Effects of Wildfire on Soil CO2 Emission and Bacterial Community in Plantations
Abstract
1. Introduction
2. Materials and Methods
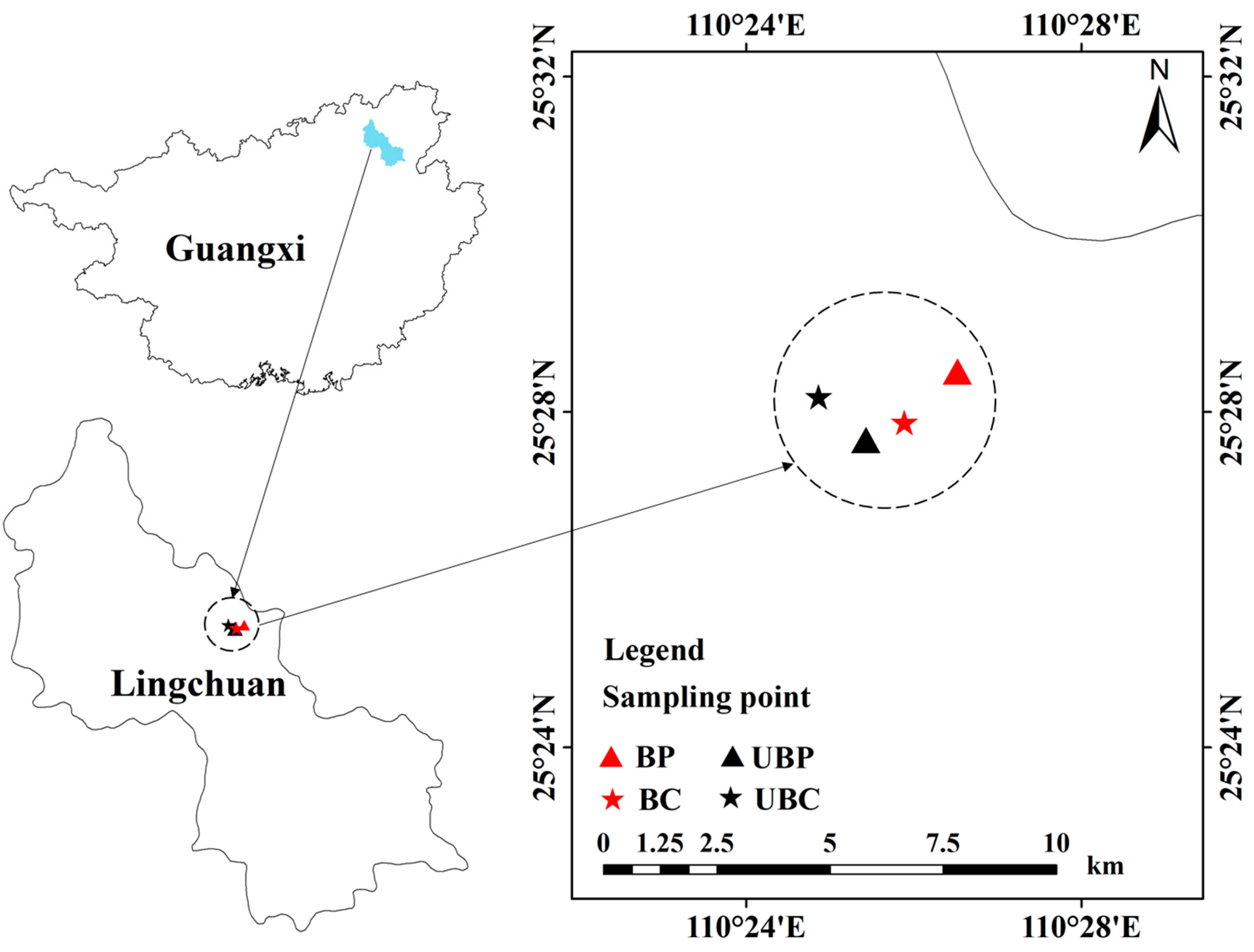
2.1. Study Area and Soil Sampling
2.2. Soil Sampling
2.3. Experimental Design
2.4. Measuring Indices
2.5. Calculations
2.6. Statistical Analyses
3. Results
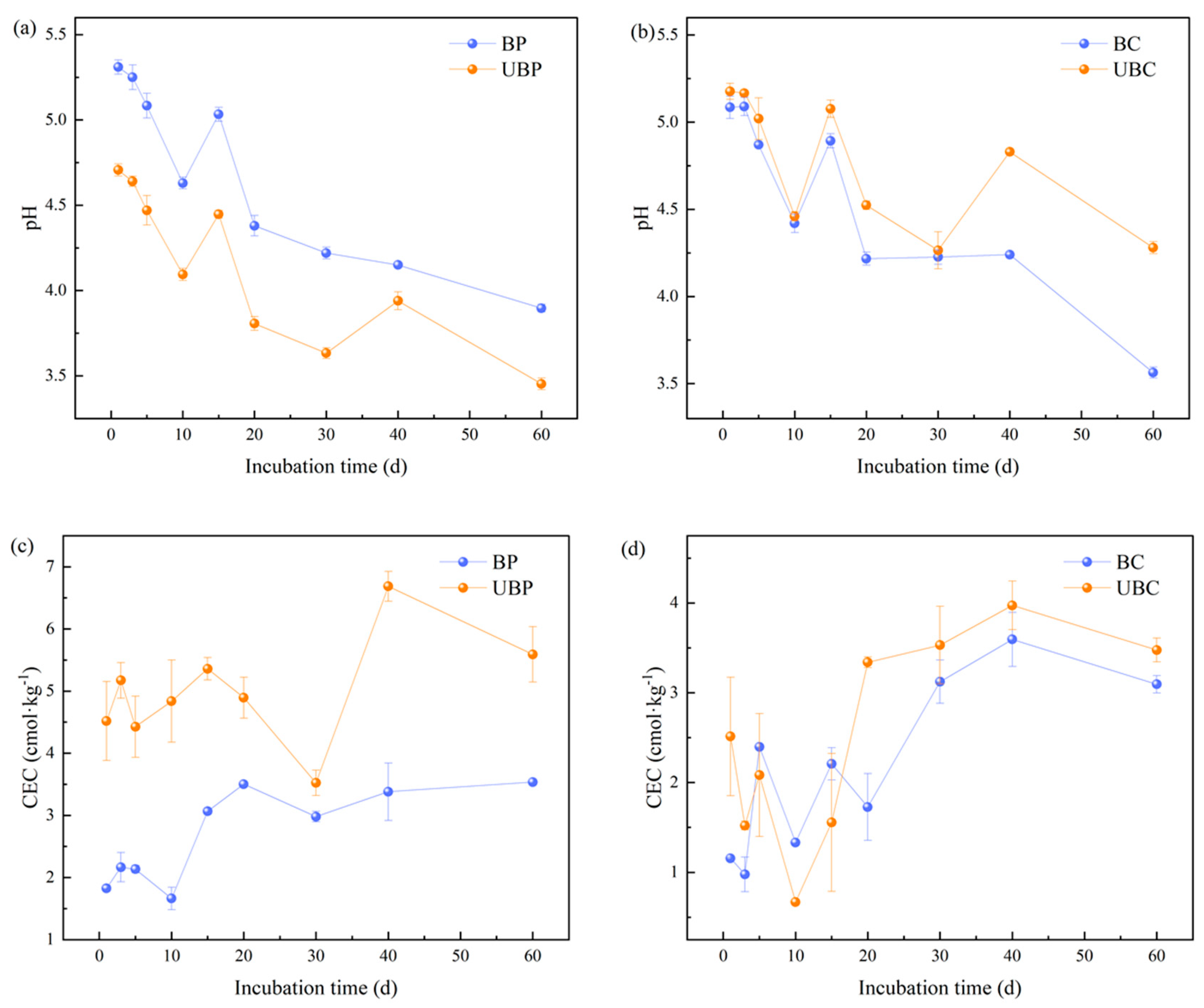
3.1. Effect of Wildfires on Soil pH and Cation Exchange Capacity (CEC)
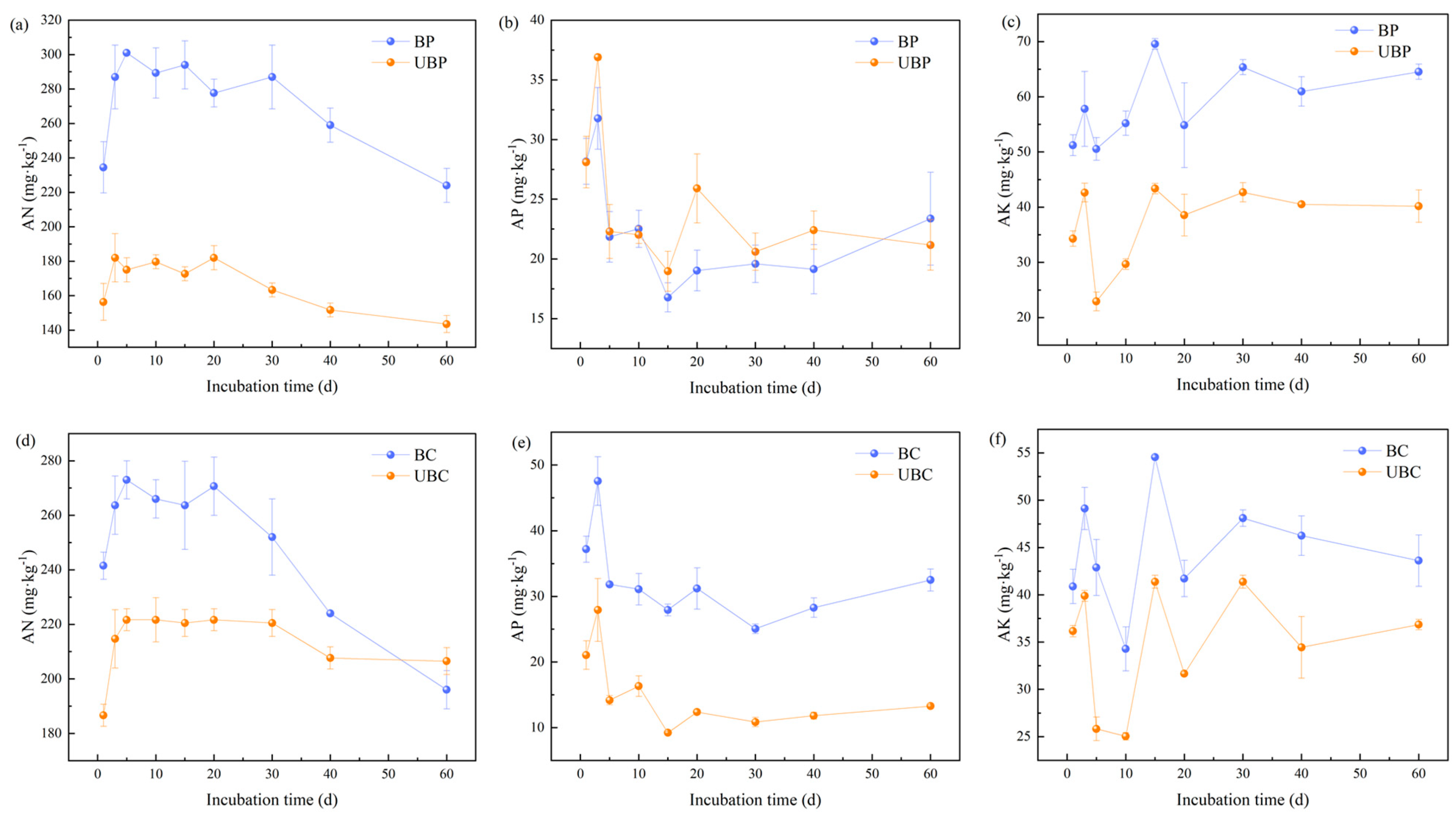
3.2. Effects of Wildfires on Soil Nutrients
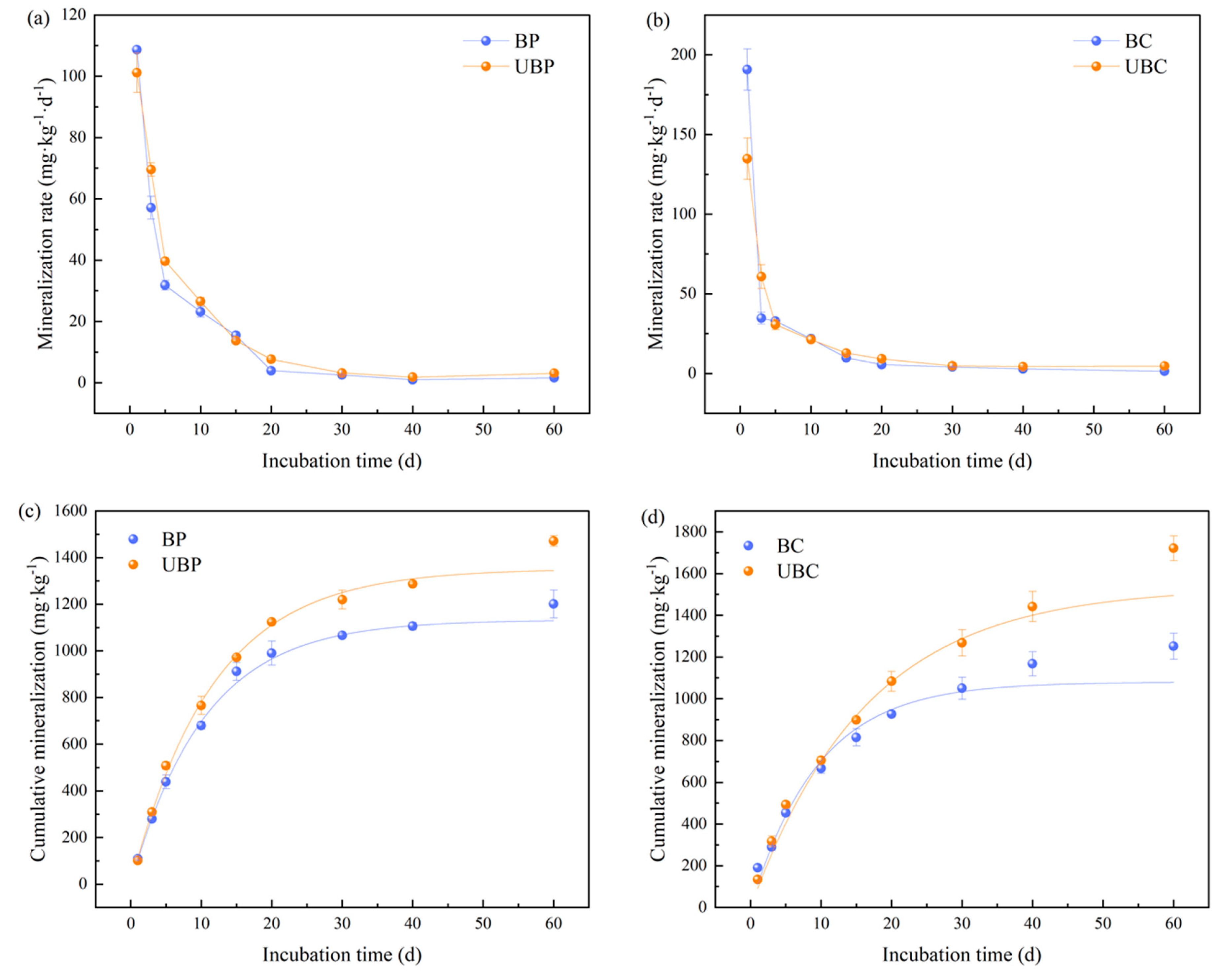
3.3. Effects of Wildfires on Soil Organic Carbon Mineralization
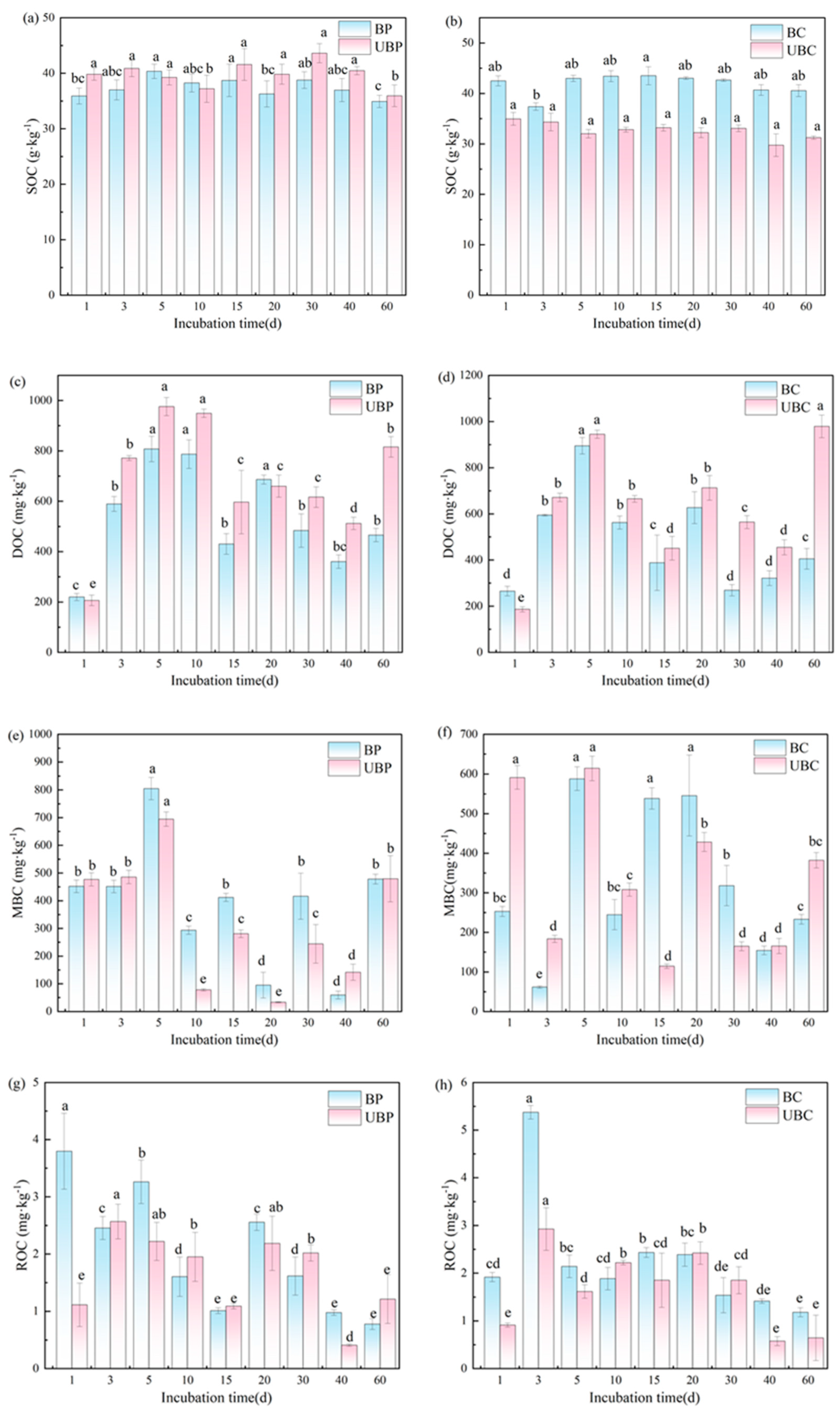
3.4. Effects of Wildfires on Different Carbon Fractions of Soils
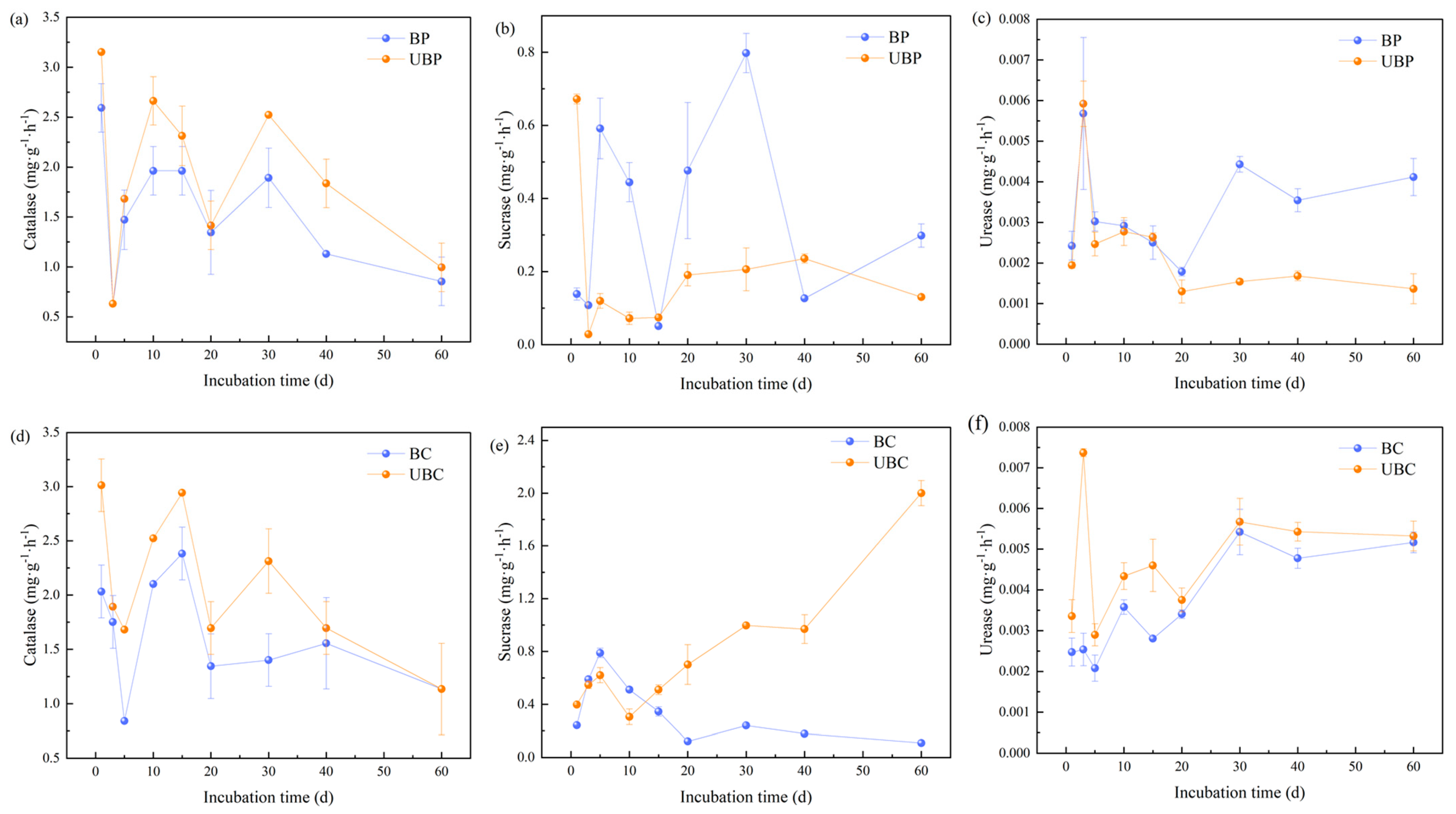
3.5. Effects of Wildfires on Soil Enzyme Activities
3.6. Effects of Wildfires on Bacterial Community Structure
3.7. Correlation Analysis
4. Discussion
4.1. Effects of Wildfires on Soil pH and CEC
4.2. Effects of Wildfires on Soil Nutrients
4.3. Effects of Wildfires on Soil Organic Carbon Mineralization
4.4. Effects of Wildfires on Different Carbon Fractions of Soils
4.5. Effects of Wildfires on Soil Enzyme Activities
4.6. Effects of Wildfires on Soil Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, S.; Song, X.; Zeng, H.; Wang, J. Soil microbial necromass carbon in forests: A global synthesis of patterns and controlling factors. Soil Ecol. Lett. 2024, 6, 240237. [Google Scholar] [CrossRef]
- Xue, L.; Li, Q.J.; Chen, H.Y. Effects of a wildfire on selected physical, chemical and biochemical soil properties in a Pinus massoniana forest in South China. Forests 2014, 5, 2947–2966. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Pan, Y. Research progress of polymer-based fire-warning sensors and self-flame-retardant sensors: A review. Polym. Bull. 2023, 36, 1263–1274. [Google Scholar] [CrossRef]
