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Groups % | Age Groups % | |||||||||
| <25 | 26–45 | 46–65 | >65 | <25 | 26–45 | 46–65 | >65 | |||
| M. avium | 41 | - | 5 | 29 | 53 | 30 | - | 26 | 40 | 33 |
| M. abscessus complex | 13 | - | 23 | 23 | 57 | 10 | - | 20 | 30 | 50 |
| M. chelonae | 11 | - | 27 | 36 | 27 | 9 | - | 22 | 44 | 33 |
| M. asiaticum | 1 | - | - | 100 | - | 1 | - | - | 100 | - |
| M. chimaera | 9 | - | 22 | - | 77 | 10 | - | 40 | 30 | 30 |
| M. fortuitum | 2 | - | 50 | 50 | - | 4 | - | - | 100 | - |
| M. gordonae | 16 | 6 | 25 | 43 | 25 | 16 | 6 | 25 | 31 | 37 |
| M. intracellulare | 10 | - | 10 | 40 | 50 | 5 | - | - | 40 | 60 |
| M. intracellulare/chimaera | 32 | - | 6 | 37 | 53 | 37 | - | 2 | 24 | 68 |
| M. kansasii | 9 | - | 33 | 44 | 22 | 4 | 25 | 50 | - | 25 |
| M. malmoense | 1 | - | - | 100 | - | 3 | - | - | 67 | 33 |
| M. mucogenicum | 2 | - | 50 | - | 50 | 1 | 100 | - | - | - |
| M. scrofulaceum | 2 | - | - | - | 100 | 1 | - | - | 100 | - |
| M. szulgai | 1 | - | - | - | 100 | - | - | - | - | - |
| M. xenopi | 4 | - | 75 | 25 | - | 4 | - | - | 50 | 50 |
| Total | 154 | 29 | 48 | 47.6 | 135 | 26 | 54.6 | 42 | ||
| Organism | N° Strain | Parameters | CLR | RFB | MXF | RIF | SXT | AMK | LZD | CIP | DOX |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M. avium | 60 | Susceptible (%) | 93 | - | 34 | - | - | 72 | 22 | - | - |
| Intermediate (%) | 5 | - | 37 | - | - | 15 | 20 | - | - | ||
| Resistant (%) | 1 | - | 27 | - | - | 3 | 58 | - | - | ||
| M. intracellulare | 23 | Susceptible (%) | 81 | - | 38 | - | - | 67 | 38 | - | - |
| Intermediate (%) | - | - | 28 | - | - | 14 | 33 | - | - | ||
| Resistant (%) | 19 | - | 28 | - | - | 14 | 28 | - | - | ||
| M. chelonae | 2 | Susceptible (%) | 100 | - | 100 | - | - | 100 | 100 | 100 | 100 |
| Intermediate (%) | - | - | - | - | - | - | - | - | - | ||
| Resistant (%) | - | - | - | - | 100 | - | - | - | - | ||
| M. abscessus complex | 12 | Susceptible (%) | 100 | - | 8 | - | 16 | 75 | 58 | 16 | - |
| Intermediate (%) | - | - | 8 | - | - | 8 | 33 | 25 | 25 | ||
| Resistant (%) | - | - | 83 | - | 83 | 16 | 8 | 58 | 75 | ||
| M. chimaera | 30 | Susceptible (%) | 100 | - | 10 | - | - | 63 | 16 | - | - |
| Intermediate (%) | - | - | 40 | - | - | 23 | 36 | - | - | ||
| Resistant (%) | - | - | 46 | - | - | 13 | 50 | - | - | ||
| M. fortuitum | 2 | Susceptible (%) | 50 | - | 100 | - | 100 | 100 | 100 | - | 100 |
| Intermediate (%) | - | - | - | - | - | - | - | - | - | ||
| Resistant (%) | 50 | - | - | - | - | - | - | 100 | - | ||
| M. gordonae | 2 | Susceptible (%) | 100 | 100 | 100 | 50 | 50 | 100 | 100 | 50 | - |
| Intermediate (%) | - | - | - | - | - | - | - | 50 | 100 | ||
| Resistant (%) | - | - | - | 50 | 50 | - | - | - | - | ||
| M. kansasii | 9 | Susceptible (%) | 100 | 78 | 67 | 22 | 11 | 67 | 44 | 11 | - |
| Intermediate (%) | - | - | 33 | - | - | 22 | 22 | 44 | 44 | ||
| Resistant (%) | - | 22 | - | 78 | 89 | 11 | 22 | 44 | 55 |
| Molecular DST (N°, %) | pDST, CLR/AMK (N°, %) | |||
|---|---|---|---|---|
| S | I | R | ||
| WT rrl (CLR-S) | 57/66 (86.3) | 56/66 (84.8) | - | 1/66 (1.5) |
| Mutant rrl (CLR-r) | 7/66 (10.6) | 7/66 (10.6) | - | - |
| WT rrs (AMK-S) | 62/66 (94) | 38/66 (57.5) | 16/66 (24.2) | 8/66 (12) |
| Mutant rrs (AMK-R) | 4/66 (6) | 1/66 (1.5) | 2/66 (3) | 1/66 (1.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzarelli, A.; Nisii, C.; Cannas, A.; Vulcano, A.; Bartolini, B.; Turchi, F.; Butera, O.; Rossi, A.; De Giuli, C.; Massimino, C.; et al. The Drug Susceptibility of Non-Tuberculous Mycobacteria (NTM) in a Referral Hospital in Rome from 2018 to 2023. Microorganisms 2024, 12, 1615. https://doi.org/10.3390/microorganisms12081615
Mazzarelli A, Nisii C, Cannas A, Vulcano A, Bartolini B, Turchi F, Butera O, Rossi A, De Giuli C, Massimino C, et al. The Drug Susceptibility of Non-Tuberculous Mycobacteria (NTM) in a Referral Hospital in Rome from 2018 to 2023. Microorganisms. 2024; 12(8):1615. https://doi.org/10.3390/microorganisms12081615
Chicago/Turabian StyleMazzarelli, Antonio, Carla Nisii, Angela Cannas, Antonella Vulcano, Barbara Bartolini, Federica Turchi, Ornella Butera, Alberto Rossi, Chiara De Giuli, Chiara Massimino, and et al. 2024. "The Drug Susceptibility of Non-Tuberculous Mycobacteria (NTM) in a Referral Hospital in Rome from 2018 to 2023" Microorganisms 12, no. 8: 1615. https://doi.org/10.3390/microorganisms12081615
APA StyleMazzarelli, A., Nisii, C., Cannas, A., Vulcano, A., Bartolini, B., Turchi, F., Butera, O., Rossi, A., De Giuli, C., Massimino, C., Stellitano, C., Antonelli, V., Petriccione, I., Girardi, E., Gualano, G., Palmieri, F., & Fontana, C. (2024). The Drug Susceptibility of Non-Tuberculous Mycobacteria (NTM) in a Referral Hospital in Rome from 2018 to 2023. Microorganisms, 12(8), 1615. https://doi.org/10.3390/microorganisms12081615








