The Role of Postbiotics in Asthma Treatment
Abstract
:1. Introduction
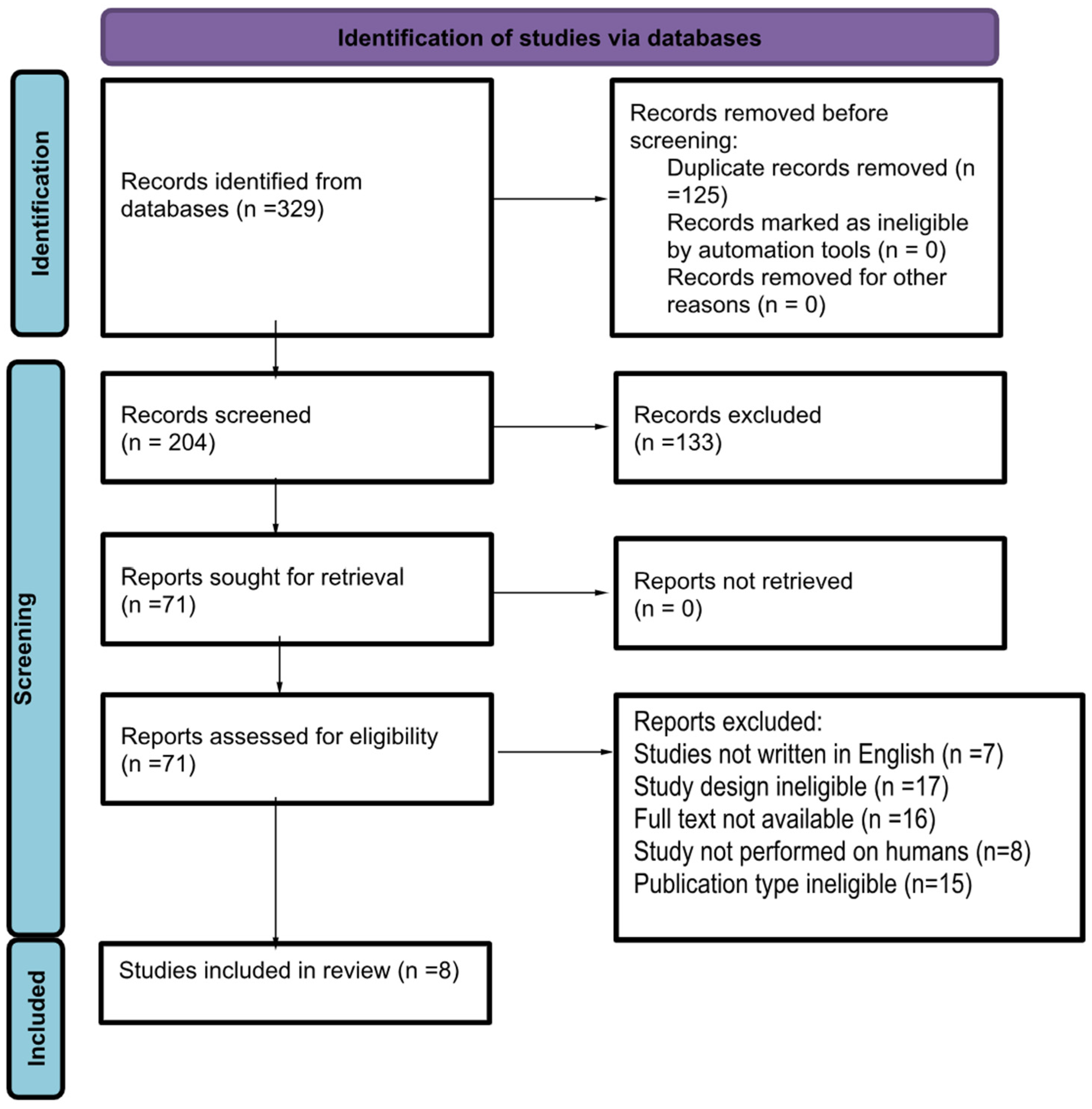
2. Methods of Acquiring Data
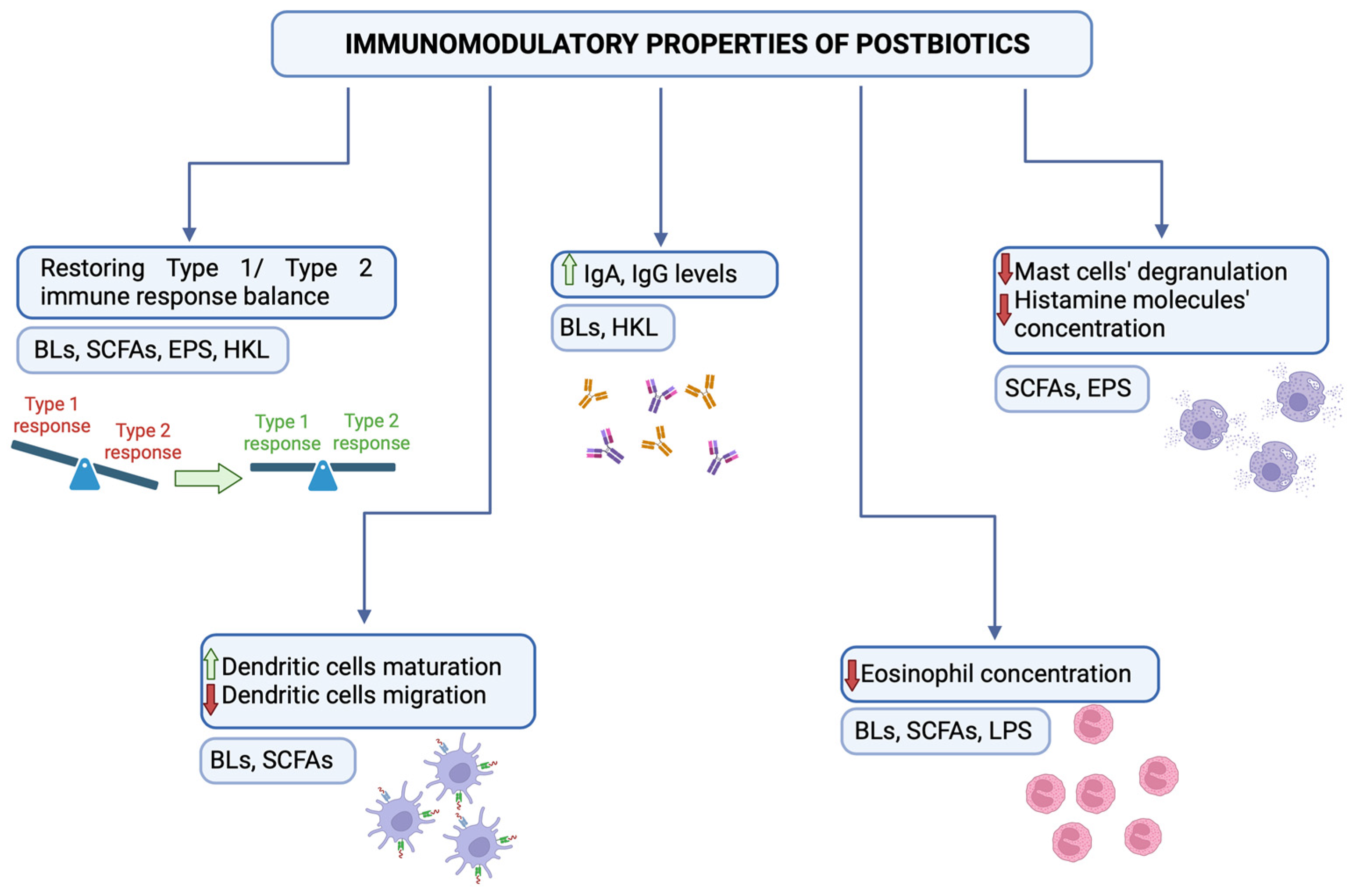
3. Bacterial Lysates
4. Short-Chained Fatty Acids
5. Exopolysaccharides
6. Heat-Killed Lactobacillus
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinberger, M. Can we prevent exacerbations of asthma caused by common cold viruses? J. Allergy Clin. Immunol. 2010, 126, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Zolkiewicz, J.; Marzec, A.; Ruszczynski, M.; Feleszko, W. Postbiotics—A Step beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Naja, A.S.; Permaul, P.; Phipatanakul, W. Taming Asthma in School-Aged Children: A Comprehensive Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Gans, M.D.; Gavrilova, T. Understanding the immunology of asthma: Pathophysiology, biomarkers, and treatments for asthma endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Nobs, S.P.; Zmora, N.; Elinav, E. Nutrition Regulates Innate Immunity in Health and Disease. Annu. Rev. Nutr. 2020, 40, 189–219. [Google Scholar] [CrossRef]
- Bessler, W.G.; Vor dem Esche, U.; Masihi, N. The bacterial extract OM-85 BV protects mice against influenza and Salmonella infection. Int. Immunopharmacol. 2010, 10, 1086–1090. [Google Scholar] [CrossRef]
- Suarez, N.; Ferrara, F.; Rial, A.; Dee, V.; Chabalgoity, J.A. Bacterial Lysates as Immunotherapies for Respiratory Infections: Methods of Preparation. Front. Bioeng. Biotechnol. 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Anapurapu, S.; Page, C.P. Polyvalent mechanical bacterial lysate for the prevention of recurrent respiratory infections: A meta-analysis. Pulm. Pharmacol. Ther. 2012, 25, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pivniouk, V.; Gimenes-Junior, J.A.; Ezeh, P.; Michael, A.; Pivniouk, O.; Hahn, S.; VanLinden, S.R.; Malone, S.P.; Abidov, A.; Anderson, D.; et al. Airway administration of OM-85, a bacterial lysate, blocks experimental asthma by targeting dendritic cells and the epithelium/IL-33/ILC2 axis. J. Allergy Clin. Immunol. 2022, 149, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.; Pohunek, P.; Feleszko, W.; Ballarini, S.; Colin, A.A. Viral infections and wheezing-asthma inception in childhood: Is there a role for immunomodulation by oral bacterial lysates? Clin. Transl. Allergy 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Ver Heul, A.; Planer, J.; Kau, A.L. The Human Microbiota and Asthma. Clin. Rev. Allergy Immunol. 