Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection
Abstract
:1. Introduction
2. Local Microbiota
Microbiota Recurrent Implantation Failure and Recurrent Pregnancy Loss
3. Impact of Gut Microbiota on Vaginal and Endometrial Microbiota
4. Immune Cells in the Female Reproductive Tract
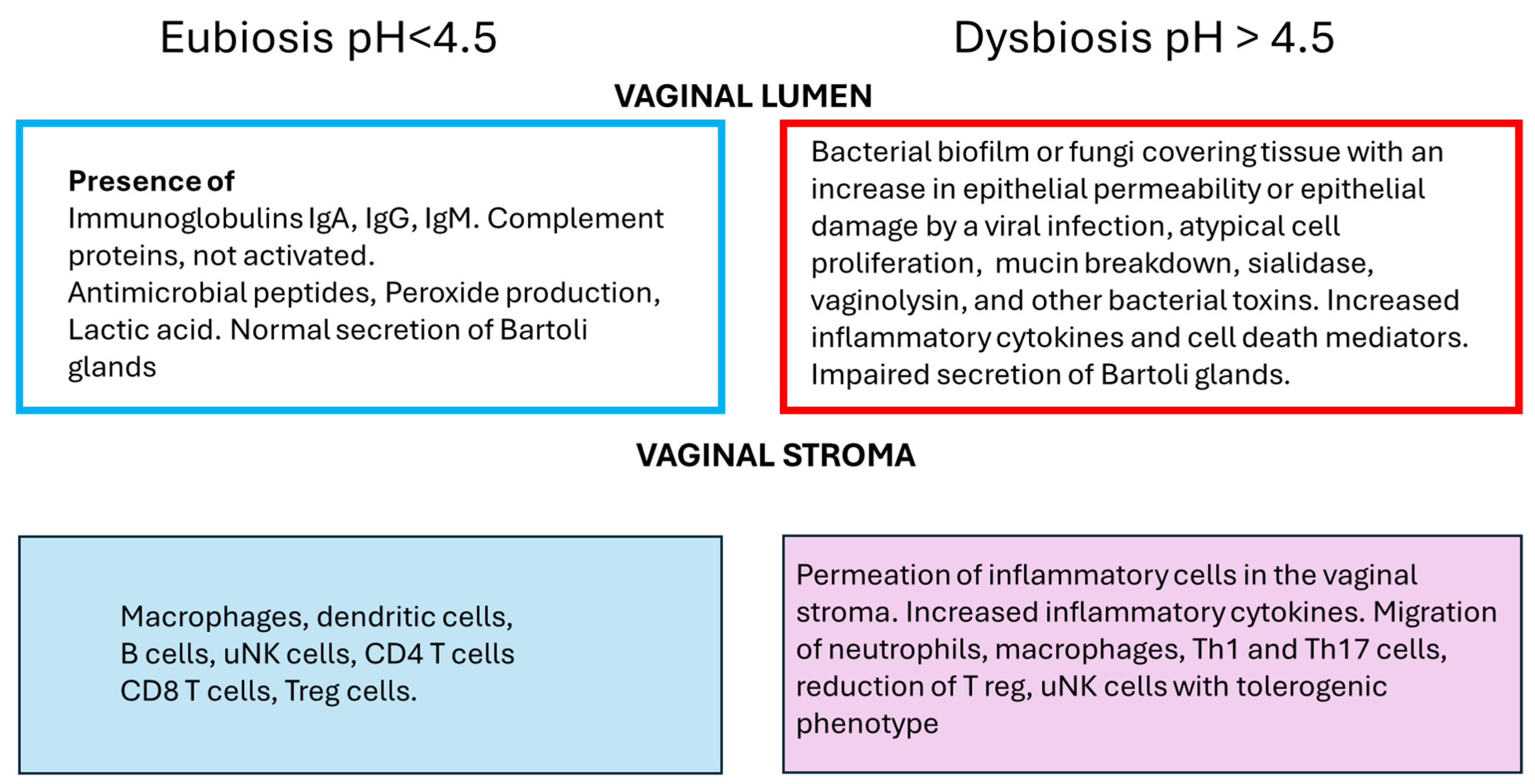
4.1. Innate Immunity
4.2. Adaptative Immunity
5. Perspectives of Microbiota Modulation on RPL
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stephenson, M.D. Frequency of factors associated with habitual abortion in 197 couples. Fertil. Steril. 1996, 66, 24–29. [Google Scholar]
- Ford, H.B.; Schust, D.J. Recurrent pregnancy loss: Etiology, diagnosis, and therapy. Rev. Obstet. Gynecol. 2009, 2, 76–83. [Google Scholar]
- Pillarisetty, L.S.; Mahdy, H. Recurrent Pregnancy Loss. [Updated 2023 August 28]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554460/ (accessed on 4 June 2024).
- Gao, H.; Liu, Q.; Wang, X.; Li, T.; Li, H.; Li, G.; Tan, L.; Chen, Y. Deciphering the role of female reproductive tract microbiome in reproductive health: A review. Front. Cell. Infect. Microbiol. 2024, 14, 1351540. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Gao, X.; Louwers, Y.V.; Laven, J.S.E.; Schoenmakers, S. Clinical Relevance of Vaginal and Endometrial Microbiome Investigation in Women with Repeated Implantation Failure and Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2024, 25, 622. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.J.; Kang, J.H. Immune cells in the female reproductive tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Mendz, G.L. The Vaginal Microbiome during Pregnancy in Health and Disease. Appl. Microbiol. 2023, 3, 1302–1338. [Google Scholar] [CrossRef]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How uterine microbiota might be responsible for a receptive, fertile endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The interplay between reproductive tract microbiota and immunological system in human reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.; Black, N.; Bennett, P.R.; Brosens, J.; Quenby, S.; MacIntyre, D.A. The endometrial microbiota and early pregnancy loss. Hum. Reprod. 2024, 39, 638–646. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; De Seta, F.; Verstraelen, H.; Ventolini, G.; Lonnee-Hoffmann, R.; Vieira-Baptista, P. The Vaginal Microbiome: II. Vaginal Dysbiotic Conditions. J. Low. Genit. Tract Dis. 2022, 26, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, J.H.; Ardizzone, C.M.; Cerca, N.; Toh, E.; Łaniewski, P.; Lillis, R.A.; Herbst-Kralovetz, M.M.; Quayle, A.J.; Muzny, C.A.; Taylor, C.M. A novel Gardnerella, Prevotella, and Lactobacillus standard that improves accuracy in quantifying bacterial burden in vaginal microbial communities. Front. Cell. Infect. Microbiol. 2023, 13, 1198113. [Google Scholar] [CrossRef] [PubMed]
- Sola-Leyva, A.; Andrés-León, E.; Molina, N.M.; Terron-Camero, L.C.; Plaza-Díaz, J.; Sáez-Lara, M.J.; Gonzalvo, M.C.; Sánchez, R.; Ruíz, S.; Martínez, L.; et al. Mapping the entire functionally active endometrial microbiota. Hum. Reprod. 2021, 36, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Blazheva, S.; Pachkova, S.; Bodurska, T.; Ivanov, P.; Blazhev, A.; Lukanov, T.; Konova, E. Unlocking the Uterine Code: Microbiota, Immune Cells, and Therapy for Recurrent Reproductive Failure. Microorganisms 2024, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Al-Memar, M.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Chan, D.; Lewis, H.; Kindinger, L.; Terzidou, V.; Bourne, T.; et al. Establishment of vaginal microbiota composition in early pregnancy and its association with subsequent preterm prelabor rupture of the fetal membranes. Transl. Res. 2019, 207, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.S.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. mSphere 2020, 5, e00593-20. [Google Scholar] [CrossRef] [PubMed]
- Toson, B.; Simon, C.; Moreno, I. The Endometrial Microbiome and Its Impact on Human Conception. Int. J. Mol. Sci. 2022, 23, 485. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.M.T.; Bernstein, K.T.; Aral, S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef]
- Ma, Z.S. Microbiome Transmission During Sexual Intercourse Appears Stochastic and Supports the Red Queen Hypothesis. Front. Microbiol. 2022, 12, 789983. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.S.; Lingappa, J.R.; Srinivasan, S.; Kinuthia, J.; John-Stewart, G.C.; Jaoko, W.; Richardson, B.A.; Yuhas, K.; Fiedler, T.L.; Mandaliya, K.N.; et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: A nested case-control study. Lancet Infect. Dis. 2018, 18, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Chacra, L.A.; Ly, C.; Hammoud, A.; Iwaza, R.; Mediannikov, O.; Bretelle, F.; Fenollar, F. Relationship between Bacterial Vaginosis and Sexually Transmitted Infections: Coincidence, Consequence or Co-Transmission? Microorganisms 2023, 11, 2470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thakur, R.; Shen, Q.; He, Y.; Chen, C. Influences of vaginal microbiota on human papillomavirus infection and host immune regulation: What we have learned? Decod. Infect. Transm. 2023, 1, 100002. [Google Scholar] [CrossRef]
