Analysis of the Probiotic Potential of Lactiplantibacillus plantarum LB1_P46 Isolated from the Mexican Fermented Pulque Beverage: A Functional and Genomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultivation Conditions
2.2. Safety Traits of L. plantarum
2.2.1. Hemolytic Activity
2.2.2. Antibiotic Resistance
2.3. Functional Traits
2.3.1. Cholesterol Reduction
2.3.2. β-Galactosidase Activity
2.4. Cell Surface Properties
2.4.1. Hydrophobicity
2.4.2. Auto-Aggregation
2.4.3. Coaggregation
2.5. In Vitro Assays
2.5.1. Bile Salt and Acid Resistance
2.5.2. Antibacterial Assays against Pathogenic Bacteria
2.5.3. In Vivo Preventive Anti-Infective Activity of L. plantarum LB1_P46 Isolate against Salmonella Typhimurium
2.6. Statistical Analysis
3. Results
3.1. Safety Traits of L. plantarum
3.2. Functional Traits and Cell Surface Properties
3.3. In Vitro Assays
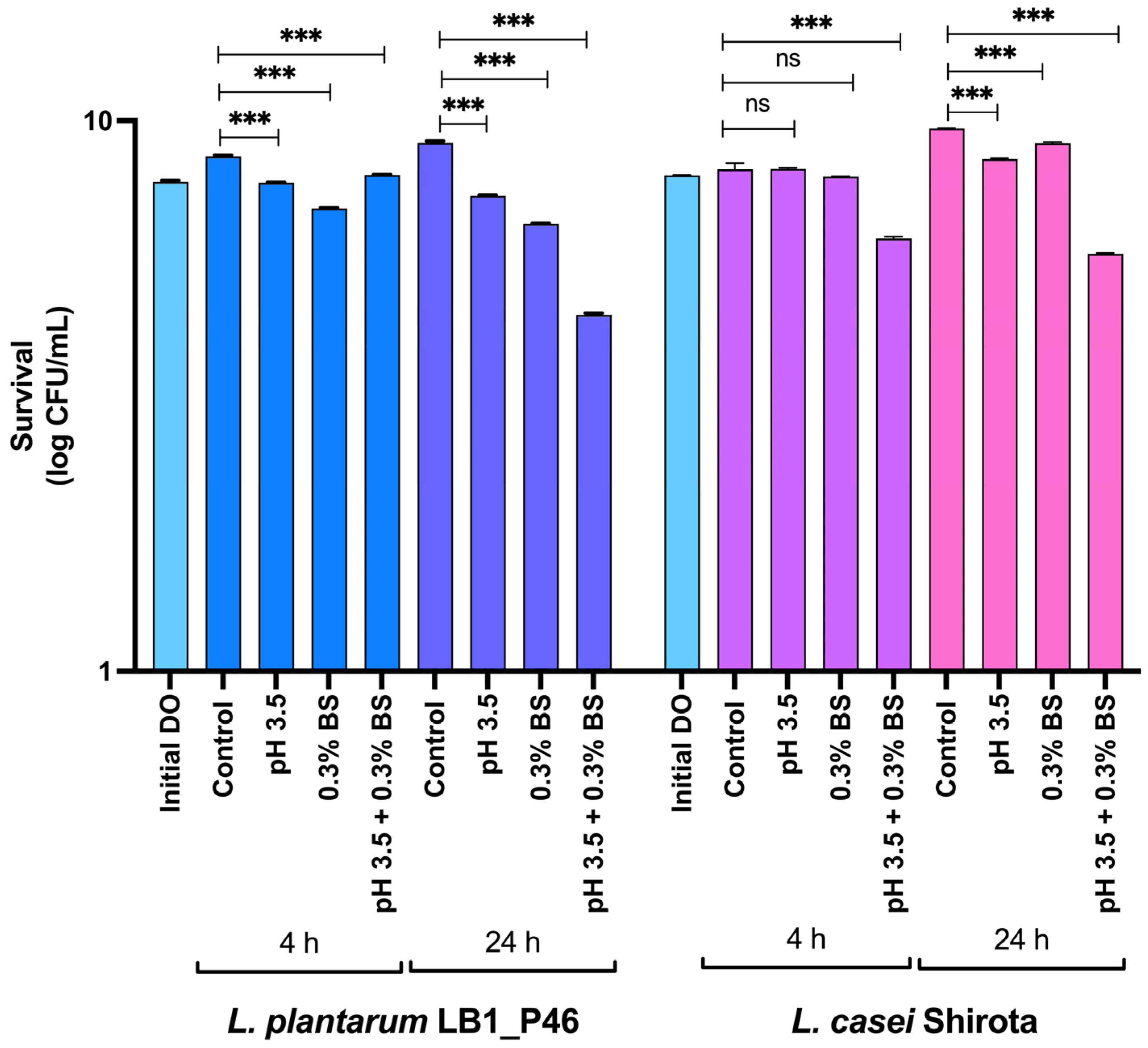
Bile Salt and Acid Resistance
3.4. Antibacterial Assays
3.4.1. In Vitro Antibacterial Assays
3.4.2. In Vivo Anti-Infective Activity of L. plantarum LB1_P46 Isolate against Salmonella Typhimurium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno-Terrazas, R.; Escalante, A.; Verdugo-Valdez, A.G.; De la Rosa, M.; Ortiz Basurto, R.I.; Orantes-García, C.; Palafox-González, L.; Castro Díaz, A.S.; Lappe-Oliveras, P. Bebidas refrescantes y alcohólicas de agave. In Panorama del Aprovechamiento de Agaves en México; CONACYT, CIATEJ, AGARED: Guadalajara, Mexico, 2017; pp. 123–163. ISBN 978-607-97548-2-2. [Google Scholar]
- Ramírez Rancaño, M. El Rey Del Pulque: Ignacio Torres Adalid y La Industria Pulquera; Plaza y Valdes, UNAM, Instituto de Investigaciones Sociales: Ciudad de México, Mexico, 2000; ISBN 968-856-812-0. [Google Scholar]
- Valdivieso Solís, D.G.; Vargas Escamilla, C.A.; Mondragón Contreras, N.; Galván Valle, G.A.; Gilés-Gómez, M.; Bolívar, F.; Escalante, A. Sustainable production of pulque and maguey in Mexico: Current situation and perspectives. Front. Sustain. Food Syst. 2021, 5, 678168. [Google Scholar] [CrossRef]
- Sánchez-Marroquín, A.; Hope, P.H. Agave juice, fermentation and chemical composition studies of some species. J. Agric. Food Chem. 1953, 1, 246–249. [Google Scholar] [CrossRef]
- Escalante, A.; Giles Gomez, M.; Hernandez, G.; Cordova Aguilar, M.; Lopez Munguia, A.; Gosset, G.; Bolivar, F. Analysis of bacterial community during the fermentation of pulque, a traditional Mexican alcoholic beverage, using a polyphasic approach. Int. J. Food Microbiol. 2008, 124, 126–134. [Google Scholar] [CrossRef]