- Liang, T.; He, H.; Fan, D.; Zeng, Y.; Fu, B. Temporal and spatial variation of forest fire carbon emissions in Guangxi from 2011 to 2021 based on GEE. Radio Eng. 2023, 53, 2529–2539. [Google Scholar]
- Ping, X.Y.; Chang, Y.; Liu, M.; Hu, Y.M.; Huang, W.T.; Shi, S.X.; Jia, Y.C.; Li, D.K. Carbon emission and redistribution among forest carbon pools, and change in soil nutrient content after different severities of forest fires in Northeast China. Forests 2022, 13, 110. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Heinonsalo, J.; Pumpanen, J.; Berninger, F. Microbial biodiversity contributes to soil carbon release: A case study on fire disturbed boreal forests. FEMS Microbiol. Ecol. 2022, 98, fiac074. [Google Scholar] [CrossRef] [PubMed]
- Dooley, S.R.; Treseder, K.K. The effect of fire on microbial biomass: A meta-analysis of field studies. Biogeochemistry 2012, 109, 49–61. [Google Scholar] [CrossRef]
- Cao, J.B.; He, X.X.; Chen, Y.Q.; Chen, Y.P.; Zhang, Y.J.; Yu, S.Q.; Zhou, L.X.; Liu, Z.F.; Zhang, C.L.; Fu, S.L. Leaf litter contributes more to soil organic carbon than fine roots in two 10-year-old subtropical plantations. Sci. Total Environ. 2020, 704, 135341. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Z.; Yuan, H.Y.; Kimberley, M.O.; Jiang, H.; Zhou, G.M.; Wang, H.L. Soil CO2 flux dynamics in the two main plantation forest types in subtropical China. Sci. Total Environ. 2013, 444, 363–368. [Google Scholar] [CrossRef]
- Yang, Z.; Du, H.; Zeng, F.; Peng, W.; Guo, S.; Song, T.; He, X. Nitrogen storage patterns of main plantation ecosystems in Guangxi. Acta Ecol. Sin. 2022, 42, 5446–5457. [Google Scholar]
- Wang, Q.-K.; Wang, S.-L.; Zhong, M.-C. Ecosystem carbon storage and soil organic carbon stability in pure and mixed stands of Cunninghamia lanceolata and Michelia macclurei. Plant Soil 2013, 370, 295–304. [Google Scholar] [CrossRef]
- Sun, Y.; Zhong, L.; Yang, X.; Zhang, X. Effects of short-term precipitation reduction on soil organic carbon mineralization and organic carbon fractions in Hainan rubber forests. J. Trop. Biol. 2024, 15, 1–9. [Google Scholar] [CrossRef]