- Zeng, M.; Li, X.; Jiao, X.; Cai, X.; Yao, F.; Xu, S.; Huang, X.; Zhang, Q.; Chen, J. Roles of vaginal flora in human papillomavirus infection, virus persistence and clearance. Front. Cell. Infect. Microbiol. 2023, 12, 1036869. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, A.; Bruyere, D.; Roncarati, P.; Peixoto, P.; Hervouet, E.; Cobraiville, G.; Taminiau, B.; Masson, M.; Gallego, C.; Mazzucchelli, G.; et al. HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources. Nat. Commun. 2022, 13, 1076. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.D.; Nandi, D.; Agingu, W.; Green, S.J.; Bhaumik, D.K.; Bailey, R.C.; Otieno, F. Vaginal and Penile Microbiome Associations With Herpes Simplex Virus Type 2 in Women and Their Male Sex Partners. J. Infect. Dis. 2022, 226, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Brotman, R.M.; Klebanoff, M.A.; Nansel, T.R.; Yu, K.F.; Andrews, W.W.; Zhang, J.; Schwebke, J.R. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J. Infect. Dis. 2010, 202, 1907–1915. [Google Scholar] [CrossRef]
- Van Gerwen, O.T.; Muzny, C.A.; Marrazzo, J.M. Sexually transmitted infections and female reproductive health. Nat. Microbiol. 2022, 7, 1116–1126. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Ness, R.B.; Totten, P.A.; Farooq, F.; Tang, G.; Ko, D.B.; Hou, X.; Fiedler, T.L.B.; Srinivasan, S.; Astete, S.G.; et al. Presence and Concentrations of Select Bacterial Vaginosis-Associated Bacteria Are Associated With Increased Risk of Pelvic Inflammatory Disease. Sex. Transm. Dis. 2020, 47, 344–346. [Google Scholar] [CrossRef]
- Brown, S.E.; Schwartz, J.A.; Robinson, C.K.; O’Hanlon, D.E.; Bradford, L.L.; He, X.; Mark, K.S.; Bruno, V.M.; Ravel, J.; Brotman, R.M. The Vaginal Microbiota and Behavioral Factors Associated With Genital Candida albicans Detection in Reproductive-Age Women. Sex. Transm. Dis. 2019, 46, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Sobstyl, A.; Chałupnik, A.; Mertowska, P.; Grywalska, E. How Do Microorganisms Influence the Development of Endometriosis? Participation of Genital, Intestinal and Oral Microbiota in Metabolic Regulation and Immunopathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 10920. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Fan, T.; Luo, S.; Zheng, J.; Zhang, L.; Cao, L.; Zhang, Z.; Li, L.; Huang, Z.; Zhang, H.; et al. Lactobacillus gasseri LGV03 isolated from the cervico-vagina of HPV-cleared women modulates epithelial innate immune responses and suppresses the growth of HPV-positive human cervical cancer cells. Transl. Oncol. 2023, 35, 101714. [Google Scholar] [CrossRef]
- Krog, M.C.; Hugerth, L.W.; Fransson, E.; Bashir, Z.; Andersen, A.N.; Edfeldt, G.; Engstrand, L.; Schuppe-Koistinen, I.; Nielsen, H.S. The healthy female microbiome across body sites: Effect of hormonal contraceptives and the menstrual cycle. Hum. Reprod. 2022, 37, 1525–1543. [Google Scholar] [CrossRef]
- van den Tweel, M.M.; van den Munckhof, E.H.A.; van der Zanden, M.; Molijn, A.C.; van Lith, J.M.M.; Le Cessie, S.; Boers, K.E. Bacterial vaginosis in a subfertile population undergoing fertility treatments: A prospective cohort study. J. Assist. Reprod. Genet. 2024, 41, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, P.; Bai, X.; Tian, S.; Yang, M.; Leng, D.; Kui, H.; Zhang, S.; Yan, X.; Zheng, Q.; et al. Vaginal microbiota are associated with in vitro fertilization during female infertility. iMeta 2024, 3, e185. [Google Scholar] [CrossRef] [PubMed]
- Elnashar, A.M. Impact of endometrial microbiome on fertility. Middle East Fertil. Soc. J. 2021, 26, 4. [Google Scholar] [CrossRef]
- Hugon, A.M.; Golos, T.G. Non-human primate models for understanding the impact of the microbiome on pregnancy and the female reproductive tract†. Biol. Reprod. 2023, 109, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H.J.; Bos, A.M.; Himschoot, L.; van Eekelen, R.; Matamoros, S.P.; de Boer, M.A.; Oudijk, M.A.; Ris-Stalpers, C.; Cools, P.; Savelkoul, P.H.; et al. Vaginal microbiota and spontaneous preterm birth in pregnant women at high risk of recurrence. Heliyon 2024, 10, e30685. [Google Scholar] [CrossRef]
- Sun, S.; Serrano, M.G.; Fettweis, J.M.; Basta, P.; Rosen, E.; Ludwig, K.; Sorgen, A.A.; Blakley, I.C.; Wu, M.C.; Dole, N.; et al. Race, the Vaginal Microbiome, and Spontaneous Preterm Birth. mSystems 2022, 7, e0001722. [Google Scholar] [CrossRef]
- Saadaoui, M.; Singh, P.; Ortashi, O.; Al Khodor, S. Role of the vaginal microbiome in miscarriage: Exploring the relationship. Front. Cell. Infect. Microbiol. 2023, 13, 1232825. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Bellamy, S.; Nachamkin, I.; Ness, R.B.; Macones, G.A.; Allen-Taylor, L. First trimester bacterial vaginosis, individual microorganism levels, and risk of second trimester pregnancy loss among urban women. Fertil. Steril. 2007, 88, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.B.; Hanlon, A.; Nachamkin, I.; Haggerty, C.; Mastrogiannis, D.S.; Liu, C.; Fredricks, D.N. Early Pregnancy Changes in Bacterial Vaginosis-Associated Bacteria and Preterm Delivery. Paediatr. Perinat. Epidemiology 2014, 28, 88–96. [Google Scholar] [CrossRef]
- Nelson, D.B.; Hanlon, A.L.; Wu, G.; Liu, C.; Fredricks, D.N. First Trimester Levels of BV-Associated Bacteria and Risk of Miscarriage Among Women Early in Pregnancy. Matern. Child Health J. 2015, 19, 2682–2687. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Khodabandehloo, M.; Ramazanzadeh, R.; Farhadifar, F.; Nikkhoo, B.; Soofizade, N.; Rezaii, M. Association between Ureaplasma urealyticum endocervical infection and spontaneous abortion. Iran. J. Microbiol. 2014, 6, 392–397. [Google Scholar] [PubMed]
- McPherson, E. Recurrence of stillbirth and second trimester pregnancy loss. Am. J. Med Genet. A 2016, 170, 1174–1180. [Google Scholar] [CrossRef]
- Işik, G.; Demirezen, Ş.; Dönmez, H.G.; Beksaç, M.S. Bacterial vaginosis in association with spontaneous abortion and recurrent pregnancy losses. J. Cytol. 2016, 33, 135–140. [Google Scholar]