- Escalante, A.; Elena Rodríguez, M.; Martínez, A.; López-Munguía, A.; Bolívar, F.; Gosset, G. Characterization of bacterial diversity in Pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 2004, 235, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Astudillo-Melgar, F.; Hernández-Chávez, G.; Rodríguez-Alegría, M.E.; Bolívar, F.; Escalante, A. Analysis of the microbial diversity and population dynamics during the pulque fermentation process. Fermentation 2023, 9, 342. [Google Scholar] [CrossRef]
- Chacón-Vargas, K.; Torres, J.; Giles-Gómez, M.; Escalante, A.; Gibbons, J.G. Genomic profiling of bacterial and fungal communities and their predictive functionality during pulque fermentation by whole-genome shotgun sequencing. Sci. Rep. 2020, 10, 15115. [Google Scholar] [CrossRef] [PubMed]
- Huezo-Sánchez, A.R.; Ortega-Rodríguez, E.M.; Pérez-Armendáriz, B.; El-Kassis, E.G. Characterization of Bacterial Diversity in Aguamiel and Two Types of Pulque from the Zacatlán Region, México. Fermentation 2023, 9, 564. [Google Scholar] [CrossRef]
- Rocha-Arriaga, C.; Espinal-Centeno, A.; Martinez-Sánchez, S.; Caballero-Pérez, J.; Alcaraz, L.D.; Cruz-Ramírez, A. Deep microbial community profiling along the fermentation process of pulque, a biocultural resource of Mexico. Microbiol. Res. 2020, 241, 126593. [Google Scholar] [CrossRef]
- Goel, A.; Halami, P.M.; Tamang, J.P. Genome Analysis of Lactobacillus plantarum isolated from some Indian fermented foods for bacteriocin production and probiotic marker genes. Front. Microbiol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Zielińska, D.; Kolożyn-Krajewska, D. Food-origin lactic acid bacteria may exhibit probiotic properties: Review. BioMed Res. Int. 2018, 2018, 5063185. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Zielińska, D.; Neffe-Skocińska, K.; Kołożyn-Krajewska, D. Beneficial bacteria isolated from food in relation to the next generation of probiotics. Microorganisms 2023, 11, 1714. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; DeCesare, A.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 19: Suitability of taxonomic units notified to EFSA until September 2023. EFSA J. 2024, 22, e8517. [Google Scholar] [CrossRef]
- FDA. Recently Published GRAS Notices and FDA Letters; FDA: Silver Spring, MD, USA, 2024.
