Prevalence of Antimicrobial Resistance in Klebsiella pneumoniae, Enterobacter cloacae, and Escherichia coli Isolates among Stillbirths and Deceased Under-Five Children in Sierra Leone: Data from the Child Health and Mortality Prevention Surveillance Sites from 2019 to 2022
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Site and Settings
2.3. Study Population and Sample Collection
2.4. Inclusion Criteria
2.5. Exclusion Criteria
2.6. Culture and Susceptibility Testing
2.7. Data Analysis
2.8. Ethics
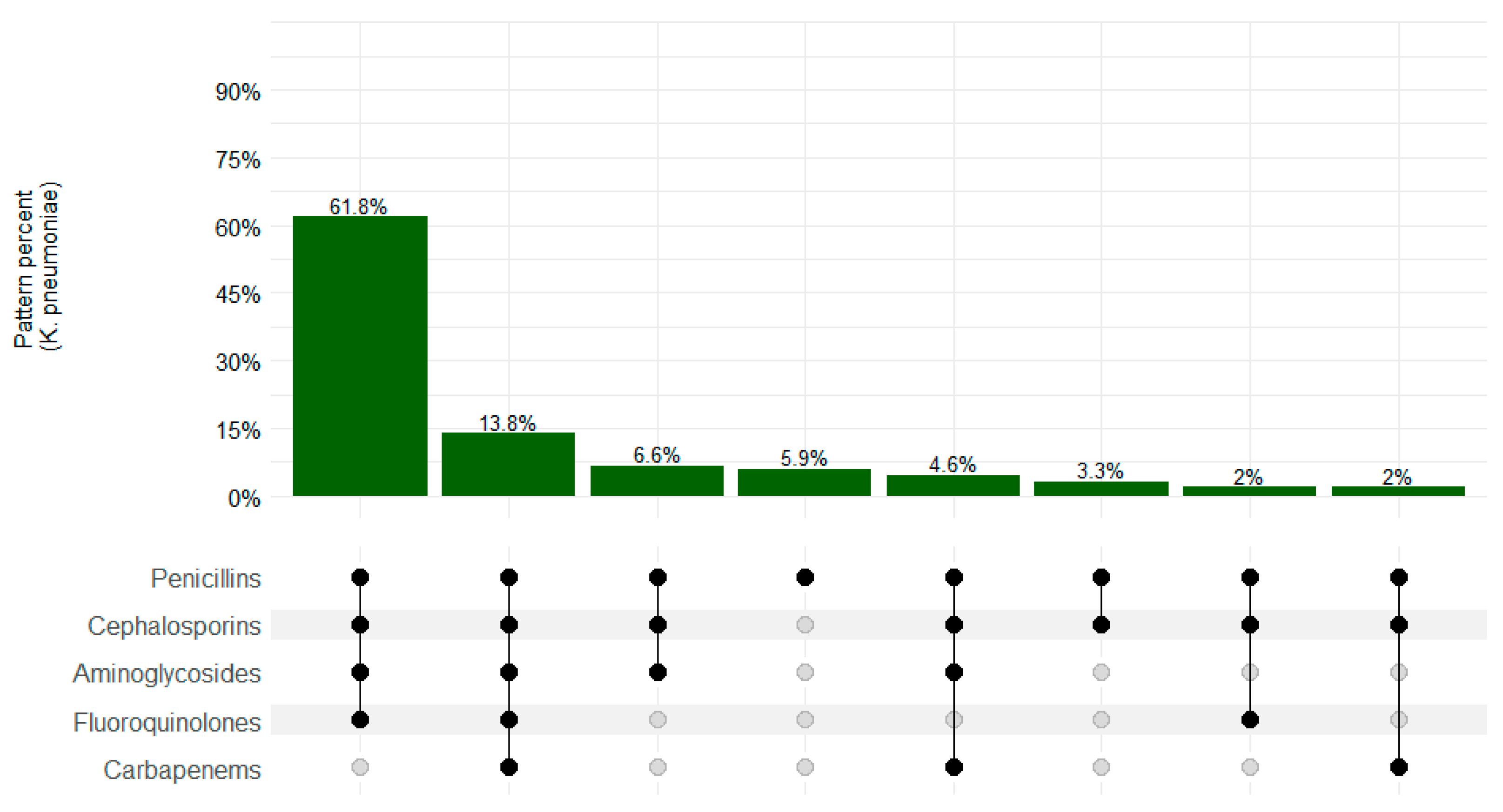
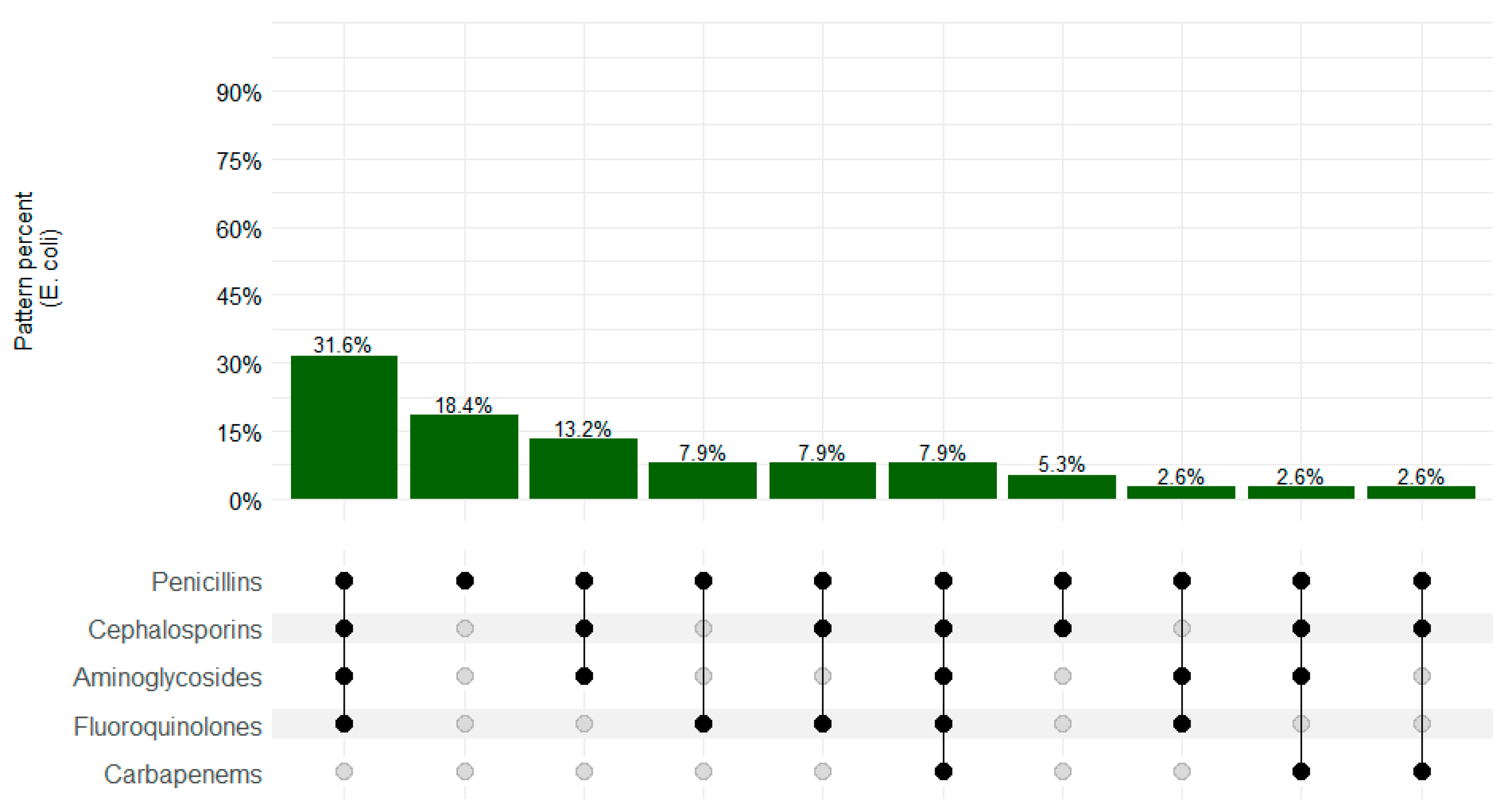
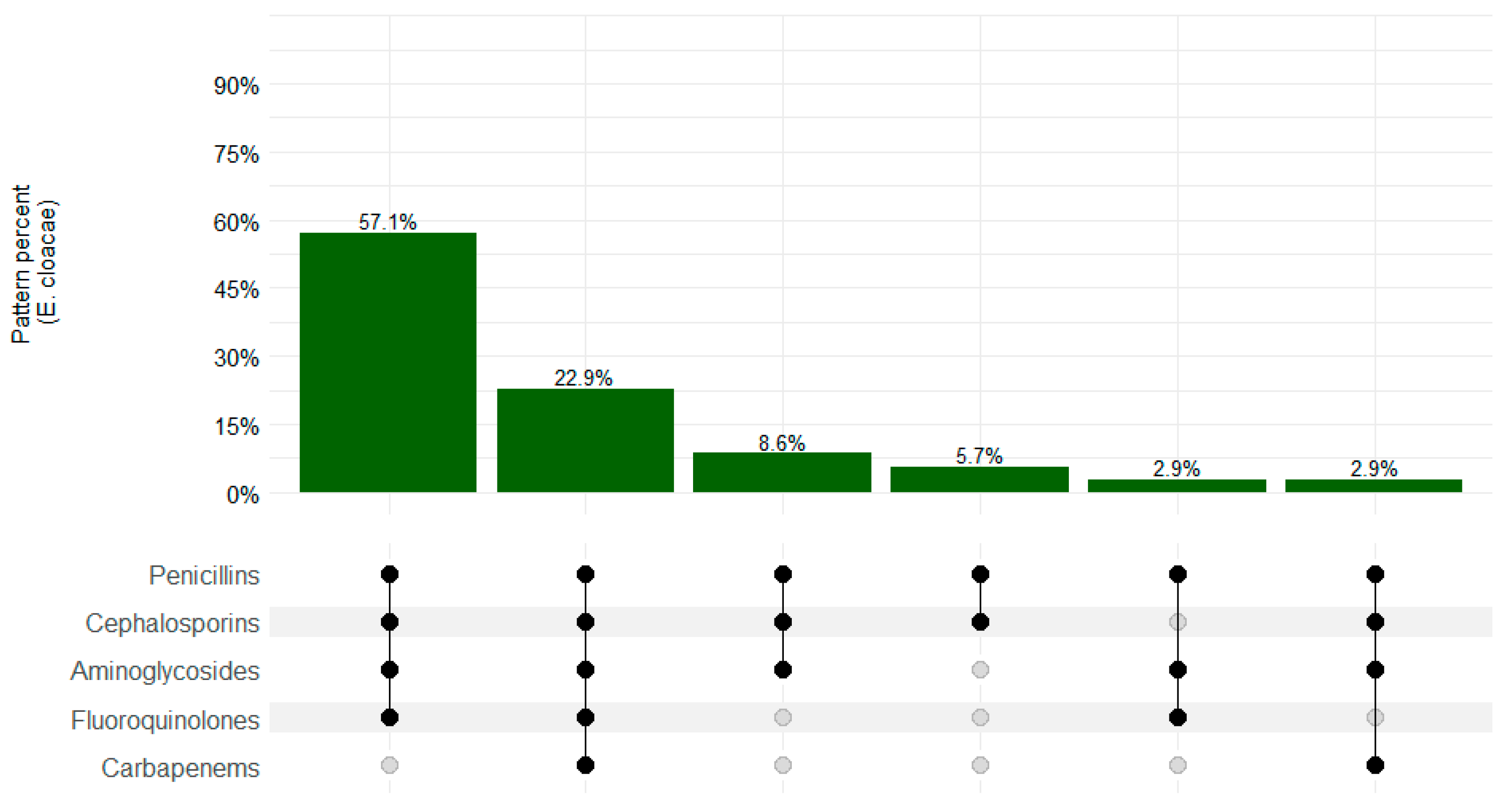
3. Results
| Characteristic | N = 367 1 |
|---|---|
| Isolated organisms | |
| Klebsiella pneumonia | 152 (41.4%) |
| Escherichia coli | 40 (10.9%) |
| Enterobacter cloacae | 35 (9.5%) |
| Other * | 140 (38.1%) |