- Kuon, R.J.; Togawa, R.; Vomstein, K.; Weber, M.; Goeggl, T.; Strowitzki, T.; Markert, U.R.; Zimmermann, S.; Daniel, V.; Dalpke, A.H.; et al. Higher prevalence of colonization with Gardnerella vaginalis and gram-negative anaerobes in patients with recurrent miscarriage and elevated peripheral natural killer cells. J. Reprod. Immunol. 2017, 120, 15–19. [Google Scholar] [CrossRef]
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.; Smith, A.; Marchesi, J.; Timmerman, D.; Bourne, T.; Bennett, P.; et al. The association between vaginal bacterial composition and miscarriage: A nested case-control study. BJOG 2020, 127, 264–274. [Google Scholar] [CrossRef]
- Chang, D.H.; Shin, J.; Rhee, M.S.; Park, K.R.; Cho, B.K.; Lee, S.K.; Kim, B.C. Vaginal Microbiota Profiles of Native Korean Women and Associations with High-Risk Pregnancy. J. Microbiol. Biotechnol. 2020, 30, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, L.; Lian, C.; Xue, H.; Lu, Y.; Chen, X.; Xia, Y. Vaginal Microbiota Diversity of Patients with Embryonic Miscarriage by Using 16S rDNA High-Throughput Sequencing. Int. J. Genom. 2020, 2020, 1764959. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yamada, H.; Sasagawa, Y.; Tanimura, K.; Deguchi, M. Uterine endometrium microbiota and pregnancy outcome in women with recurrent pregnancy loss. J. Reprod. Immunol. 2022, 152, 103653. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Quinlivan, J.A.; Peek, M.; Castaño-Rodríguez, N.; Mendz, G.L. Is there an association between the vaginal microbiome and first-trimester miscarriage? A prospective observational study. J. Obstet. Gynaecol. Res. 2022, 48, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Ncib, K.; Bahia, W.; Leban, N.; Mahdhi, A.; Trifa, F.; Mzoughi, R.; Haddad, A.; Jabeur, C.; Donders, G. Microbial Diversity and Pathogenic Properties of Microbiota Associated with Aerobic Vaginitis in Women with Recurrent Pregnancy Loss. Diagnostics 2022, 12, 2444. [Google Scholar] [CrossRef]
- Vomstein, K.; Reider, S.; Böttcher, B.; Watschinger, C.; Kyvelidou, C.; Tilg, H.; Moschen, A.R.; Toth, B. Uterine microbiota plasticity during the menstrual cycle: Differences between healthy controls and patients with recurrent miscarriage or implantation failure. J. Reprod. Immunol. 2022, 151, 103634. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Garcia-Grau, I.; Perez-Villaroya, D.; Gonzalez-Monfort, M.; Bahçeci, M.; Barrionuevo, M.J.; Taguchi, S.; Puente, E.; Dimattina, M.; Lim, M.W.; et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome 2022, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Severgnini, M.; Morselli, S.; Camboni, T.; Ceccarani, C.; Laghi, L.; Zagonari, S.; Patuelli, G.; Pedna, M.F.; Sambri, V.; Foschi, C.; et al. A Deep Look at the Vaginal Environment During Pregnancy and Puerperium. Front. Cell. Infect. Microbiol. 2022, 12, 838405. [Google Scholar] [CrossRef] [PubMed]
- Peuranpää, P.; Holster, T.; Saqib, S.; Kalliala, I.; Tiitinen, A.; Salonen, A.; Hautamäki, H. Female reproductive tract microbiota and recurrent pregnancy loss: A nested case-control study. Reprod. Biomed. Online 2022, 45, 1021–1031. [Google Scholar] [CrossRef]
- Shu, J.; Lin, S.; Wu, Y.; Zhu, J.; Gong, D.; Zou, X.; Zhu, H.; Gao, J. A potential role for the uterine microbiome in missed abortions. J. Biol. Regul. Homeost. Agents 2022, 36, 1055–1063. [Google Scholar]
- Tanaka, S.E.; Sakuraba, Y.; Kitaya, K.; Ishikawa, T. Differential Vaginal Microbiota Profiling in Lactic-Acid-Producing Bacteria between Infertile Women with and without Chronic Endometritis. Diagnostics 2022, 12, 878. [Google Scholar] [CrossRef]
- Dong, M.; Dong, Y.; Bai, J.; Li, H.; Ma, X.; Li, B.; Wang, C.; Li, H.; Qi, W.; Wang, Y.; et al. Interactions between microbiota and cervical epithelial, immune, and mucus barrier. Front. Cell. Infect. Microbiol. 2023, 13, 1124591. [Google Scholar] [CrossRef]
- Mori, R.; Hayakawa, T.; Hirayama, M.; Ozawa, F.; Yoshihara, H.; Goto, S.; Kitaori, T.; Ozaki, Y.; Sugiura-Ogasawara, M. Cervicovaginal microbiome in patients with recurrent pregnancy loss. J. Reprod. Immunol. 2023, 157, 103944. [Google Scholar] [CrossRef]
- Masucci, L.; D’Ippolito, S.; De Maio, F.; Quaranta, G.; Mazzarella, R.; Bianco, D.M.; Castellani, R.; Inversetti, A.; Sanguinetti, M.; Gasbarrini, A.; et al. Celiac Disease Predisposition and Genital Tract Microbiota in Women Affected by Recurrent Pregnancy Loss. Nutrients 2023, 15, 221. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zhu, M.; Ge, L.; Liu, X.; Su, K.; Chen, Z.; Zhao, W. The Interplay Between Cervicovaginal Microbial Dysbiosis and Cervicovaginal Immunity. Front. Immunol. 2022, 13, 857299. [Google Scholar] [CrossRef]
- Celicanin, M.M.; Haahr, T.; Humaidan, P.; Skafte-Holm, A. Vaginal dysbiosis—The association with reproductive outcomes in IVF patients: A systematic review and meta-analysis. Curr. Opin. Obstet. Gynecol. 2024, 36, 155–164. [Google Scholar] [CrossRef]
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P.R. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022, 20, 38. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Feng, L.; Zhang, J. Interactions between gut microbiota and metabolites modulate cytokine network imbalances in women with unexplained miscarriage. NPJ Biofilms Microbiomes 2021, 7, 24. [Google Scholar] [CrossRef]
- Vomstein, K.; Krog, M.C.; Wrønding, T.; Nielsen, H.S. The microbiome in recurrent pregnancy loss—A scoping review. J. Reprod. Immunol. 2024, 163, 104251. [Google Scholar] [CrossRef]