- Fuentes-Ramirez, A.; Barrientos, M.; Almonacid, L.; Arriagada-Escamilla, C.; Salas-Eljatib, C. Short-term response of soil microorganisms, nutrients and plant recovery in fire-affected Araucaria araucana forests. Appl. Soil Ecol. 2018, 131, 99–106. [Google Scholar] [CrossRef]
- García-Carmona, M.; Lepinay, C.; Mataix-Solera, J.; Baldrian, P.; Arcenegui, V.; Cajthaml, T.; García-Orenes, F. Post-fire wood mulch negatively affects the moss biocrust cover and its positive effects on microbial diversity in a semi-arid Mediterranean forest. Appl. Soil Ecol. 2023, 191, 105026. [Google Scholar] [CrossRef]
- Guo, J.; Chen, G.; Xie, J.; Yang, Z.; Yang, Y. Effect of heat-disturbance on microbial biomass carbon and microbial respiration in Chinese fir (Cunninghamia lanceolata) forest soils. J. For. Res. 2015, 26, 933–939. [Google Scholar] [CrossRef]
- Hewelke, E.; Górska, E.B.; Gozdowski, D.; Korc, M.; Olejniczak, I.; Predecka, A. Soil functional responses to natural ecosystem restoration of a pine forest peucedano-pinetum after a fire. Forests 2020, 11, 286. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Liu, S.H.; Kong, F.L.; Li, Y.; Jiang, Z.X.; Xi, M.; Wu, J. Mineral-ions modified biochars enhance the stability of soil aggregate and soil carbon sequestration in a coastal wetland soil. Catena 2020, 193, 104618. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.; Wang, Y.; Liu, X.; Zheng, Y.; Zheng, Y.; Wang, T.; Liu, Y. Effects of fire on the soil chemical properties in the Jinyun Mountain of Chongqing. Sci. Soil Water Conserv. 2023, 21, 52–59. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, Z.; Xiao, X.; Leng, M.; Wang, X.; Pan, F. Investigation of soil fungal communities and functionalities within karst paddy fields. Environ. Sci. 2021, 42, 4005–4014. [Google Scholar] [CrossRef]