- Escalante, A.; López Soto, D.R.; Velázquez Gutiérrez, J.E.; Giles-Gómez, M.; Bolívar, F.; López-Munguía, A. Pulque, a traditional Mexican alcoholic fermented beverage: Historical, microbiological, and technical aspects. Front. Microbiol. 2016, 7, 1026. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; Giles-Gómez, M.; Esquivel Flores, G.; Matus Acuña, V.; Moreno-Terrazas, R.; López-Munguía, A.; Lappe-Oliveras, P. Pulque Fermentation. In Handbook of Plant-Based Fermented Food and Beverage Technology; Hui, Y.H., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 691–706. ISBN 978-1-4398-7069-3. [Google Scholar]
- Giles-Gómez, M.; Sandoval García, J.G.; Matus, V.; Campos Quintana, I.; Bolívar, F.; Escalante, A. In vitro and in vivo probiotic assessment of Leuconostoc mesenteroides P45 isolated from pulque, a Mexican traditional alcoholic beverage. SpringerPlus 2016, 5, 708. [Google Scholar] [CrossRef] [PubMed]
- Torres-Maravilla, E.; Lenoir, M.; Mayorga-Reyes, L.; Allain, T.; Sokol, H.; Langella, P.; Sánchez-Pardo, M.E.; Bermúdez-Humarán, L.G. Identification of novel anti-inflammatory probiotic strains isolated from pulque. Appl. Microbiol. Biotechnol. 2016, 100, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Elizarrarás, A.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Vega-Sánchez, V.; Velázquez-Guadarrama, N.; Zafra-Rojas, Q.; Piloni-Martini, J. In vitro probiotic potential of lactic acid bacteria isolated from aguamiel and pulque and antibacterial activity against pathogens. Appl. Sci. 2019, 9, 601. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, Y.; Valadez-Blanco, R.; Calderón-García, C.; Chikindas, M.L.; Ponce-Alquicira, E. Probiotic and functional potential of lactic acid bacteria isolated from pulque and evaluation of their safety for food applications. Front. Microbiol. 2023, 14, 1241581. [Google Scholar] [CrossRef] [PubMed]
- Backstrand, J.R.; Goodman, A.H.; Allen, L.H.; Pelto, G.H. Pulque intake during pregnancy and lactation in rural Mexico: Alcohol and child growth from 1 to 57 months. Eu. J. Clin. Nutr. 2004, 58, 1626–1634. [Google Scholar] [CrossRef]
- Backstrand, J.R.; Allen, L.H.; Black, A.K.; de Mata, M.; Pelto, G.H. Diet and iron status of nonpregnant women in rural Central Mexico. Am. J. Clin. Nutr. 2002, 76, 156–164. [Google Scholar] [CrossRef]
- Backstrand, J.R.; Allen, L.H.; Martinez, E.; Pelto, G.H. Maternal Consumption of pulque, a traditional Central Mexican alcoholic beverage: Relationships to infant growth and development. Public Health Nutr. 2001, 4, 883–891. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- De Vries, M.C.; Vaughan, E.E.; Kleerebezem, M.; De Vos, W.M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy. J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Abdulhussain Kareem, R.; Razavi, S.H. Plantaricin bacteriocins: As safe alternative antimicrobial peptides in food preservation—A review. J. Food Saf. 2020, 40, e12735. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Sarıyıldız, S.; Cholakov, R.; Nalbantsoy, A.; Baler, B.; Aslan, E.; Düzel, A.; Sargın, S.; Göksungur, Y.; Kışla, D. A Novel Lactiplantibacillus plantarum strain: Probiotic properties and optimization of the growth conditions by response surface methodology. World J. Microbiol. Biotechnol. 2024, 40, 66. [Google Scholar] [CrossRef]