| Characteristic | N | ESBL 2 (n = 243) 1 | Non-ESBL (n = 124) 1 |
|---|---|---|---|
| Isolated organisms | 367 | ||
| Klebsiella pneumonia | 143 (94.1%) | 9 (5.9%) | |
| Escherichia coli | 27 (67.5%) | 13 (32.5%) | |
| Enterobacter cloacae | 34 (97.1%) | 1 (2.9%) | |
| Other * | 39 (27.8%) | 101 (72.1%) |
| Characteristic | N | CRO 2 (n = 71) 1 | Non-CRO (n = 296) 1 |
|---|---|---|---|
| Isolated organisms | 367 | ||
| Klebsiella pneumonia | 31 (20.4%) | 121 (79.6%) | |
| Escherichia coli | 5 (12.5%) | 35 (87.5%) | |
| Enterobacter cloacae | 9 (25.7%) | 26 (74.3%) | |
| Other * | 26 (18.6%) | 114 (81.4%) |
| Characteristic | N | MDR 2 (n = 258) | Non-MDR (n = 109) 1 |
|---|---|---|---|
| Total isolated organisms | 367 | ||
| Klebsiella pneumonia | 138 (90.8%) | 14 (9.2%) | |
| Escherichia coli | 26 (65.0%) | 14 (35.0%) | |
| Enterobacter cloacae | 33 (94.3%) | 2 (5.7%) | |