- Cheng, Z.C.; Wu, S.; Du, J.; Liu, Y.Z.; Sui, X.; Yang, L.B. Reduced arbuscular mycorrhizal fungi (AMF) diversity in light and moderate fire sites in taiga forests, Northeast China. Microorganisms 2023, 11, 1836. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, F.; Ren, J.; Zhu, J.; Luo, X.Q.; Xiang, Y.Z. Effects of dominant plant growth on the nutrient composition and bacterial community structure of manganese residues. Int. J. Phytoremediat. 2022, 24, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Cheng, H.; Twagirayezu, G.; Fang, X.; Huang, L.; Deng, L.; Ji, B. Synergistic adsorption of Sb(V) using wet ball milling of manganese slag supported by biochar. China Environ. Sci. 2024, 44, 3741–3748. [Google Scholar] [CrossRef]
- Kong, J.J.; Yang, J.; Cai, W.H. Topography controls post-fire changes in soil properties in a Chinese boreal forest. Sci. Total Environ. 2019, 651, 2662–2670. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, D.; Zhou, M.; Zhao, P.; Tian, J.; Shu, D. Effects of different degrees of fire disturbance on soil carbon composition in the Greater Khingan Mountains permafrost region. J. Northwest For. Univ. 2022, 37, 141–145+201. [Google Scholar]
- Wang, L.H.; Fu, Q. Soil quality assessment of vegetation restoration after a large forest fire in Daxing’anling, northeast China. Can. J. Soil Sci. 2020, 100, 162–174. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, L.; Liu, F.; Ren, J.; Guo, J.; Yan, X. Effects of DA- 6 and EDDS on growth and Cd uptake by Solanum nigrum L. and on the soil bacterial community structure. Environ. Sci. 2022, 43, 1641–1648. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Liu, X.H.; Li, S.; Li, K.; Deng, H. Effects of bagasse biochar application on soil organic carbon fixation in manganese-contaminated sugarcane fields. Chem. Biol. Technol. Agric. 2023, 10, 46. [Google Scholar] [CrossRef]