- Umanets, A.; Surono, I.S.; Venema, K. I Am Better than I Look: Genome based safety assessment of the probiotic Lactiplantibacillus plantarum IS-10506. BMC Genom. 2023, 24, 518. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Rodríguez, E.K.; Astudillo-Melgar, F.; Larios, V.; Bolívar, F.; Escalante, A.; Giles-Gómez, M. The complete genome of two Lactiplantibacillus plantarum isolates from the traditional Mexican fermented pulque beverage assembled with a combination of PacBio and Illumina platforms. Microbiol. Resour. Announc. 2024, 13, e00985-23. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Bourgin, M.; Labarthe, S.; Kriaa, A.; Lhomme, M.; Gérard, P.; Lesnik, P.; Laroche, B.; Maguin, E.; Rhimi, M. Exploring the bacterial impact on cholesterol cycle: A numerical study. Front. Microbiol. 2020, 11, 1121. [Google Scholar] [CrossRef]
- Dawson, J.A.; Mallonee, D.H.; Björkhem, I.; Hylemon, P.B. Expression and characterization of a C24 bile acid 7 alpha-dehydratase from Eubacterium sp. strain VPI 12708 in Escherichia coli. J. Lipid Res. 1996, 37, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, G.; Chen, P.; Cai, K.; Dong, W.; Peng, N.; Zhao, S. Uncovering acid resistance genes in lactic acid bacteria and impact of non-viable bacteria on bacterial community during Chinese strong-flavor baijiu fermentation. Food Res. Int. 2023, 167, 112741. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [PubMed]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A User-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, B.; Kyriakou, P.K.; Oppegård, C.; Nissen-Meyer, J.; Kaznessis, Y.N.; Kristiansen, P.E. Structure–function analysis of the two-peptide bacteriocin plantaricin EF. Biochemistry 2016, 55, 5106–5116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Li, L.; Jiang, X.; Chen, Z.; Zhao, F.; Yi, Y. Biosynthesis and production of class II bacteriocins of food-associated lactic acid bacteria. Fermentation 2022, 8, 217. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Campedelli, I.; Mathur, H.; Salvetti, E.; Clarke, S.; Rea, M.C.; Torriani, S.; Ross, R.P.; Hill, C.; O’Toole, P.W. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2018, 85, e01738-18. [Google Scholar] [CrossRef]
- Selim, S. Mechanisms of Gram-positive vancomycin resistance (Review). Biomed. Rep. 2022, 16, 7. [Google Scholar] [CrossRef]
- Clark, D.P.; Pazdernik, N.J.; McGehee, M.R. Plasmids. In Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 712–748. ISBN 978-0-12-813288-3. [Google Scholar]
- D’Andrea, M.M.; Arena, F.; Pallecchi, L.; Rossolini, G.M. CTX-M-Type β-Lactamases: A successful story of antibiotic resistance. Int. J. Med. Microbiol. 2013, 303, 305–317. [Google Scholar] [CrossRef]
- Li, W.; Atkinson, G.C.; Thakor, N.S.; Allas, Ü.; Lu, C.; Chan, K.-Y.; Tenson, T.; Schulten, K.; Wilson, K.S.; Hauryliuk, V.; et al. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O). Nat. Commun. 2013, 4, 1477. [Google Scholar] [CrossRef]
- Orelle, C.; Mathieu, K.; Jault, J.-M. Multidrug ABC transporters in Bacteria. Res. Microbiol. 2019, 170, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, M.; Chan, E.W.C.; Chen, S. Membrane transporters of the major facilitator superfamily are essential for long-term maintenance of phenotypic tolerance to multiple antibiotics in E. coli. Microbiol. Spectr. 2021, 9, e01846-21. [Google Scholar] [CrossRef] [PubMed]