- Liu, F.T.; Yang, S.; Yang, Z.; Zhou, P.; Peng, T.; Yin, J.; Ye, Z.; Shan, H.; Yu, Y.; Li, R. An Altered Microbiota in the Lower and Upper Female Reproductive Tract of Women with Recurrent Spontaneous Abortion. Microbiol. Spectr. 2022, 10, e0046222. [Google Scholar]
- Wang, L.; Chen, J.; He, L.; Liu, H.; Liu, Y.; Luan, Z.; Li, H.; Liu, W.; Luo, M. Association between the vaginal and uterine microbiota and the risk of early embryonic arrest. Front. Microbiol. 2023, 14, 1137869. [Google Scholar] [CrossRef]
- Takimoto, K.; Yamada, H.; Shimada, S.; Fukushi, Y.; Wada, S. Chronic Endometritis and Uterine Endometrium Microbiota in Recurrent Implantation Failure and Recurrent Pregnancy Loss. Biomedicines 2023, 11, 2391. [Google Scholar] [CrossRef]
- Palomino, M.M.; Allievi, M.C.; Gordillo, T.B.; Bockor, S.S.; Fina Martin, J.; Ruzal, S.M. Surface layer proteins in species of the family Lactobacillaceae. Microb. Biotechnol. 2023, 16, 1232–1249. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Mendes-Soares, H.; Suzuki, H.; Hickey, R.J.; Forney, L.J. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J. Bacteriol. 2014, 196, 1458–1470. [Google Scholar] [CrossRef]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Cela, V.; Daniele, S.; Obino, M.E.R.; Ruggiero, M.; Zappelli, E.; Ceccarelli, L.; Papini, F.; Marzi, I.; Scarfò, G.; Tosi, F.; et al. Endometrial Dysbiosis Is Related to Inflammatory Factors in Women with Repeated Implantation Failure: A Pilot Study. J. Clin. Med. 2022, 11, 2481. [Google Scholar] [CrossRef]
- Santoro, A.; Travaglino, A.; Inzani, F.; Angelico, G.; Raffone, A.; Maruotti, G.M.; Straccia, P.; Arciuolo, D.; Castri, F.; D’Alessandris, N.; et al. The Role of Plasma Cells as a Marker of Chronic Endometritis: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 1714. [Google Scholar] [CrossRef]
- Ma, N.; Li, J.; Zhang, J.; Jin, Y.; Wang, J.; Qin, W.; Hang, F.; Qin, A. Combined oral antibiotics and intrauterine perfusion can improve in vitro fertilization and embryo transfer pregnancy outcomes in patients with chronic endometritis and repeated embryo implantation failure. BMC Women’s Health 2023, 23, 344. [Google Scholar] [CrossRef]
- Kitaya, K.; Yasuo, T. Commonalities and Disparities between Endometriosis and Chronic Endometritis: Therapeutic Potential of Novel Antibiotic Treatment Strategy against Ectopic Endometrium. Int. J. Mol. Sci. 2023, 24, 2059. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Steffensen, R.; Nielsen, H.S.; Varming, K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol. Obstet. Investig. 2008, 66, 257–267. [Google Scholar] [CrossRef]
- Ishimwe, J.A. Maternal microbiome in preeclampsia pathophysiology and implications on offspring health. Physiol. Rep. 2021, 9, e14875. [Google Scholar] [CrossRef]
- Rafat, D.; Singh, S.; Nawab, T.; Khan, F.; Khan, A.U.; Khalid, S. Association of vaginal dysbiosis and gestational diabetes mellitus with adverse perinatal outcomes. Int. J. Gynecol. Obstet. 2022, 158, 70–78. [Google Scholar] [CrossRef]
- Kan, H.; He, Y.; Li, Q.; Mu, Y.; Dong, Y.; Fan, W.; Zhang, M.; Wang, T.; Li, Y.; Liu, H.; et al. Differential Effect of Vaginal Microbiota on Spontaneous Preterm Birth among Chinese Pregnant Women. BioMed Res. Int. 2022, 2022, 3536108. [Google Scholar] [CrossRef]
- Esmaeili, S.A.; Mahmoudi, M.; Rezaieyazdi, Z.; Sahebari, M.; Tabasi, N.; Sahebkar, A.; Rastin, M. Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics. J. Cell. Biochem. 2018, 119, 7865–7872. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, S.; Ye, C.; Zhao, W. Gut microbiota dysbiosis in polycystic ovary syndrome: Mechanisms of progression and clinical applications. Front. Cell. Infect. Microbiol. 2023, 13, 1142041. [Google Scholar] [CrossRef]
- Corrie, L.; Awasthi, A.; Kaur, J.; Vishwas, S.; Gulati, M.; Kaur, I.P.; Gupta, G.; Kommineni, N.; Dua, K.; Singh, S.K. Interplay of Gut Microbiota in Polycystic Ovarian Syndrome: Role of Gut Microbiota, Mechanistic Pathways and Potential Treatment Strategies. Pharmaceuticals 2023, 16, 197. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Zhu, J.; Jin, J.; Qi, Q.; Li, L.; Zhou, J.; Cao, L.; Wang, L. The association of gut microbiome with recurrent pregnancy loss: A comprehensive review. Drug Discov. Ther. 2023, 17, 157–169. [Google Scholar] [CrossRef]
- Soyer Caliskan, C.; Yurtcu, N.; Celik, S.; Sezer, O.; Kilic, S.S.; Cetin, A. Derangements of vaginal and cervical canal microbiota determined with real-time PCR in women with recurrent miscarriages. J. Obstet. Gynaecol. 2022, 42, 2105–2114. [Google Scholar] [CrossRef]
- Song, D.; He, Y.; Wang, Y.; Liu, Z.; Xia, E.; Huang, X.; Xiao, Y.; Li, T.-C. Impact of antibiotic therapy on the rate of negative test results for chronic endometritis: A prospective randomized control trial. Fertil. Steril. 2021, 115, 1549–1556. [Google Scholar] [CrossRef]
- Salmeri, N.; Sinagra, E.; Dolci, C.; Buzzaccarini, G.; Sozzi, G.; Sutera, M.; Candiani, M.; Ungaro, F.; Massimino, L.; Danese, S.; et al. Microbiota in Irritable Bowel Syndrome and Endometriosis: Birds of a Feather Flock Together—A Review. Microorganisms 2023, 11, 2089. [Google Scholar] [CrossRef]
- Peelen, M.J.; Luef, B.M.; Lamont, R.F.; de Milliano, I.; Jensen, J.S.; Limpens, J.; Hajenius, P.J.; Jørgensen, J.S.; Menon, R.; PREBIC Biomarker Working Group 2014–2018. The influence of the vaginal microbiota on preterm birth: A systematic review and recommendations for a minimum dataset for future research. Placenta 2019, 79, 30–39. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Tersigni, C.; D’Ippolito, S.; Di Nicuolo, F.; Marana, R.; Valenza, V.; Masciullo, V.; Scaldaferri, F.; Malatacca, F.; de Waure, C.; Gasbarrini, A.; et al. Recurrent pregnancy loss is associated to leaky gut: A novel pathogenic model of endometrium inflammation? J. Transl. Med. 2018, 16, 102. [Google Scholar] [CrossRef]