- Etchells, H.; O’Donnell, A.J.; McCaw, W.L.; Grierson, P.F. Fire severity impacts on tree mortality and post-fire recruitment in tall eucalypt forests of southwest Australia. For. Ecol. Manag. 2020, 459, 117850. [Google Scholar] [CrossRef]
- Campos, I.; Abrantes, N.; Keizer, J.J.; Vale, C.; Pereira, P. Major and trace elements in soils and ashes of eucalypt and pine forest plantations in Portugal following a wildfire. Sci. Total Environ. 2016, 572, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Shu, H. Soil physicochemical property and enzymatic activity of Chinese fir plantation. Guizhou Agric. Sci. 2010, 38, 81–83+87. [Google Scholar]
- Ji, G.; Xu, M.; Wen, S.; Wang, B.; Zhang, L.; Liu, L. Characteristics of soil pH and exchangeable acidity in red soil profile under different vegetation types. Chin. J. Appl. Ecol. 2015, 26, 2639–2645. [Google Scholar] [CrossRef]
- Guedes, B.S.; Olsson, B.A.; Karltun, E. Effects of 34-year-old Pinus taeda and Eucalyptus grandis plantations on soil carbon and nutrient status in former miombo forest soils. Glob. Ecol. Conserv. 2016, 8, 190–202. [Google Scholar] [CrossRef]
- Wos, B.; Józefowska, A.; Likus-Cieslik, J.; Chodak, M.; Pietrzykowski, M. Effect of tree species and soil texture on the carbon stock, macronutrient content, and physicochemical properties of regenerated postfire forest soils. Land. Degrad. Dev. 2021, 32, 5227–5240. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Goforth, B.R.; Hubbert, K.R. Fire effects on cation exchange capacity of California forest and woodland soils. Geoderma 2017, 286, 125–130. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, G.; Ran, Q. Effects of different soil amendments on yield and quality of pepper. J. Yangzhou Univ. 2023, 44, 105–111. [Google Scholar] [CrossRef]
- Qin, Q.Q.; Zhang, Y.J.; Zhu, Q.; Bai, Y.S.; Sun, X.Y.; Liu, Y.H. Wildfire decouples soil multi-element cycles: Contributions of legacy effects and variations. Geoderma 2022, 424, 116012. [Google Scholar] [CrossRef]