- Vimberg, V.; Zieglerová, L.; Buriánková, K.; Branny, P.; Balíková Novotná, G. VanZ reduces the binding of lipoglycopeptide antibiotics to Staphylococcus aureus and Streptococcus pneumoniae cells. Front. Microbiol. 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, C.; Xie, J. The underling mechanism of bacterial TetR/AcrR family transcriptional repressors. Cell Signal 2013, 25, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-X.; Zhang, J.-F.; Sun, Y.-H.; Li, R.-S.; Lin, X.-L.; Yang, L.; Webber, M.A.; Jiang, H.-X. contribution of different mechanisms to ciprofloxacin resistance in Salmonella spp. Front. Microbiol. 2021, 12, 663731. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, S.; Tian, K.; Zhou, D.; Wang, B.; Liu, J.; Zhu, H.; Qiao, J. A Novel small RNA S042 increases acid tolerance in Lactococcus lactis F44. Biochem. Biophys. Res. Commun. 2018, 500, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Gänzle, M.G. Influence of cyclopropane fatty acids on heat, high pressure, acid and oxidative resistance in Escherichia coli. Int. J. Food Microbiol. 2016, 222, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Keseler, I.M.; Gama-Castro, S.; Mackie, A.; Billington, R.; Bonavides-Martínez, C.; Caspi, R.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Muñiz-Rascado, L.; et al. The EcoCyc Database in 2021. Front Microbiol 2021, 12, 711077. [Google Scholar] [CrossRef]
- Viola, R.E. L-Aspartase: New tricks from an old enzyme. Adv. Enzymol. Relat. Areas Mol. Biol. 2000, 74, 295–341. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, P.; Zhang, Y.; Li, L.; Chen, S. Characterization of an aspartate-dependent acid survival system in Yersinia pseudotuberculosis. FEBS Lett. 2010, 584, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Santiago, B.; MacGilvray, M.; Faustoferri, R.C.; Quivey, R.G. The branched-chain amino acid aminotransferase encoded by ilvE is involved in acid tolerance in Streptococcus mutans. J. Bacteriol. 2012, 194, 2010–2019. [Google Scholar] [CrossRef]
- Domínguez-Ramírez, L.L.; Rodríguez-Sanoja, R.; Tecante, A.; García-Garibay, M.; Sainz, T.; Wacher, C. Tolerance to acid and alkali by Streptococcus infantarius subsp. infantarius strain 25124 isolated from fermented nixtamal dough: Pozol. studies in APT broth. Food Microbiol. 2020, 90, 103458. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Ran, J.; Xu, X.; Wang, J. Heat, Acid and Cold Stresses Enhance the Expression of DnaK Gene in Alicyclobacillus Acidoterrestris. Food Res. Int. 2015, 67, 183–192. [Google Scholar] [CrossRef]
- Lee, K.; Pi, K.; Kim, E.B.; Rho, B.-S.; Kang, S.-K.; Lee, H.G.; Choi, Y.-J. Glutathione-mediated response to acid stress in the probiotic bacterium Lactobacillus salivarius. Biotechnol. Lett. 2010, 32, 969–972. [Google Scholar] [CrossRef]
- Pophaly, S.D.; Singh, R.; Pophaly, S.D.; Kaushik, J.K.; Tomar, S.K. current status and emerging role of glutathione in food grade lactic acid bacteria. Microb. Cell Fact. 2012, 11, 114. [Google Scholar] [CrossRef]
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef]
