Escherichia coli Nissle 1917 Protects against Sepsis-Induced Intestinal Damage by Regulating the SCFA/GPRs Signaling Pathway
Abstract
:1. Introduction
2. Method
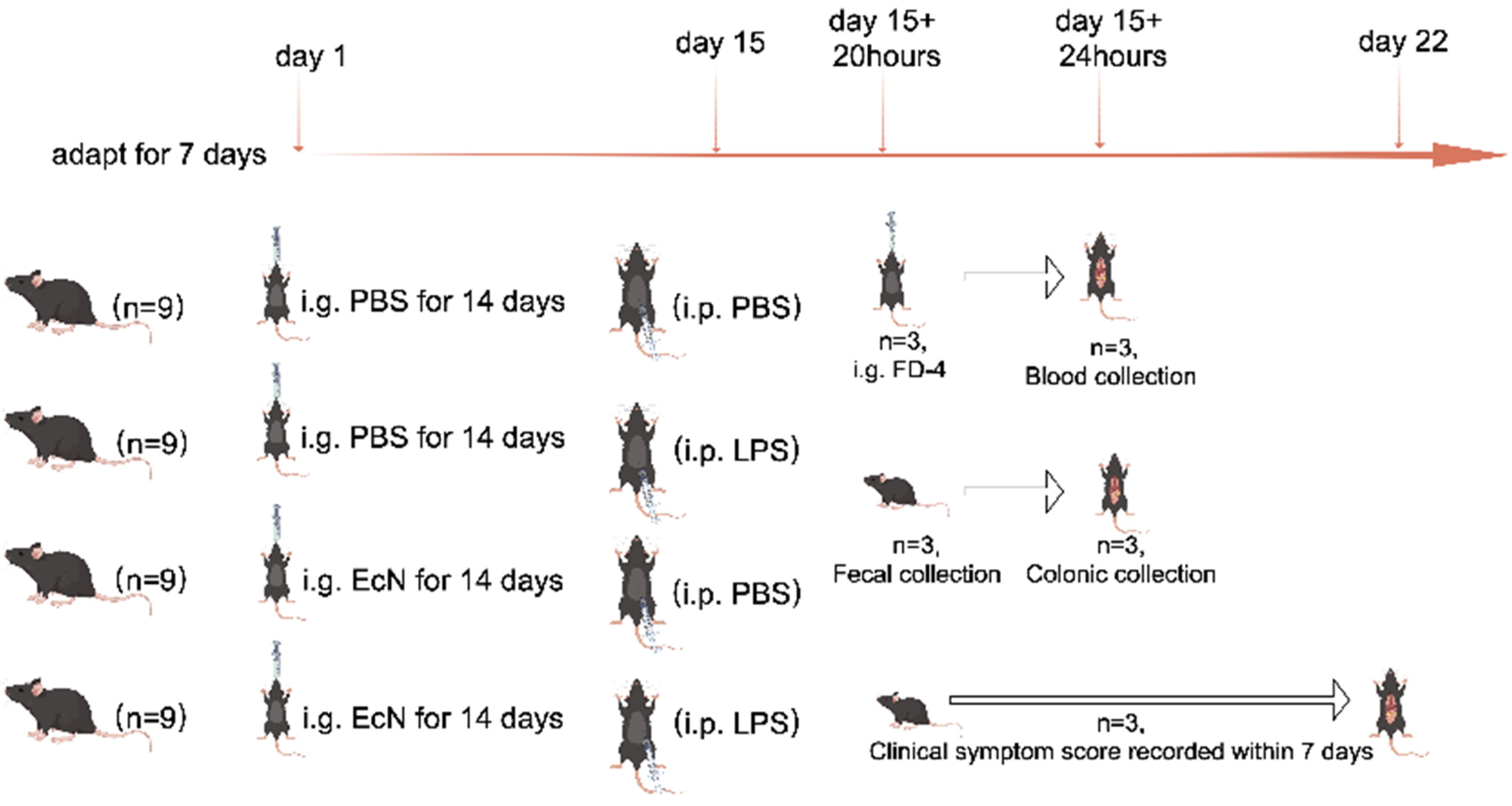
2.1. Animals and Experimental Design
2.2. Clinical Status and FD-4 Flux Measurement of the Intestinal Barrier of Mice
2.3. Morphological Examination and Histopathological Analysis
2.4. Immunofluorescence of Tight Junction Proteins in Animals
2.5. 16S rRNA Sequence Analysis of Mouse Feces
2.6. Determination of SCFAs Content
2.7. Cell Culture
2.8. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
3.1. LPS-Induced Acute Septic Shock in Mice
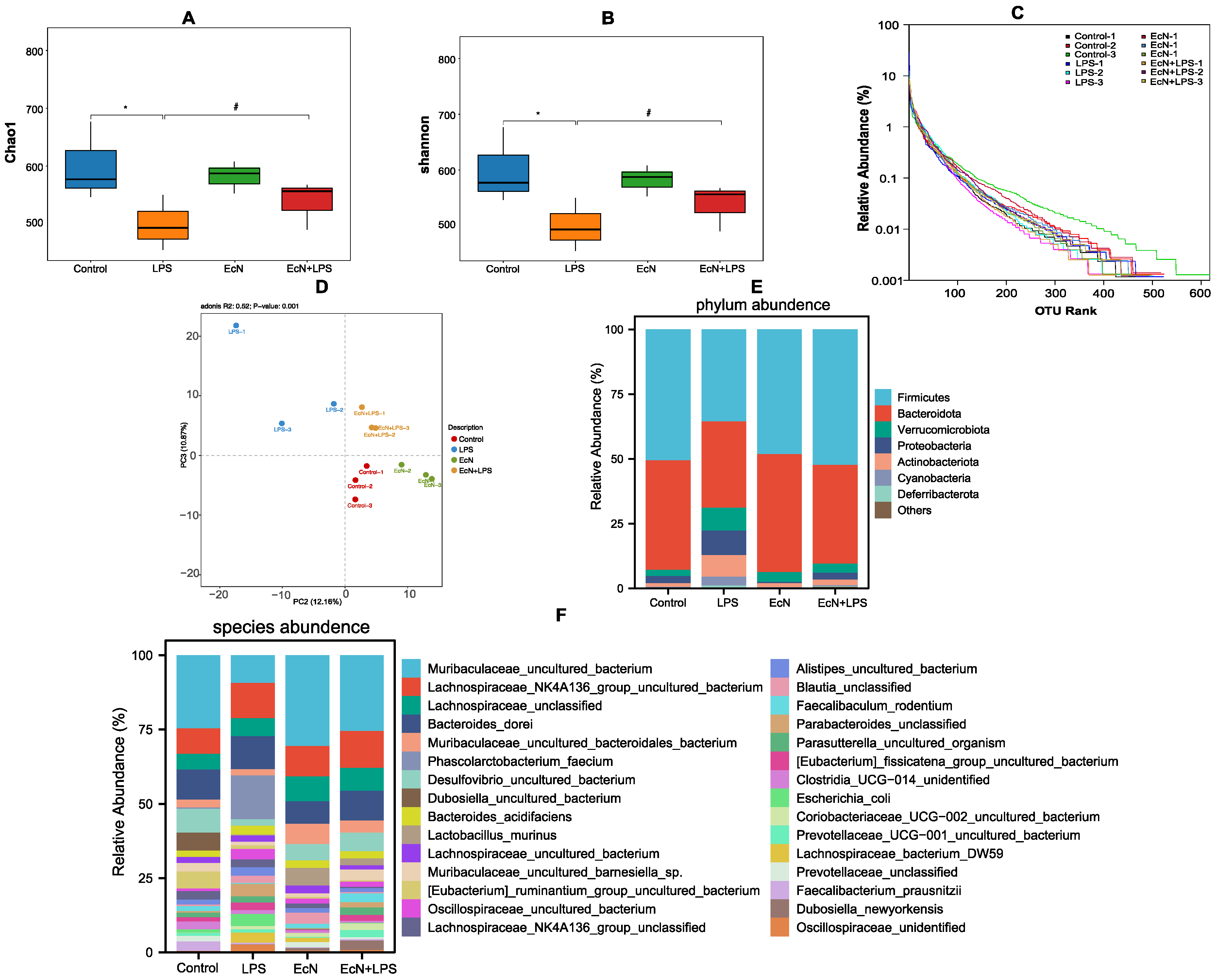
3.2. EcN Increased the Number of Bacteria in the Gut That Produce SCFAs in Mice
3.3. EcN Increased the Content of Short-Chain Fatty Acids Produced in the Gut of Mice and Increased the Expression of Short-Chain Fatty Acid Receptor Proteins GPR41 and GPR43 in Colon Tissue
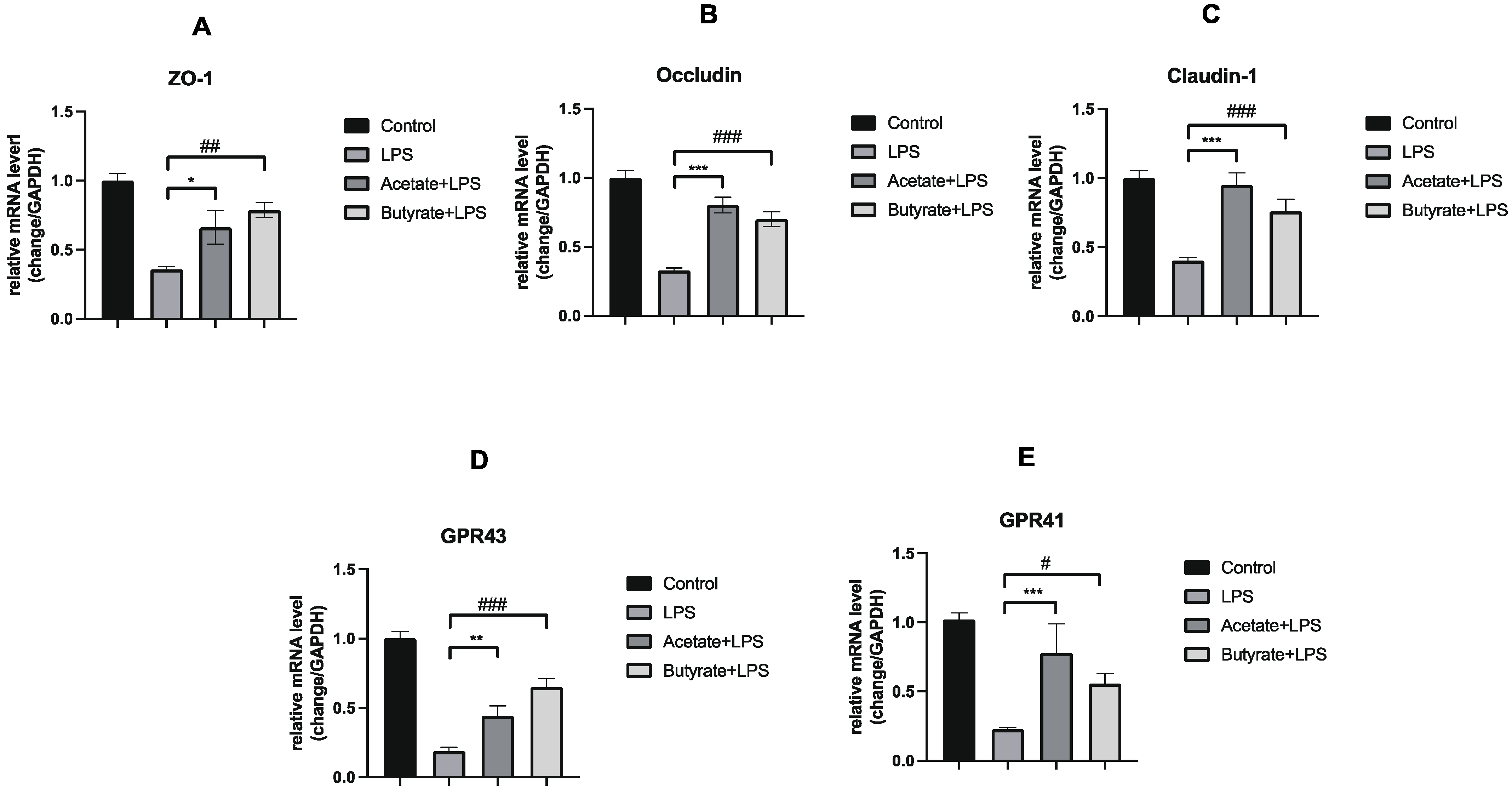
3.4. Acetate and Butyrate Increased the Expression of Expression of Tight Junction Proteins and GPRs in Caco-2 Monolayer Colonic Intestinal Epithelium Models
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Huguet, J.M.; Ferrer-Barceló, L.; Suárez, P.; Sanchez, E.; Prieto, J.D.; Garcia, V.; Sempere, J. Colorectal cancer screening and surveillance in patients with inflammatory bowel disease in 2021. World J. Gastroenterol. 2022, 28, 502–516. [Google Scholar] [CrossRef]
- Kruis, W.; Frič, P.; Pokrotnieks, J.; Lukáš, M.; Fixa, B.; Kaščák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Fernández-Caballero, J.A.; García, F.; Rodríguez-Cabezas, M.E.; Gálvez, J. The Administration of Escherichia coli Nissle 1917 Ameliorates Development of DSS-Induced Colitis in Mice. Front. Pharmacol. 2018, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Ukena, S.N.; Singh, A.; Dringenberg, U.; Engelhardt, R.; Seidler, U.; Hansen, W.; Bleich, A.; Bruder, D.; Franzke, A.; Rogler, G.; et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE 2007, 2, e1308. [Google Scholar] [CrossRef]