- Ran, Y.; Zhou, J.; Zhang, Q.; Fan, J.; Li, X.; Ma, Y. Effects of burning intensity on soil chemical and physical proper-ties in southwest subtropical forests. Acta Agrestia Sin. 2023, 31, 2796–2804. [Google Scholar]
- Lei, J.; Wu, H.B.; Li, X.Y.; Guo, W.F.; Duan, A.G.; Zhang, J.G. Response of rhizosphere bacterial communities to near-natural forest management and tree species within Chinese fir plantations. Microbiol. Spectr. 2023, 11, e02328-22. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Shinjo, H.; Noro, Y.; Takenaka, S.; Miura, R.; Sokotela, S.B.; Funakawa, S. Short-term effects of fire intensity on soil organic matter and nutrient release after slash-and-burn in Eastern Province, Zambia. Soil Sci. Plant Nutr. 2014, 60, 173–182. [Google Scholar] [CrossRef]
- Liu, X.; Chu, J.; Zhang, Y.; Shan, Q. Effects of environmental factors and fire disturbance factors on distribution of Chamerion an gustifolium in Kanas taiga. Ecol. Environ. Sci. 2022, 31, 37–43. [Google Scholar] [CrossRef]
- Gui, H.R.; Wang, J.L.; Hu, M.J.; Zhou, Z.X.; Wan, S.Q. Impacts of fire on soil respiration and its components A global meta-analysis. Agric. For. Meteorol. 2023, 336, 109496. [Google Scholar] [CrossRef]
- Ross, C.S.; Kaye, J.P.; Kaye, M.W.; Kurth, V.J.; Brimmer, R.; Hart, S.C.; Fulé, P.Z. Ecosystem carbon remains low for three decades following fire and constrains soil CO2 responses to precipitation in Southwestern Ponderosa Pine Forests. Ecosystems 2012, 15, 725–740. [Google Scholar] [CrossRef]
- Sullivan, B.W.; Kolb, T.E.; Hart, S.C.; Kaye, J.P.; Hungate, B.A.; Dore, S.; Montes-Helu, M. Wildfire reduces carbon dioxide efflux and increases methane uptake in ponderosa pine forest soils of the southwestern USA. Biogeochemistry 2011, 104, 251–265. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Yang, X.; Cui, J.; Sui, P. The effect of different organic materials amendment on soil bacteria communities in barren sandy loam soil. Environ. Sci. Pollut. Res. 2017, 24, 24019–24028. [Google Scholar] [CrossRef] [PubMed]