2019, 57, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Kearney, S.C.; Dziekiewicz, M.; Feleszko, W. Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann. Allergy Asthma Immunol. 2015, 114, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Kirtland, M.E.; Tsitoura, D.C.; Durham, S.R.; Shamji, M.H. Toll-Like Receptor Agonists as Adjuvants for Allergen Immunotherapy. Front. Immunol. 2020, 11, 599083. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Karisola, P.; Fyhrquist, N.; Savinko, T.; Lehtimaki, S.; Wolff, H.; Lauerma, A.; Alenius, H. Toll-like receptor activation during cutaneous allergen sensitization blocks development of asthma through IFN-gamma-dependent mechanisms. J. Investig. Dermatol. 2013, 133, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Coviello, S.; Wimmenauer, V.; Polack, F.P.; Irusta, P.M. Bacterial lysates improve the protective antibody response against respiratory viruses through Toll-like receptor 4. Hum. Vaccines Immunother. 2014, 10, 2896–2902. [Google Scholar] [CrossRef]
- Li, Y.; Tu, C.; Chen, M.; Tan, C.; Zheng, X.; Wang, Z.; Liang, Y.; Wang, K.; Wu, J.; Li, H.; et al. Establishing a high microbial load maternal-offspring asthma model in adult mice. Int. Immunopharmacol. 2020, 83, 106453. [Google Scholar] [CrossRef]
- de Boer, G.M.; Braunstahl, G.J.; van der Ploeg, E.K.; van Zelst, C.M.; van Bruggen, A.; Epping, G.; van Nimwegen, M.; Verhoeven, G.; Birnie, E.; Boxma-de Klerk, B.M.; et al. Bacterial lysate add-on therapy to reduce exacerbations in severe asthma: A double-blind placebo-controlled trial. Clin. Exp. Allergy 2021, 51, 1172–1184. [Google Scholar] [CrossRef]
- Han, L.; Zheng, C.P.; Sun, Y.Q.; Xu, G.; Wen, W.; Fu, Q.L. A bacterial extract of OM-85 Broncho-Vaxom prevents allergic rhinitis in mice. Am. J. Rhinol. Allergy 2014, 28, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Han, R.F.; Li, H.Y.; Wang, J.W.; Cong, X.J. Study on clinical effect and immunologic mechanism of infants capillary bronchitis secondary bronchial asthma treated with bacterial lysates Broncho-Vaxom. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2151–2155. [Google Scholar] [PubMed]
- Lu, Y.; Li, Y.; Xu, L.; Xia, M.; Cao, L. Bacterial lysate increases the percentage of natural killer T cells in peripheral blood and alleviates asthma in children. Pharmacology 2015, 95, 139–144. [Google Scholar] [CrossRef]