- Charoensappakit, A.; Sae-Khow, K.; Leelahavanichkul, A. Gut Barrier Damage and Gut Translocation of Pathogen Molecules in Lupus, an Impact of Innate Immunity (Macrophages and Neutrophils) in Autoimmune Disease. Int. J. Mol. Sci. 2022, 23, 8223. [Google Scholar] [CrossRef]
- Poggi, A.; Benelli, R.; Venè, R.; Costa, D.; Ferrari, N.; Tosetti, F.; Zocchi, M.R. Human Gut-Associated Natural Killer Cells in Health and Disease. Front. Immunol. 2019, 10, 961. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Leeuwenhoek 2018, 111, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Tersigni, C.; Barbaro, G.; Castellani, R.; Onori, M.; Granieri, C.; Scambia, G.; Di Simone, N. Oral administration of Bifidobacterium longum ES1 reduces endometrial inflammation in women with recurrent pregnancy loss. Am. J. Reprod. Immunol. 2024, 91, e13804. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Thonusin, C.; Chattipakorn, N.; Chattipakorn, S.C. Impacts of gut microbiota on gestational diabetes mellitus: A comprehensive review. Eur. J. Nutr. 2021, 60, 2343–2360. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.T.; Roesch, L.F.W.; Ördberg, M.; Ilonen, J.; Atkinson, M.A.; Schatz, D.A.; Triplett, E.W.; Ludvigsson, J. Genetic risk for autoimmunity is associated with distinct changes in the human gut microbiome. Nat. Commun. 2019, 10, 3621. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lu, T.; Liang, X.; Huang, T.; Wu, L.; He, Z.; Xiao, X.; Fan, S. The influence of placenta microbiota of normal term pregnant women on immune regulation during pregnancy. BMC Pregnancy Childbirth 2024, 24, 171. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Li, D.; Li, M. An Estrogen-NK Cells Regulatory Axis in Endometriosis, Related Infertility, and Miscarriage. Int. J. Mol. Sci. 2024, 25, 3362. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, A.; Aguilera, M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Nutrients 2020, 12, 757. [Google Scholar] [CrossRef]
- Murphy, K.; Gromisch, M.; Srinivasan, S.; Wang, T.; Wood, L.; Proll, S.; Liu, C.; Fiedler, T.; Valint, D.J.; Fredricks, D.N.; et al. IgA coating of vaginal bacteria is reduced in the setting of bacterial vaginosis (BV) and preferentially targets BV-associated species. Infect. Immun. 2023, 92, e0037323. [Google Scholar] [CrossRef]
- Azkargorta, M.; Bregón-Villahoz, M.; Escobes, I.; Ibáñez-Pérez, J.; Iloro, I. In-depth proteomics and natural peptidomics analyses reveal antibacterial peptides in human endometrial fluid. J. Proteom. 2020, 216, 103652. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, J.V.; De Sanctis, J.B. A Brief Analysis of Tissue-Resident NK Cells in Pregnancy and Endometrial Diseases: The Importance of Pharmacologic Modulation. Immuno 2021, 1, 174–193. [Google Scholar] [CrossRef]
- Dai, M.; Xu, Y.; Gong, G.; Zhang, Y. Roles of immune microenvironment in the female reproductive maintenance and regulation: Novel insights into the crosstalk of immune cells. Front. Immunol. 2023, 14, 1109122. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P. The Immunophysiology of Male Reproduction. In Knobil and Neill’s Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 2015; pp. 805–892. [Google Scholar] [CrossRef]
- Solders, M.; Gorchs, L.; Erkers, T.; Lundell, A.C.; Nava, S.; Gidlöf, S.; Tiblad, E.; Magalhaes, I.; Kaipe, H. MAIT cells accumulate in placental intervillous space and display a highly cytotoxic phenotype upon bacterial stimulation. Sci. Rep. 2017, 7, 6123. [Google Scholar] [CrossRef] [PubMed]
- Favaro, R.R.; Phillips, K.; Delaunay-Danguy, R.; Ujčič, K.; Markert, U.R. Emerging Concepts in Innate Lymphoid Cells, Memory, and Reproduction. Front. Immunol. 2022, 13, 824263. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; Leeansyah, E.; Introini, A.; Paquin-Proulx, D.; Hasselrot, K.; Andersson, E.; Broliden, K.; Sandberg, J.K.; Tjernlund, A. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017, 10, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.M.; Hladik, F.; Prlic, M. Advances and challenges in studying the tissue-resident T cell compartment in the human female reproductive tract. Immunol. Rev. 2023, 316, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Prašnikar, E.; Kunej, T.; Gorenjak, M.; Potočnik, U.; Kovačič, B.; Knez, J. Transcriptomics of receptive endometrium in women with sonographic features of adenomyosis. Reprod. Biol. Endocrinol. 2022, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Jewanraj, J.; Ngcapu, S.; Osman, F.; Mtshali, A.; Singh, R.; Mansoor, L.E.; Abdool Karim, S.S.; Abdool Karim, Q.; Passmore, J.S.; Liebenberg, L.J.P. The Impact of Semen Exposure on the Immune and Microbial Environments of the Female Genital Tract. Front. Reprod. Health 2020, 2, 566559. [Google Scholar] [CrossRef]
- Koga, K.; Izumi, G.; Mor, G.; Fujii, T.; Osuga, Y. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am. J. Reprod. Immunol. 2014, 72, 192–205. [Google Scholar] [CrossRef]
- Benjelloun, F.; Quillay, H.; Cannou, C.; Marlin, R.; Madec, Y.; Fernandez, H.; Chrétien, F.; Le Grand, R.; Barré-Sinoussi, F.; Nugeyre, M.T.; et al. Activation of Toll-Like Receptors Differentially Modulates Inflammation in the Human Reproductive Tract: Preliminary Findings. Front. Immunol. 2020, 11, 1655. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado-Torroglosa, I.; García-Velasco, J.A.; Alecsandru, D. The Impacts of Inflammatory and Autoimmune Conditions on the Endometrium and Reproductive Outcomes. J. Clin. Med. 2024, 13, 3724. [Google Scholar] [CrossRef] [PubMed]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The female reproductive tract microbiotas, inflammation, and gynecological conditions. Front. Reprod. Health 2022, 4, 963752. [Google Scholar] [CrossRef] [PubMed]