- Auwal, M.; Sun, H.; Adamu, U.K.; Meng, J.; Van Zwieten, L.; Singh, B.P.; Luo, Y.; Xu, J.M. The phosphorus limitation in the post-fire forest soils increases soil CO2 emission via declining cellular carbon use efficiency and increasing extracellular phosphatase. Catena 2023, 224, 106968. [Google Scholar] [CrossRef]
- Shen, J.; He, Z.; Dong, Q.; Gao, S.; Lin, Y. Effects of mild fire on soil respiration rate and abiotic factors in coastal sandy plantation. Chin. J. Plant Ecol. 2023, 47, 1032–1042. [Google Scholar] [CrossRef]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Mansilla-Salinero, P.; Rodríguez-Soalleiro, R.; Merino, A. Influence of tree species on carbon sequestration in afforested pastures in a humid temperate region. Plant Soil 2012, 353, 333–353. [Google Scholar] [CrossRef]
- Zhou, L.; Li, S.; Liu, B.; Wu, P.; Heal, K.V.; Ma, X. Tissue-specific carbon concentration, carbon stock, and distribution in Cunninghamia lanceolata (Lamb.) Hook plantations at various developmental stages in subtropical China. Ann. For. Sci. 2019, 76, 70. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, M.; Wang, L.; Cui, F.; Fan, L. Soil stoichiometry characteristics in different vegetation restoration pattermns in Xishan post-mining area in Shanxi Province. Chin. J. Soil Sci. 2020, 51, 634–640. [Google Scholar] [CrossRef]
- Yao, Z.; Jiao, P.; Wu, X.; Yan, Q.; Liu, X.; Hu, Y.; Wang, Y. Effect of fire-deposited charcoal on soil organic carbon pools and associated enzyme activities in a recently harvested Pinus massoniana plantation subjected to broadcast burning. Environ. Sci. 2023, 44, 4201–4210. [Google Scholar] [CrossRef]
- Näthe, K.; Levia, D.F.; Tischer, A.; Michalzik, B. Low-intensity surface fire effects on carbon and nitrogen cycling in soil and soil solution of a Scots pine forest in central Germany. Catena 2018, 162, 360–375. [Google Scholar] [CrossRef]