| Other * | 61 (43.6%) | 79 (56.4%) |
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kamara, I.F.; Kumar, A.M.V.; Maruta, A.; Fofanah, B.D.; Njuguna, C.K.; Shongwe, S.; Moses, F.; Tengbe, S.M.; Kanu, J.S.; Lakoh, S.; et al. Antibiotic Use in Suspected and Confirmed COVID-19 Patients Admitted to Health Facilities in Sierra Leone in 2020–2021: Practice Does Not Follow Policy. Int. J. Environ. Res. Public Health 2022, 19, 4005. [Google Scholar] [CrossRef] [PubMed]
- Lakoh, S.; Li, L.; Sevalie, S.; Guo, X.; Adekanmbi, O.; Yang, G.; Adebayo, O.; Yi, L.; Coker, J.M.; Wang, S.; et al. Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: A cross-sectional study. Antimicrob. Resist. Infect. Control 2020, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A., III; Forrest, A.; Bulitta, J.B.; Tsuji, B.T. Resurgence of Colistin: A Review of Resistance, Toxicity, Pharmacodynamics, and Dosing. Pharmacotherapy 2010, 30, 1279–1291. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E. Safety profiles of old and new antimicrobials for the treatment of MRSA infections. Expert Opin. Drug Saf. 2016, 15, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, A.; Ogundipe, O.F.; Repetto, E.C.; Mekiedje, C.; Sanke-Waigana, H.; Ngaya, G.; Ingelbeen, B.; Gil-Cuesta, J. When first line treatment of neonatal infection is not enough: Blood culture and resistance patterns in neonates requiring second line antibiotic therapy in Bangui, Central African Republic. BMC Pediatr. 2021, 21, 570. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- World Health Organization Priority Pathogen List; Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. 2024. Available online: https://creativecommons.org/licenses/by-nc-sa/3.0/igo (accessed on 30 July 2024).