- Rodrigues, A.; Gualdi, L.P.; de Souza, R.G.; Vargas, M.H.; Nunez, N.K.; da Cunha, A.A.; Jones, M.H.; Pinto, L.A.; Stein, R.T.; Pitrez, P.M. Bacterial extract (OM-85) with human-equivalent doses does not inhibit the development of asthma in a murine model. Allergol. Immunopathol. 2016, 44, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak-Emeryk, M.; Emeryk, A.; Rolinski, J.; Wawryk-Gawda, E.; Markut-Miotla, E. Impact of Polyvalent Mechanical Bacterial Lysate on lymphocyte number and activity in asthmatic children: A randomized controlled trial. Allergy Asthma Clin. Immunol. 2021, 17, 10. [Google Scholar] [CrossRef]
- Luan, H.; Zhang, Q.; Wang, L.; Wang, C.; Zhang, M.; Xu, X.; Zhou, H.; Li, X.; Xu, Q.; He, F.; et al. OM85-BV induced the productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-mediated ERK1/2/NF-κB pathway in RAW264.7 cells. J. Interferon Cytokine Res. 2014, 34, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Y.; Zhang, T. Influence of OM-85 BV on hBD-1 and immunoglobulin in children with asthma and recurrent respiratory tract infection. Zhongguo Dang Dai Er Ke Za Zhi 2014, 16, 508–512. [Google Scholar]
- Liu, C.; Huang, R.; Yao, R.; Yang, A. The Immunotherapeutic Role of Bacterial Lysates in a Mouse Model of Asthma. Lung 2017, 195, 563–569. [Google Scholar] [CrossRef]
- Kaczynska, A.; Klosinska, M.; Janeczek, K.; Zarobkiewicz, M.; Emeryk, A. Promising Immunomodulatory Effects of Bacterial Lysates in Allergic Diseases. Front. Immunol. 2022, 13, 907149. [Google Scholar] [CrossRef]
- Weiss, S.; Fux, T. Effect of Broncho-Vaxom on serum IgE and IgG levels in patients with bronchial asthma and chronic obstructive lung disease. A placebo-controlled double-blind study. Schweiz. Med. Wochenschr. 1987, 117, 1514–1518. [Google Scholar]
- Emeryk, A.; Bartkowiak-Emeryk, M.; Raus, Z.; Braido, F.; Ferlazzo, G.; Melioli, G. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children—The EOLIA study. Pediatr. Allergy Immunol. 2018, 29, 394–401. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Hu, C.; Di Nardo, M.; Srinivasan, V.; Adamko, D.J.; Sun, J.; Du, Y.; Zeng, X. Effectiveness of polyvalent bacterial lysate for pediatric asthma control: A retrospective propensity score-matched cohort study. Transl. Pediatr. 2022, 11, 1697–1703. [Google Scholar] [CrossRef]