- Haghshenas, B.; Kiani, A.; Mansoori, S.; Mohammadi-noori, E.; Nami, Y. Probiotic properties and antimicrobial evaluation of silymarin-enriched Lactobacillus bacteria isolated from traditional curd. Sci. Rep. 2023, 13, 10916. [Google Scholar] [CrossRef]
- Sadeghi, M.; Panahi, B.; Mazlumi, A.; Hejazi, M.A.; Komi, D.E.A.; Nami, Y. Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superior bacteria for application as biocontrol using machine learning models. LWT 2022, 162, 113471. [Google Scholar] [CrossRef]
- Debabov, D.V.; Kiriukhin, M.Y.; Neuhaus, F.C. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: Role of DltD in d-alanylation. J. Bacteriol. 2000, 182, 2855–2864. [Google Scholar] [CrossRef]
- Vélez, M.P.; Verhoeven, T.L.A.; Draing, C.; Von Aulock, S.; Pfitzenmaier, M.; Geyer, A.; Lambrichts, I.; Grangette, C.; Pot, B.; Vanderleyden, J.; et al. Functional analysis of d-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2007, 73, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Galicia Robles, E.M.; Giles Gómez, M. Elaboración de Bebidas Lácteas Fermentadas con las Cepas Probióticas Leuconostoc mesenteroides P45 y Lactobacillus plantarum P46; Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2020. [Google Scholar]
- Prabhurajeshwar, C.; Chandrakanth, K. Evaluation of antimicrobial properties and their substances against pathogenic bacteria in-vitro by probiotic Lactobacilli strains isolated from commercial yoghurt. Clin. Nutr. Exp. 2019, 23, 97–115. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Shen, Y.; Sun, Z. Using probiotics to mute Salmonella enteric serovar Typhimurium: An opinion. Front. Bioeng. Biotechnol. 2020, 8, 558. [Google Scholar] [CrossRef] [PubMed]
- Petrariu, O.-A.; Barbu, I.C.; Niculescu, A.-G.; Constantin, M.; Grigore, G.A.; Cristian, R.-E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 2024, 14, 1296447. [Google Scholar] [CrossRef] [PubMed]


| Antibiotic | Concentration (mg/L) | Antibiotic Susceptibility |
|---|---|---|
| Penicillin (PEP) | 0.031–0.5 | R |
| 0.063–1.0 | R | |
| 0.125–2.0 | R | |
| 0.25–4.0 | I | |
| Penicillin–Streptomycin (PES) | 0.12–2.0 | I |
| Amoxicillin (AMOP) | 2.0–4.0 | S |
| Cefotaxime (CTXP) | 0.5–2.0 | R |
| 0.5–1.0 | R | |
| Erythromycin (ERY) | 0.25 | S |
| Quinupristin/dalfopristin (QDA) | 1.0 | S |
| Clindamycin (CLI) | 0.25 | S |
| Tetracycline (TET) | 2.0 | R |
| Levofloxacin (LVX) | 2.0–4.0 | I |
| Chloramphenicol (CMP) | 4.0 | S |
| Vancomycin (VAN) | 1.0 | R |
| Cotrimoxazole (TSU) (trimetoprima y sulfametoxazol) | 0.5/9.5–2/38 | S |
| Gene/locus_tag 1 | Encoded Protein | Protein_id 1 |
|---|---|---|
| RUO99_00065 | MFS transporter 1, Multidrug resistance protein MdtG 2 | WNW15827.1 |
| RUO99_00280 | TetR/AcrR family transcriptional regulator 1 | WNW15867.1 |
| RUO99_00405 | TetM/TetW/TetO/TetS family tetracycline resistance ribosomal protection protein 1, Tetracycline resistance protein TetO 2 | WNW15891.1 |
| RUO99_00755 | TetR/AcrR family transcriptional regulator 1 | WNW15960.1 |
| RUO99_01090 | Small multidrug resistance protein 1 | WNW16015.1 |
| RUO99_01335 | TetR/AcrR family transcriptional regulator 1 | WNW16064.1 |
| RUO99_01430 | TetR-like C-terminal domain-containing protein 1 | WNW16081.1 |