- World Health Organization. Global Antimicrobial Resistance Surveillance System: Manual for Early Implementation; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/188783 (accessed on 22 September 2023).
- Son, J.S.; Song, J.-H.; Ko, K.S.; Yeom, J.S.; Ki, H.K.; Kim, S.-W.; Chang, H.-H.; Ryu, S.Y.; Kim, Y.-S.; Jung, S.-I.; et al. Bloodstream Infections and Clinical Significance of Healthcare-associated Bacteremia: A Multicenter Surveillance Study in Korean Hospitals. J. Korean Med. Sci. 2010, 25, 992–998. [Google Scholar] [CrossRef]
- Zou, H.; Jia, X.; He, X.; Su, Y.; Zhou, L.; Shen, Y.; Sheng, C.; Liao, A.; Li, C.; Li, Q. Emerging Threat of Multidrug Resistant Pathogens from Neonatal Sepsis. Front. Cell. Infect. Microbiol. 2021, 11, 694093. [Google Scholar] [CrossRef]
- Cosgrove, S.E. The Relationship between Antimicrobial Resistance and Patient Outcomes: Mortality, Length of Hospital Stay, and Health Care Costs. Clin. Infect. Dis. 2006, 42 (Suppl. 2), S82–S89. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef]
- Final-results_-2015_population_and_housing_census.pdf. 2015. Available online: https://www.statistics.sl/images/StatisticsSL/Documents/final-results_-2015_population_and_housing_census.pdf (accessed on 15 February 2023).
- Matheron, H.; Westendorp, J.; Av, D.; Mansaray, M.; Daskalska, L.; Sankoh, O.; Groen, R. The burden of miscarriages and perinatal deaths in Sierra Leone, data from a nation-wide household survey (PRESSCO 2020). 2022; Preprints. [Google Scholar] [CrossRef]
- Madhi, S.A.; Pathirana, J.; Baillie, V.L.; Izu, A.; Bassat, Q.; Blau, D.M.; Breiman, R.F.; Hale, M.; Mathunjwa, A.; Martines, R.B.; et al. Unraveling Specific Causes of Neonatal Mortality Using Minimally Invasive Tissue Sampling: An Observational Study. Clin. Infect. Dis. 2019, 69 (Suppl. 4), S351–S360. [Google Scholar] [CrossRef]
- Emory Global Health Institute. Child Health and Mortality Prevention Surveillance (CHAMPS) Network. Mortality Surveillance Protocol. Version 1.3. 2016. Available online: https://champshealth.org/wp-content/uploads/2021/01/CHAMPS-Manual-v3.pdf (accessed on 25 January 2024).