- Koatz, A.M.; Coe, N.A.; Ciceran, A.; Alter, A.J. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections. Lung 2016, 194, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.A.; Hanna, K.M.; El Attar, S.; Abdel Nabi, E.; Hatem, A.; Abdel Ghaffar, M. Influence of a bacterial extract, broncho-vaxom, on clinical and immunological parameters in patients with intrinsic asthma. Int. J. Immunother. 1993, 9, 127–133. [Google Scholar]
- Roßberg, S.; Keller, T.; Icke, K.; Siedmann, V.; Lau, I.; Keil, T.; Lau, S. Orally applied bacterial lysate in infants at risk for atopy does not prevent atopic dermatitis, allergic rhinitis, asthma or allergic sensitization at school age: Follow-up of a randomized trial. Allergy 2020, 75, 2020–2025. [Google Scholar] [CrossRef]
- Yip, W.; Hughes, M.R.; Li, Y.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Ang, Z.; Ding, J.L. GPR41 and GPR43 in Obesity and Inflammation—Protective or Causative? Front. Immunol. 2016, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Suaini, N.H.A.; Afghani, J.; Heye, K.N.; O’Mahony, L.; Venter, C.; Lauener, R.; Frei, R.; Roduit, C. Systematic review of the association between short-chain fatty acids and allergic diseases. Allergy 2024, 79, 1789–1811. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.M.M.; Pelgrom, L.R.; van der Ham, A.J.; Yazdanbakhsh, M.; Everts, B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells via both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109A Signaling. Front. Immunol. 2017, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Kozielski, J.; Bartolik, K.; Kabicz, P.; Targowski, T. The incidence of pneumonia in the paediatric population in Poland in light of the maps of health needs. J. Public Health 2023, 31, 457–465. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Hu, M.; Alashkar Alhamwe, B.; Santner-Nanan, B.; Miethe, S.; Harb, H.; Renz, H.; Potaczek, D.P.; Nanan, R.K. Short-Chain Fatty Acids Augment Differentiation and Function of Human Induced Regulatory T Cells. Int. J. Mol. Sci. 2022, 23, 5740. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.; Kim, W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef]
- Theiler, A.; Barnthaler, T.; Platzer, W.; Richtig, G.; Peinhaupt, M.; Rittchen, S.; Kargl, J.; Ulven, T.; Marsh, L.M.; Marsche, G.; et al. Butyrate ameliorates allergic airway inflammation by limiting eosinophil trafficking and survival. J. Allergy Clin. Immunol. 2019, 144, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xu, M.; Pan, S.; Gao, S.; Ren, J.; Bai, R.; Li, H.; He, C.; Zhao, S.; Shi, Z.; et al. Induction of the apoptosis, degranulation and IL-13 production of human basophils by butyrate and propionate via suppression of histone deacetylation. Immunology 2021, 164, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.J.; Junt, T.; Tam, S.Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.B.; Li, M.; Folkerts, G.; Henricks, P.A.J.; Garssen, J.; van Esch, B. Butyrate and Propionate Restore the Cytokine and House Dust Mite Compromised Barrier Function of Human Bronchial Airway Epithelial Cells. Int. J. Mol. Sci. 2020, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Bottcher, M.F.; Nordin, E.K.; Sandin, A.; Midtvedt, T.; Bjorksten, B. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin. Exp. Allergy 2000, 30, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Juraskova, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef]
- Schiavi, E.; Plattner, S.; Rodriguez-Perez, N.; Barcik, W.; Frei, R.; Ferstl, R.; Kurnik-Lucka, M.; Groeger, D.; Grant, R.; Roper, J.; et al. Exopolysaccharide from Bifidobacterium longum subsp. longum 35624 modulates murine allergic airway responses. Benef. Microbes 2018, 9, 761–773. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, H. An exopolysaccharide from Bacillus subtilis alleviates airway inflammatory responses via the NF-κB and STAT6 pathways in asthmatic mice. Biosci. Rep. 2022, 42, BSR20212461. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Hariu, M.; Nakashima, Y.; Watanabe, K.; Yasuda, S.; Igoshi, K. Lactic acid bacterial exopolysaccharides strongly bind histamine and can potentially be used to remove histamine contamination in food. Microbiology 2021, 167, mic000936. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Kanno, K.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Plant-Derived Lactobacillus paracasei IJH-SONE68 Improves Chronic Allergy Status: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2021, 13, 4022. [Google Scholar] [CrossRef] [PubMed]
- Oberg, T.S.; McMahon, D.J.; Culumber, M.D.; McAuliffe, O.; Oberg, C.J. Invited review: Review of taxonomic changes in dairy-related lactobacilli. J. Dairy Sci. 2022, 105, 2750–2770. [Google Scholar] [CrossRef] [PubMed]
- Kalliomaki, M.; Salminen, S.; Arvilommi, H.; Kero, P.; Koskinen, P.; Isolauri, E. Probiotics in primary prevention of atopic disease: A randomised placebo-controlled trial. Lancet 2001, 357, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhang, Y.; Zheng, L.; Rong, N.; Yang, Y.; Gong, P.; Yang, Y.; Siwu, X.; Zhang, C.; Zhu, L.; et al. Bifidobacterium and Lactobacillus improve inflammatory bowel disease in zebrafish of different ages by regulating the intestinal mucosal barrier and microbiota. Life Sci. 2023, 324, 121699. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, F.R.; Tomokiyo, M.; Fukuyama, K.; Elean, M.; Moyano, R.O.; Yamamuro, H.; Shibata, R.; Quilodran-Vega, S.; Kurata, S.; Villena, J.; et al. Post-immunobiotics increase resistance to primary respiratory syncytial virus infection and secondary pneumococcal pneumonia. Benef. Microbes 2023, 14, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.H.; Li, H.Y.; Huang, C.H.; Lee, B.W.; Lee, Y.K.; Chua, K.Y. The effects of heat-killed wild-type Lactobacillus casei Shirota on allergic immune responses in an allergy mouse model. Int. Arch. Allergy Immunol. 2009, 148, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Kim, Y.H.; Oh, S.; Lee, H.J.; Kim, J.H.; Park, S.H.; Kim, H.J.; Lee, S.J.; Chun, T. Anti-inflammatory potential of a heat-killed Lactobacillus strain isolated from Kimchi on house dust mite-induced atopic dermatitis in NC/Nga mice. J. Appl. Microbiol. 2017, 123, 535–543. [Google Scholar] [CrossRef]
- Hong, H.J.; Kim, E.; Cho, D.; Kim, T.S. Differential suppression of heat-killed lactobacilli isolated from kimchi, a Korean traditional food, on airway hyper-responsiveness in mice. J. Clin. Immunol. 2010, 30, 449–458. [Google Scholar] [CrossRef]
- Lee, Y.D.; Hong, Y.F.; Jeon, B.; Jung, B.J.; Chung, D.K.; Kim, H. Differential Cytokine Regulatory Effect of Three Lactobacillus Strains Isolated from Fermented Foods. J. Microbiol. Biotechnol. 2016, 26, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.; Wu, K.G.; Pai, C.; Hsieh, P.S.; Tsai, J.J.; Yen, J.H.; Lin, M.Y. Heat-killed cells of lactobacilli skew the immune response toward T helper 1 polarization in mouse splenocytes and dendritic cell-treated T cells. J. Agric. Food Chem. 2007, 55, 11080–11086. [Google Scholar] [CrossRef] [PubMed]
- Li, A.L.; Meng, X.C.; Duan, C.C.; Huo, G.C.; Zheng, Q.L.; Li, D. Suppressive effects of oral administration of heat-killed Lactobacillus acidophilus on T helper-17 immune responses in a bovine β-lactoglobulin-sensitized mice model. Biol. Pharm. Bull. 2013, 36, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.; Kim, M.; Jeon, S.A.; Kim, Y.H.; Lee, S. A randomized trial of Lactobacillus rhamnosus IDCC 3201 tyndallizate (RHT3201) for treating atopic dermatitis. Pediatr. Allergy Immunol. 2020, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Study Design | Subject (BLs/Control) | Mean Age | Treatment Regimen | Clinical Outcomes | Immunological and Other Outcomes |