| RUO99_02075 | Multidrug efflux MFS transporter 1 | WNW16202.1 |
| RUO99_03140 | Tetracycline resistance M.F.S. efflux pump 1 | WNW16395.1 |
| RUO99_03385 | TetR/AcrR family transcriptional regulator 1 | WNW16441.1 |
| RUO99_03440 | VanZ family protein 1 | WNW16450.1 |
| RUO99_03670 | TetR/AcrR family transcriptional regulator 1 | WNW16492.1 |
| RUO99_03685 (pseudo) | Multidrug efflux SMR transporter 1 | |
| RUO99_04150 | TetR/AcrR family transcriptional regulator 1 | WNW16574.1 |
| RUO99_04695 | TetR/AcrR family transcriptional regulator 1 | WNW16678.1 |
| RUO99_05535 | MDR family MFS transporter 1, Multidrug resistance protein 3 2 | WNW16825.1 |
| RUO99_06080 | TetR family transcriptional regulator 1 | WNW16925.1 |
| RUO99_06590 | TetR/AcrR family transcriptional regulator 1 | WNW17024.1 |
| RUO99_06840 | TetR/AcrR family transcriptional regulator 1 | WNW17073.1 |
| RUO99_07040 (pseudo) | TetR/AcrR family transcriptional regulator 1 | |
| RUO99_07045 | MFS transporter/Putative multidrug resistance protein MdtD 1 | WNW17111.1 |
| RUO99_07060 | TetR family transcriptional regulator 1 | WNW17114.1 |
| RUO99_07745 | MFS transporter 1, Multidrug resistance protein 3 2 | WNW14477.1 |
| RUO99_07940 | ABC transporter permease 1, Daunorubicin/doxorubicin resistance ABC transporter permease protein DrrB 2 | WNW14514.1 |
| RUO99_07945 | Daunorubicin resistance protein DrrA family ABC transporter ATP-binding protein 1 | WNW14515.1 |
| RUO99_09860 | Serine hydrolase (GO:0030655—beta-lactam antibiotic catabolic process) 1 | WNW14850.1 |
| RUO99_10165 | ABC transporter ATP-binding protein 1, Multidrug resistance ABC transporter ATP-binding/permease protein BmrA 2 | WNW14908.1 |
| RUO99_10430 | VanZ family protein 1 | WNW14958.1 |
| RUO99_11265 | ABC transporter ATP-binding protein 1, Linearmycin resistance ATP-binding protein LnrL 2 | WNW15116.1 |
| RUO99_11750 | A.B.C. transporter ATP-binding protein 1, Putative multidrug resistance A.B.C. transporter ATP-binding/permease protein YheH 2 | WNW15206.1 |
| RUO99_11750 | A.B.C. transporter ATP-binding protein 1, Putative multidrug resistance A.B.C. transporter ATP-binding/permease protein YheI 2 | WNW15206.1 |
| RUO99_11780 | TetR/AcrR family transcriptional regulator 1 | WNW15212.1 |
| RUO99_12010 | TetR-like C-terminal domain-containing protein 1 | WNW15254.1 |
| RUO99_12025 | ABC transporter ATP-binding protein 1, Multidrug resistance A.B.C. transporter ATP-binding and permease protein 2 | WNW15256.1 |
| RUO99_12525 | TetR/AcrR family transcriptional regulator 1 | WNW15353.1 |
| RUO99_12560 | TetR/AcrR family transcriptional regulator 1 | WNW15360.1 |
| RUO99_12610 | TetR/AcrR family transcriptional regulator 1 | WNW17292.1 |
| RUO99_12660 | TetR/AcrR family transcriptional regulator 1 | WNW15377.1 |
| RUO99_12810 | TetR/AcrR family transcriptional regulator 1 | WNW15405.1 |
| RUO99_13645 | Multidrug efflux SMR transporter 1 | WNW17299.1 |
| RUO99_13760 | VanZ family protein 1 | WNW15579.1 |
| RUO99_14075 | TetR family transcriptional regulator 1 | WNW15636.1 |
| RUO99_14165 | TetR/AcrR family transcriptional regulator 1 | WNW15651.1 |
| RUO99_14295 | TetR/AcrR family transcriptional regulator 1 | WNW15675.1 |
| Assay | L. plantarum LB1_P46 | L. casei Shirota |
|---|---|---|