- Berryman, M.A.; Ilonen, J.; Triplett, E.W.; Ludvigsson, J. Important denominator between autoimmune comorbidities: A review of class II HLA, autoimmune disease, and the gut. Front. Immunol. 2023, 14, 1270488. [Google Scholar] [CrossRef] [PubMed]
- Ludgate, M.E.; Masetti, G.; Soares, P. The relationship between the gut microbiota and thyroid disorders. Nat. Rev. Endocrinol. 2024, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Godines-Enriquez, M.S.; Miranda-Velásquez, S.; Enríquez-Pérez, M.M.; Arce-Sánchez, L.; Martínez-Cruz, N.; Flores-Robles, C.M.; Aguayo-González, P.; Morales-Hernández, F.V.; Villarreal-Barranca, A.; Suárez-Rico, B.V.; et al. Prevalence of Thyroid Autoimmunity in Women with Recurrent Pregnancy Loss. Medicina 2021, 57, 96. [Google Scholar] [CrossRef] [PubMed]
- Turesheva, A.; Aimagambetova, G.; Ukybassova, T.; Marat, A.; Kanabekova, P.; Kaldygulova, L.; Amanzholkyzy, A.; Ryzhkova, S.; Nogay, A.; Khamidullina, Z.; et al. Recurrent Pregnancy Loss Etiology, Risk Factors, Diagnosis, and Management. Fresh Look into a Full Box. J. Clin. Med. 2023, 12, 4074. [Google Scholar] [CrossRef] [PubMed]
- Buendia-Roldan, I.; Ponce-Gallegos, M.A.; Lara-Beltrán, D.; Del Ángel-Pablo, A.D.; Pérez-Rubio, G.; Mejía, M.; Selman, M.; Falfán-Valencia, R. The HLA-DRB1*07 Allele Is Associated with Interstitial Lung Abnormalities (ILA) and Subpleural Location in a Mexican Mestizo Population. Biomolecules 2022, 12, 1662. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Barakonyi, A. The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health. Antioxidants 2023, 12, 1055. [Google Scholar] [CrossRef]
- Vanstokstraeten, R.; Callewaert, E.; Blotwijk, S.; Rombauts, E.; Crombé, F.; Emmerechts, K.; Soetens, O.; Vandoorslaer, K.; De Geyter, D.; Allonsius, C.; et al. Comparing Vaginal and Endometrial Microbiota Using Culturomics: Proof of Concept. Int. J. Mol. Sci. 2023, 24, 5947. [Google Scholar] [CrossRef]
- Osadchiy, V.; Belarmino, A.; Kianian, R.; Sigalos, J.T.; Ancira, J.S.; Kanie, T.; Mangum, S.F.; Tipton, C.D.; Hsieh, T.-C.M.; Mills, J.N.; et al. Semen microbiota are dramatically altered in men with abnormal sperm parameters. Sci. Rep. 2024, 14, 1068. [Google Scholar] [CrossRef]
- Doroftei, B.; Ilie, O.D.; Armeanu, T.; Stoian, I.L.; Anton, N.; Babici, R.G.; Ilea, C. A Narrative Review Discussing the Obstetric Repercussions Due to Alterations of Personalized Bacterial Sites Developed within the Vagina, Cervix, and Endometrium. J. Clin. Med. 2023, 12, 5069. [Google Scholar] [CrossRef]
- Faught, B.M.; Reyes, S. Characterization and Treatment of Recurrent Bacterial Vaginosis. J. Women’s Health 2019, 28, 1218–1226. [Google Scholar] [CrossRef]
- Rahman, N.; Mian, M.F.; Nazli, A.; Kaushic, C. Human vaginal microbiota colonization is regulated by female sex hormones in a mouse model. Front. Cell. Infect. Microbiol. 2023, 13, 1307451. [Google Scholar] [CrossRef]
- Shen, J.; Song, N.; Williams, C.J.; Brown, C.J.; Yan, Z.; Xu, C.; Forney, L.J. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci. Rep. 2016, 6, 24380. [Google Scholar] [CrossRef]
- Gustin, A.T.; Thurman, A.R.; Chandra, N.; Schifanella, L.; Alcaide, M.; Fichorova, R.; Doncel, G.F.; Gale, M., Jr.; Klatt, N.R. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am. J. Obstet. Gynecol. 2022, 226, 225.e1–225.e15. [Google Scholar] [CrossRef]
- Tuniyazi, M.; Zhang, N. Possible Therapeutic Mechanisms and Future Perspectives of Vaginal Microbiota Transplantation. Microorganisms 2023, 11, 1427. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Zhang, G. Vaginal microbiota transplantation is a truly opulent and promising edge: Fully grasp its potential. Front. Cell. Infect. Microbiol. 2024, 14, 1280636. [Google Scholar] [CrossRef]
- Martinelli, S.; Nannini, G.; Cianchi, F.; Staderini, F.; Coratti, F.; Amedei, A. Microbiota Transplant and Gynecological Disorders: The Bridge between Present and Future Treatments. Microorganisms 2023, 11, 2407. [Google Scholar] [CrossRef]
- Wrønding, T.; Vomstein, K.; Bosma, E.F.; Mortensen, B.; Westh, H.; Heintz, J.E.; Mollerup, S.; Petersen, A.M.; Ensign, L.M.; DeLong, K.; et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: A proof of concept case study. eClinicalMedicine 2023, 61, 102070. [Google Scholar] [CrossRef]
- Lyra, A.; Ala-Jaakkola, R.; Yeung, N.; Datta, N.; Evans, K.; Hibberd, A.; Lehtinen, M.J.; Forssten, S.D.; Ibarra, A.; Pesonen, T.; et al. A Healthy Vaginal Microbiota Remains Stable during Oral Probiotic Supplementation: A Randomised Controlled Trial. Microorganisms 2023, 11, 499. [Google Scholar] [CrossRef]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.M.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.M.; et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG 2020, 127, 275–284. [Google Scholar] [CrossRef]
- Marcotte, H.; Larsson, P.G.; Andersen, K.K.; Zuo, F.; Mikkelsen, L.S.; Brandsborg, E.; Gray, G.; Laher, F.; Otwombe, K. An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infect. Dis. 2019, 19, 824. [Google Scholar] [CrossRef]
- Rafiee, M.; Sereshki, N.; Alipour, R.; Ahmadipanah, V.; Pashoutan Sarvar, D.; Wilkinson, D. The effect of probiotics on immunogenicity of spermatozoa in couples suffering from recurrent spontaneous abortion. BMC Immunol. 2022, 23, 32. [Google Scholar] [CrossRef]