- Teng, G.; Liu, Z.; Liu, Y.; Wu, T.; Dai, Y.; Wang, H.; Wang, W. Probiotic Escherichia coli Nissle 1917 Expressing Elafin Protects Against Inflammation and Restores the Gut Microbiota. Front. Microbiol. 2022, 13, 819336. [Google Scholar] [CrossRef]
- Wu, H.; Wei, J.; Zhao, X.; Liu, Y.; Chen, Z.; Wei, K.; Lu, J.; Chen, W.; Jiang, M.; Li, S.; et al. Neuroprotective effects of an engineered Escherichia coli Nissle 1917 on Parkinson’s disease in mice by delivering GLP-1 and modulating gut microbiota. Bioeng. Transl. Med. 2023, 8, e10351. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, S.; Ma, J.; Ma, Y.; Zhu, J.; Ma, Y.; Liu, Y.; Wang, P.; Pan, Y. Escherichia coli Nissle 1917 Protects Intestinal Barrier Function by Inhibiting NF-κB-Mediated Activation of the MLCK-P-MLC Signaling Pathway. Mediat. Inflamm. 2019, 2019, 5796491. [Google Scholar] [CrossRef]
- Xu, H.; Hou, Q.; Zhu, J.; Feng, M.; Wang, P.; Pan, Y. The protective effect of Escherichia coli Nissle 1917 on the intestinal barrier is mediated by inhibition of RhoA/ROCK2/MLC signaling via TLR-4. Life Sci. 2022, 292, 120330. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Chen, S.; Guo, S.; Yue, T.; Hou, Q.; Feng, M.; Xu, H.; Liu, Y.; Wang, P.; et al. The administration of Escherichia coli Nissle 1917 ameliorates irinotecan-induced intestinal barrier dysfunction and gut microbial dysbiosis in mice. Life Sci. 2019, 231, 116529. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.D.; Oliveira, C.S.; Azevedo-Silva, J.; Casanova, M.R.; Barreto, J.; Pereira, H.; Chaves, S.R.; Rodrigues, L.R.; Casal, M.; Côrte-Real, M.; et al. The Role of Diet Related Short-Chain Fatty Acids in Colorectal Cancer Metabolism and Survival: Prevention and Therapeutic Implications. Curr. Med. Chem. 2020, 27, 4087–4108. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal. Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Hwang, D.; Kim, J.K.; Lim, Y.H. Oxyresveratrol improves tight junction integrity through the PKC and MAPK signaling pathways in Caco-2 cells. Food Chem. Toxicol. 2017, 108 Pt A, 203–213. [Google Scholar] [CrossRef]
- Lima-Júnior, R.C.; Figueiredo, A.A.; Freitas, H.C.; Melo, M.L.; Wong, D.V.; Leite, C.A.; Medeiros, R.P.; Marques-Neto, R.D.; Vale, M.L.; Brito, G.A.; et al. Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: Role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother. Pharmacol. 2012, 69, 931–942. [Google Scholar] [CrossRef]
- Chou, H.C.; Chen, C.M. Neonatal hyperoxia disrupts the intestinal barrier and impairs intestinal function in rats. Exp. Mol. Pathol. 2017, 102, 415–421. [Google Scholar] [CrossRef]
- Ochiai, T.; Honsawa, T.; Yamaguchi, K.; Sasaki, Y.; Yokoyama, C.; Kuwata, H.; Hara, S. Prostacyclin synthase deficiency exacerbates systemic inflammatory responses in lipopolysaccharide-induced septic shock in mice. Inflamm. Res. 2024, 73, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Fábrega, M.J.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Algieri, F.; Badía, J.; Giménez, R.; Gálvez, J.; Baldomà, L. Intestinal Anti-inflammatory Effects of Outer Membrane Vesicles from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Utrilla, P.; Comalada, M.; Zorrilla, P.; Garrido-Mesa, J.; Zarzuelo, A.; Rodríguez-Cabezas, M.E.; Gálvez, J. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem. Pharmacol. 2011, 82, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Lepage, P.; Häsler, R.; Spehlmann, M.E.; Rehman, A.; Zvirbliene, A.; Begun, A.; Ott, S.; Kupcinskas, L.; Doré, J.; Raedler, A.; et al. Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology 2011, 141, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cao, F.; Hu, Z.; Zhou, Y.; Guo, T.; Yan, S.; Xie, Q.; Xia, X.; Yuan, H.; Li, G.; et al. Cyanidin-3-O-Glucoside Alleviates Alcoholic Liver Injury via Modulating Gut Microbiota and Metabolites in Mice. Nutrients 2024, 16, 694. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Zhang, K.K.; Shen, H.W.; Liu, Y.; Li, X.W.; Chen, L.J.; Liu, J.L.; Li, J.H.; Zhao, D.; Wang, Q.; et al. Sigma-1 receptor knockout disturbs gut microbiota, remodels serum metabolome, and exacerbates isoprenaline-induced heart failure. Front. Microbiol. 2023, 14, 1255971. [Google Scholar] [CrossRef]
- Satokari, R. High Intake of Sugar and the Balance between Pro- and Anti-Inflammatory Gut Bacteria. Nutrients 2020, 12, 1348. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, C.; Song, B.; Guo, Q.; Zhong, Y.; Zhang, S.; Zhang, L.; Duan, G.; Li, F.; Duan, Y. HMB Improves Lipid Metabolism of Bama Xiang Mini-Pigs via Modulating the Bacteroidetes-Acetic Acid-AMPKα Axis. Front. Microbiol. 2021, 12, 736997. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Y.; Xu, Q.; Mao, L. Short Chain Fatty Acids: Essential Weapons of Traditional Medicine in Treating Inflammatory Bowel Disease. Molecules 2024, 29, 379. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Shaheen, N.; Miao, J.; Li, D.; Xia, B.; Baoyinna, B.; Zhao, Y.; Zhao, J. Indole-3-Acetic Acid Protects Against Lipopolysaccharide-induced Endothelial Cell Dysfunction and Lung Injury through the Activation of USP40. Am. J. Respir. Cell Mol. Biol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Gundelund Nielsen, D.S.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Yan, X.; Liu, X.Y.; Zhang, D.; Zhang, Y.D.; Li, Z.H.; Liu, X.; Wu, F.; Chen, G.Q. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell Mol. Immunol. 2021, 18, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.T.; Kim, T.; Ham, J.; Choi, J.; Lee, H.S.; Yeon, Y.J.; Choi, S.I.; Kim, N.; Kim, Y.R.; Seok, Y.J. Physiological activity of E. coli engineered to produce butyric acid. Microb. Biotechnol. 2022, 15, 832–843. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wu, T.; Li, Y.; Zhou, X.; Ruan, Z. Indole-3-propionic Acid Improved the Intestinal Barrier by Enhancing Epithelial Barrier and Mucus Barrier. J. Agric. Food Chem. 2021, 69, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, A.H.; Taghinezhad, S.S.; Fu, X. Gut microbiota-derived metabolites and colorectal cancer: New insights and updates. Microb. Pathog. 2020, 149, 104569. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.W.; Yin, F.Y.; Zhang, Z.F.; Gong, X.; Yang, Y. Butyrate Suppresses Glucose Metabolism of Colorectal Cancer Cells via GPR109a-AKT Signaling Pathway and Enhances Chemotherapy. Front Mol. Biosci. 2021, 8, 634874. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Deng, H.; Xiao, L.; Pan, Y. Escherichia coli Nissle 1917 Protects against Sepsis-Induced Intestinal Damage by Regulating the SCFA/GPRs Signaling Pathway. Microorganisms 2024, 12, 1622. https://doi.org/10.3390/microorganisms12081622
Wang Y, Deng H, Xiao L, Pan Y. Escherichia coli Nissle 1917 Protects against Sepsis-Induced Intestinal Damage by Regulating the SCFA/GPRs Signaling Pathway. Microorganisms. 2024; 12(8):1622. https://doi.org/10.3390/microorganisms12081622
Chicago/Turabian StyleWang, Yajie, Huan Deng, Lin Xiao, and Yisheng Pan. 2024. "Escherichia coli Nissle 1917 Protects against Sepsis-Induced Intestinal Damage by Regulating the SCFA/GPRs Signaling Pathway" Microorganisms 12, no. 8: 1622. https://doi.org/10.3390/microorganisms12081622