| β-galactosidase activity | + | + |
| Assayed trait 1 | ||
| Cholesterol assimilation (%) | 56.03 ± 0.96 b | 46.21 ± 0.27 |
| Hydrophobicity (%) | ||
| Chloroform | 37.65 ± 1.08 a | 64.49 ± 0.43 |
| Hexane | 29.13 ± 3.14 NS | 34.78 ± 0.27 |
| Auto-aggregation (%) | 20.67 ± 2.21 b | 30.81 ± 1.56 |
| Coaggregation (%) | ||
| EPEC | 39.12 ± 0.98 b | 54.34 ± 1.04 |
| L. monocitogenes | 30.30 ± 1.24 c | 38.86 ± 1.78 |
| Salmonella Typhi | 56.13 ± 1.71 b | 73.17 ± 1.96 |
| Gene/locus_tag 1 | Gene 1,2 | Encoded Protein | Protein_id 1 |
|---|---|---|---|
| RUO99_00625 | NA 1,2 | Hsp20/alpha-crystallin family protein 1, acid shock protein 2 | WNW15934.1 |
| RUO99_05920 | argR1, argR_1 2 | Arginine repressor 1,2 | WNW16894.1 |
| RUO99_07080 | NA 1, cfa_2 2 | Cyclopropane-fatty-acyl-phospholipid synthase family protein 1,2 | WNW17118.1 |
| RUO99_11515 | aspA2 | Aspartate ammonia-lyase 1,2 | WNW15160.1 |
| RUO99_10045 | NA 1, ilvE 2 | Branched-chain amino acid aminotransferase 1,2 | WNW14887.1 |
| RUO99_12925 | NA 1, cfa_2 2 | Cyclopropane-fatty-acyl-phospholipid synthase family protein 1,2 | WNW15428.1 |
| RUO99_08375 | dnaK1,2 | Molecular chaperone DnaK 1,2 | WNW14596.1 |
| RUO99_09755 | gshAB_2 2 | Glutamate-cysteine ligase 1, glutathione biosynthesis bifunctional protein GshAB 2 | WNW14833.1 |
| RUO99_09835 | NA 1, gshAB_1 2 | Bifunctional glutamate--cysteine ligase GshA/glutathione synthetase GshB 1, glutathione biosynthesis bifunctional protein GshAB 2 | WNW14847.1 |
| Pathogen | L. plantarum LB1_p46 | L. casei Shirota |
|---|---|---|
| Growth Inhibition Zone (mm) 1 | ||
| Salmonella Typhimurium ATCC14028 | 17.5 ± 1.8 a | 7.4 ± 0.4 |
| E. coli 1129 | 11.2 ± 0 a | 7.1 ± 0.0 |
| P. aeruginosa ATCC27853 | 6.3 ± 0.6 a | 4.9 ± 0.4 |
| L monocytogenes CFQ-B-103 | 27.2 ± 0.6 a | 9.6 ± 1.2 |
| S. pyogenes CFQ-B-218 | 13.8 ± 0.9 a | 9.9 ± 0.4 |
| S. aureus ATCC6538 | 13.7 ± 0.5 a | 9.9 ± 0.4 |
| E. faecalis CFQ-B- | 14.0 ± 0.5 b | 13.3 ± 0.5 |
| B. cereus CFQ-B-230 | 13.5 ± 1.4 a | 16.6 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giles-Gómez, M.; Morales Huerta, X.; Pastelin-Palacios, R.; López-Macías, C.; Flores Montesinos, M.S.; Astudillo-Melgar, F.; Escalante, A. Analysis of the Probiotic Potential of Lactiplantibacillus plantarum LB1_P46 Isolated from the Mexican Fermented Pulque Beverage: A Functional and Genomic Analysis. Microorganisms 2024, 12, 1652. https://doi.org/10.3390/microorganisms12081652
Giles-Gómez M, Morales Huerta X, Pastelin-Palacios R, López-Macías C, Flores Montesinos MS, Astudillo-Melgar F, Escalante A. Analysis of the Probiotic Potential of Lactiplantibacillus plantarum LB1_P46 Isolated from the Mexican Fermented Pulque Beverage: A Functional and Genomic Analysis. Microorganisms. 2024; 12(8):1652. https://doi.org/10.3390/microorganisms12081652
Chicago/Turabian StyleGiles-Gómez, Martha, Ximena Morales Huerta, Rodolfo Pastelin-Palacios, Constantino López-Macías, Mayrene Sarai Flores Montesinos, Fernando Astudillo-Melgar, and Adelfo Escalante. 2024. "Analysis of the Probiotic Potential of Lactiplantibacillus plantarum LB1_P46 Isolated from the Mexican Fermented Pulque Beverage: A Functional and Genomic Analysis" Microorganisms 12, no. 8: 1652. https://doi.org/10.3390/microorganisms12081652