- CLSI. CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2021. [Google Scholar]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef]
- Eshetie, S.; Unakal, C.; Gelaw, A.; Ayelign, B.; Endris, M.; Moges, F. Multidrug resistant and carbapenemase producing Enterobacteriaceae among patients with urinary tract infection at referral Hospital, Northwest Ethiopia. Antimicrob. Resist. Infect. Control 2015, 4, 12. [Google Scholar] [CrossRef]
- Newman, M.J.; Frimpong, E.; Donkor, E.S.; Opintan, J.A.; Asamoah-Adu, A. Resistance to antimicrobial drugs in Ghana. Infect. Drug Resist. 2011, 4, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Leski, T.A.; Taitt, C.R.; Bangura, U.; Stockelman, M.G.; Ansumana, R.; CooperIII, W.H.; Stenger, D.A.; Vora, G.J. High prevalence of multidrug resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone. BMC Infect. Dis. 2016, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Buys, H.; Muloiwa, R.; Bamford, C.; Eley, B. Klebsiella pneumoniae bloodstream infections at a South African children’s hospital 2006–2011, a cross-sectional study. BMC Infect. Dis. 2016, 16, 570. [Google Scholar] [CrossRef]
- Adeosun, I.J.; Oladipo, K.E.; Ajibade, O.A.; Olotu, T.M.; A Oladipo, A.; Awoyelu, E.H.; Alli, O.A.T.; Oyawoye, O.M. Antibiotic Susceptibility of Klebsiella pneumoniae isolated from Selected Tertiary Hospitals in Osun State, Nigeria. Iraqi J. Sci. 2019, 60, 1423–1429. [Google Scholar] [CrossRef]
- Oliveira, R.; Castro, J.; Silva, S.; Oliveira, H.; Saavedra, M.J.; Azevedo, N.F.; Almeida, C. Exploring the Antibiotic Resistance Profile of Clinical Klebsiella pneumoniae Isolates in Portugal. Antibiotics 2022, 11, 1613. [Google Scholar] [CrossRef]
- Rezai, M.S.; Pourmousa, R.; Dadashzadeh, R.; Ahangarkani, F. Multidrug resistance pattern of bacterial agents isolated from patient with chronic sinusitis. Casp. J. Intern. Med. 2016, 7, 114–119. [Google Scholar]
- Flokas, M.E.; Karanika, S.; Alevizakos, M.; Mylonakis, E. Prevalence of ESBL-Producing Enterobacteriaceae in Pediatric Bloodstream Infections: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0171216. [Google Scholar] [CrossRef]
- Najjuka, C.F.; Kateete, D.P.; Kajumbula, H.M.; Joloba, M.L.; Essack, S.Y. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res. Notes 2016, 9, 235. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojulong, J.; Gebru, G.N.; Duduyemi, B.; Gbenda, E.; Janneh, M.L.; Sharty, J.; Monteiro, L.; Kowuor, D.; Ameh, S.; Ogbuanu, I.U. Prevalence of Antimicrobial Resistance in Klebsiella pneumoniae, Enterobacter cloacae, and Escherichia coli Isolates among Stillbirths and Deceased Under-Five Children in Sierra Leone: Data from the Child Health and Mortality Prevention Surveillance Sites from 2019 to 2022. Microorganisms 2024, 12, 1657. https://doi.org/10.3390/microorganisms12081657
Ojulong J, Gebru GN, Duduyemi B, Gbenda E, Janneh ML, Sharty J, Monteiro L, Kowuor D, Ameh S, Ogbuanu IU. Prevalence of Antimicrobial Resistance in Klebsiella pneumoniae, Enterobacter cloacae, and Escherichia coli Isolates among Stillbirths and Deceased Under-Five Children in Sierra Leone: Data from the Child Health and Mortality Prevention Surveillance Sites from 2019 to 2022. Microorganisms. 2024; 12(8):1657. https://doi.org/10.3390/microorganisms12081657
Chicago/Turabian StyleOjulong, Julius, Gebrekrstos N. Gebru, Babatunde Duduyemi, Edwin Gbenda, Mohamed L. Janneh, Jack Sharty, Leonel Monteiro, Dickens Kowuor, Soter Ameh, and Ikechukwu U. Ogbuanu. 2024. "Prevalence of Antimicrobial Resistance in Klebsiella pneumoniae, Enterobacter cloacae, and Escherichia coli Isolates among Stillbirths and Deceased Under-Five Children in Sierra Leone: Data from the Child Health and Mortality Prevention Surveillance Sites from 2019 to 2022" Microorganisms 12, no. 8: 1657. https://doi.org/10.3390/microorganisms12081657