- Giannella, L.; Grelloni, C.; Quintili, D.; Fiorelli, A.; Montironi, R.; Alia, S.; Delli Carpini, G.; Di Giuseppe, J.; Vignini, A.; Ciavattini, A. Microbiome Changes in Pregnancy Disorders. Antioxidants 2023, 12, 463. [Google Scholar] [CrossRef]
- Qi, F.; Fan, S.; Fang, C.; Ge, L.; Lyu, J.; Huang, Z.; Zhao, S.; Zou, Y.; Huang, L.; Liu, X.; et al. Orally administrated Lactobacillus gasseri TM13 and Lactobacillus crispatus LG55 can restore the vaginal health of patients recovering from bacterial vaginosis. Front. Immunol. 2023, 14, 1125239. [Google Scholar] [CrossRef]
- Hertz, F.B.; Holm, J.B.; Pallejá, A.; Björnsdóttir, M.K.; Mikkelsen, L.S.; Brandsborg, E.; Frimodt-Møller, N. Vaginal microbiome following orally administered probiotic. APMIS 2022, 130, 605–611. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Y.; Zhang, M.; Wu, K.; Zhang, L.; Yao, Y.; Zheng, C. Gut microbiota-derived metabolites associate with circulating immune cell subsets in unexplained recurrent spontaneous abortion. Heliyon 2024, 10, e24571. [Google Scholar] [CrossRef]
- Zargar, M.; Ghafourian, M.; Behrahi, F.; Nikbakht, R.; Salehi, A.M. Association of recurrent implantation failure and recurrent pregnancy loss with peripheral blood natural killer cells and interferon-gamma level. Obstet. Gynecol. Sci. 2024, 67, 112–119. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, X.; Zhang, Y.; Chen, Z.; Huang, J.; Zhang, X.; Kwak-Kim, J. The characteristics of antigenic specificity of memory regulatory t cells in women with unexplained recurrent pregnancy loss. J. Reprod. Immunol. 2022, 154, 103694. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Yao, G.; Jin, J.; Zhang, T.; Sun, C.; Wang, Z.; Zhang, Q. Intestinal flora and pregnancy complications: Current insights and future prospects. iMeta 2024, 3, e167. [Google Scholar] [CrossRef]
- .Lu, X.; Shi, Z.; Jiang, L.; Zhang, S. Maternal gut microbiota in the health of mothers and offspring: From the perspective of immunology. Front. Immunol. 2024, 15, 1362784. [Google Scholar] [CrossRef]
- Esparvarinha, M.; Madadi, S.; Aslanian-Kalkhoran, L.; Nickho, H.; Dolati, S.; Pia, H.; Danaii, S.; Taghavi, S.; Yousefi, M. Dominant immune cells in pregnancy and pregnancy complications: T helper cells (TH1/TH2, TH17/Treg cells), NK cells, MDSCs, and the immune checkpoints. Cell Biol. Intern. 2023, 47, 507–519. [Google Scholar] [CrossRef]
- Jin, M.; Li, D.; Ji, R.; Liu, W.; Xu, X.; Feng, X. Changes in Gut Microorganism in Patients with Positive Immune Antibody-associated Recurrent Abortion. BioMed. Res. Int. 2020, 4673250. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Su, Y.; Zhang, X.; Qu, J.; Liao, D.; Zou, Q.; Zou, H.; Liu, X.; Li, C.; et al. Proteomic profiling analysis of human endometrium in women with unexplained recurrent spontaneous abortion. J. Proteom. 2023, 288, 104996. [Google Scholar] [CrossRef]
- Ali, S.; Majid, S.; Ali, M.N.; Taking, S.; Rehman, M.U.; Arafah, A. Cytokine imbalance at the materno-embryonic interface as a potential immune mechanism for recurrent pregnancy loss. Int. Immunopharmacol. 2021, 90, 107118. [Google Scholar] [CrossRef]
- Dingle, K.; Kassem, O.M.; Azizieh, F.; AbdulHussain, G.; Raghupathy, R. Quantitative analyses of cytokine profiles reveal hormone-mediated modulation of cytokine profiles in recurrent spontaneous miscarriage. Cytokine 2023, 164, 156160. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; He, Y.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.-N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef]
- Di Pierro, F.; Sinatra, F.; Cester, M.; Da Ros, L.; Pistolato, M.; Da Parè, V.; Fabbro, L.; Maccari, D.; Dotto, S.; Sossai, S.; et al. Effect of L. crispatus M247 Administration on Pregnancy Outcomes in Women Undergoing IVF: A Controlled, Retrospective, Observational, and Open-Label Study. Microorganisms 2023, 11, 2796. [Google Scholar] [CrossRef]
- Barati, M.; Jabbari, M.; Ghavidel, A.A.; Nikmehr, P.; Arzhang, P.; Aynehchi, A.; Babashahi, M.; Mosharkesh, E.; Roshanravan, N.; Shabani, M.; et al. The engineered probiotics for the treatment of chronic diseases: A systematic review. J. Food Biochem. 2022, 46, e14343. [Google Scholar] [CrossRef]
- Kemp, M.W.; Newnham, J.P.; Challis, J.G.; Jobe, A.H.; Stock, S.J. The clinical use of corticosteroids in pregnancy. Hum. Reprod. Update 2016, 22, 240–259. [Google Scholar] [CrossRef]
- Giulini, S.; Grisendi, V.; Sighinolfi, G.; Di Vinci, P.; Tagliasacchi, D.; Botticelli, L.; La Marca, A.; Facchinetti, F. Chronic endometritis in recurrent implantation failure: Use of prednisone and IVF outcome. J. Reprod. Immunol. 2022, 153, 103673. [Google Scholar] [CrossRef]
- Hart, R.J. Nutritional supplements and IVF: An evidence-based approach. Reprod. Biomed. Online 2023, 48, 103770. [Google Scholar] [CrossRef]
- Piekarska, K.; Dratwa, M.; Radwan, P.; Radwan, M.; Bogunia-Kubik, K.; Nowak, I. Pro- and anti-inflammatory cytokines and growth factors in patients undergoing in vitro fertilization procedure treated with prednisone. Front. Immunol. 2023, 14, 1250488. [Google Scholar] [CrossRef]
- Su, Q.; Pan, Z.; Yin, R.; Li, X. The value of G-CSF in women experienced at least one implantation failure: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1370114. [Google Scholar] [CrossRef]
- Yao, K.; Sun, Y.; Ye, X.; Wu, Y. Interferon-λ contributes to endometrial receptivity. Reproduction 2023, 165, 569–582. [Google Scholar] [CrossRef]
- Nnamonu, E.I.; Mgbenka, B.O.; Mbegbu, E.C. Impact of omega-3 fatty acids preconception intake on some fertility parameters and foetuses quality of female rats. Iran. J. Vet. Res. 2020, 21, 115–119. [Google Scholar]