|---|---|---|---|---|---|---|
| Emeryk et al. 2018 [32] | RCT | 150 (74/76) | 6–16 years | Ismigen (PMBL) vs. Placebo | The number of asthma exacerbations was lower in the PMBL group. | |
| de Boer et al. 2021 [21] | RCT | 75 (38/37) | 16–60 years | OM-85 (PCBL) vs. SC | Exacerbations were not different between groups after 18 months. | FEV1 increased in the PCBL group. |
| Lu et al. 2015 [28] | RCT | 60 (24/36) | 5–15 years | OM-85 (PCBL) vs. SC | Increased serum IFN-γ/IL-4 ratio was observed. | |
| Li et al. 2022 [33] | Retrospective PS-matched cohort study | 795 (337/458) | 6 months–14 years | QIPIAN (PMBL) vs. SC | Fewer exacerbations were observed in the PMBL group. | |
| Bartkowiak-Emeryk et al. 2021 [26] | RCT | 49 (21/28) | 6–15 years | Ismigen (PMBL) vs. placebo | Increased serum T lymphocyte, CD4+ CD25+ FOXP3+, CD8+, CD3− CD16+ CD56+. Decreased serum CD69+ and CD25+ subset of CD3+. | |
| Koatz et al. 2016 [34] | open-label, prospective, sequential | 28 | 16–65 years | 1st year SC; 2nd year OM-85 (PCBL) | Decreased symptom severity and the number of exacerbations. | Increased serum and salivary secretory IgA. |
| Han et al. 2016 [23] | RCT | 136 (74/62) | 7 months–5 years | OM-85 (PCBL) vs. inhaled corticosteroids/aminophylline/antibiotics | Decreased the frequency and duration of capillary bronchitis and asthma. | Decreased serum IL-4 and IL-17 levels. Increased serum IL-10 and IFN-g levels. |
| Abdou et al. 1993 [35] | RCT | 50(25/25) | Not applicable | OM-85 vs. SC | Reduced the duration and number of asthma attacks. | Increased FEV1/FVC% ratio. Increased serum IgA, IgM, and IgG levels. Decreased serum IgE level. Decreased eosinophil count in bronchoalveolar fluid. Increased IgA/albumin ratio. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgrzyn, K.; Jasińska, A.; Janeczek, K.; Feleszko, W. The Role of Postbiotics in Asthma Treatment. Microorganisms 2024, 12, 1642. https://doi.org/10.3390/microorganisms12081642
Węgrzyn K, Jasińska A, Janeczek K, Feleszko W. The Role of Postbiotics in Asthma Treatment. Microorganisms. 2024; 12(8):1642. https://doi.org/10.3390/microorganisms12081642
Chicago/Turabian StyleWęgrzyn, Konstancja, Agnieszka Jasińska, Kamil Janeczek, and Wojciech Feleszko. 2024. "The Role of Postbiotics in Asthma Treatment" Microorganisms 12, no. 8: 1642. https://doi.org/10.3390/microorganisms12081642