- Trop-Steinberg, S.; Gal, M.; Azar, Y.; Kilav-Levin, R.; Heifetz, E.M. Effect of omega-3 supplements or diets on fertility in women: A meta-analysis. Heliyon 2024, 10, e29324. [Google Scholar] [CrossRef]
- Mu, F.; Huo, H.; Wang, M.; Wang, F. Omega-3 fatty acid supplements and recurrent miscarriage: A perspective on potential mechanisms and clinical evidence. Food Sci. Nutr. 2023, 11, 4460–4471. [Google Scholar] [CrossRef]
- Kello, N.; Cho, Y.M. Natural supplements in antiphospholipid syndrome: A case for further study. Clin. Immunol. 2024, 258, 109848. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, M.; Chen, R.; Chen, P.; Chen, L.; Fang, C.; Li, T. Endometrial microbial alterations disrupt endometrial immune homeostasis by overactivation of Eicosapentaenoic acid biosynthesis leading to altered endometrial receptivity. J. Reprod. Immunol. 2023, 155, 103787. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Cotton, J.; Chen, S.; Horvath, V.; Stejskalova, A.; Gulati, A.; LoGrande, N.T.; Budnik, B.; Shahriar, S.; Doherty, E.R.; et al. Mucus production, host-microbiome interactions, hormone sensitivity, and innate immune responses modeled in human cervix chips. Nat. Commun. 2024, 15, 4578. [Google Scholar] [CrossRef] [PubMed]
| Findings | References |
|---|---|
| Low levels of Lactobacillus spp. in the first trimester of pregnancy may be associated with a higher risk of pregnancy loss in the second trimester. | [43] |
| The risk of miscarriage increases when vaginal Lactobacillus spp. levels fall during the first or second trimester of pregnancy. | [44,45] |
| Women with abnormal vaginal microbiota, i.e., Gardnerella vaginalis and Ureaplasma spp. have a higher risk of preterm birth and probably miscarriage. | [46] |
| A higher abundance of pathogenic bacteria, such as the genera Ureaplasma and Mycoplasma, was found in women who had a miscarriage. | [47] |
| Vaginal dysbiosis frequency was higher in women who had experienced a second-trimester miscarriage compared to those who had multiple miscarriages. | [48] |
| Women who experienced one miscarriage in the previous six months had been suffering from bacterial vaginosis. | [49] |
| Lactobacillus spp. is absent in vaginal samples from RPL patients. | [50] |
| Women who had a miscarriage had higher levels of potentially pathogenic bacteria in their vaginal microbiota compared to women with successful pregnancies. | [51] |
| Reduced Lactobacillus spp. levels are associated with the growth of bacteria of the genera Streptococcus, Prevotella, and Atopobium in women with RPL. | [52] |
| The decreased amount of Lactobacillus spp. and an increased number of bacteria of the genera Gardnerella, Prevotella, Megastrobila, and Cyclospora in vaginal microbiota may be responsible for RPL. | [53] |
| Ureaplasma spp. is abundant in the endometrial microbiota of RPL patients | [54] |
| The presence of Lactobacillus iners in vaginal microbiota increases the possibility of miscarriage. | [55] |
| High prevalence of antibiotic-resistant Enterococcus spp. and Staphylococcus spp. in the vaginal flora RPL patients | [56] |
| Low quantity of Lactobacillus species in samples of uterine microbiota in RIF and RPL | [57] |
| A dysbiotic endometrial microbiota profile composed of the genera Atopobium, Bifidobacterium, Chryseobacterium, Gardnerella, Haemophilus, Klebsiella, Neisseria, Staphylococcus and Streptococcus was associated with miscarriage. | [58] |
| Lower levels of Lactobacillus crispatus and high levels of pathogenic bacteria in patients with RPL as compared to normal pregnancy | [59] |
| Gardnerella vaginalis was present in higher abundance in the endometrial samples of women with RPL than in the controls. | [60] |
| Pseudomonadota and Bacillota species were elevated in the endometrial microbiota of RPL patients. | [61] |
| Infertile women with chronic endometritis have reduced amounts of Bifidobacterium and lactic acid-producing bacteria in their vaginal microbiota, apart from Lactobacillus. | [62] |
| Patients with RPL had lower levels of Lactobacillus spp. and abundant levels of pathogenic bacteria in the cervical mucus than women with successful pregnancies. | [63] |
| Bacteria of the genera Cutibacterium and Anaerobacillus are abundant in the cervix of patients with miscarriage compared to normal pregnancy. | [64] |
| Lactobacillus acidophilus was absent, but Lactobacillus iners was abundant in the vaginal and endometrial samples of RPL women with celiac disease. | [65] |
| L. jensenii was decreased in the early embryonic arrest group compared to the normal pregnancy cohort | [66] |
| Vaginal dysbiosis correlates with a higher pregnancy loss in IVF patients | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garmendia, J.V.; De Sanctis, C.V.; Hajdúch, M.; De Sanctis, J.B. Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection. Microorganisms 2024, 12, 1641. https://doi.org/10.3390/microorganisms12081641
Garmendia JV, De Sanctis CV, Hajdúch M, De Sanctis JB. Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection. Microorganisms. 2024; 12(8):1641. https://doi.org/10.3390/microorganisms12081641
Chicago/Turabian StyleGarmendia, Jenny Valentina, Claudia Valentina De Sanctis, Marián Hajdúch, and Juan Bautista De Sanctis. 2024. "Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection" Microorganisms 12, no. 8: 1641. https://doi.org/10.3390/microorganisms12081641
APA StyleGarmendia, J. V., De Sanctis, C. V., Hajdúch, M., & De Sanctis, J. B. (2024). Microbiota and Recurrent Pregnancy Loss (RPL); More than a Simple Connection. Microorganisms, 12(8), 1641. https://doi.org/10.3390/microorganisms12081641