- Ma, H.L.; Chen, C.C.; Yin, Y.F.; Gao, R. Experimental study on carbon mineralization of different sizes particle in forest soils. Acta Pedol. Sin. 2024, 61, 1–14. [Google Scholar]
- Zhao, H.M.; Tong, D.Q.; Lin, Q.X.; Lu, X.G.; Wang, G.P. Effect of fires on soil organic carbon pool and mineralization in a Northeastern China wetland. Geoderma 2012, 189, 532–539. [Google Scholar] [CrossRef]
- Su, J.; Weng, X.; Luo, Z.; Huang, H.; Wang, W. Impact of biochar on soil properties, pore water properties, and available cadmium. Bull. Environ. Contam. Toxicol. 2021, 107, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.P.; Ai, L.; Wu, X.L.; Dai, Y.X.; Jiang, C.; Chen, X.; Song, Y.M.; Ma, J.M.; Yang, H. Effects of microplastic pollution on agricultural soil and crops based on a global meta-analysis. Land. Degrad. Dev. 2023, 35, 551–567. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, P.; Kumar, U.; Daverey, A.; Arunachalam, K. Effect of forest fire on soil microbial biomass and enzymatic activity in oak and pine forests of Uttarakhand Himalaya, India. Ecol. Process. 2021, 10, 29. [Google Scholar] [CrossRef]
- Liu, J.; Lin, W.; Wang, Y.; Jiang, J.; Fang, X.; Yi, Z. Effects of fire on soil enzyme activities and organic carbon fractions in Pinus massoniana forest. Acta Ecol. Sin. 2018, 38, 5374–5382. [Google Scholar]
- Ortega, R.; Miralles, I.; Soria, R.; Rodriguez-Berbel, N.; Villafuerte, A.B.; Zema, D.A.; Lucas-Borja, M.E. Short-term effects of post-fire soil mulching with wheat straw and wood chips on the enzymatic activities in a Mediterranean pine forest. Sci. Total Environ. 2023, 857, 159489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, Y.; He, C.; Wang, Z.; Zhu, W.; Wang, Z.; Chen, L.; Wu, L.; Du, A. Impact of native tree species introduction on soil nutrient and bacterial community in Eucalyptus plantations. Eur. J. For. Res. 2023, 142, 1369–1383. [Google Scholar] [CrossRef]
- Guo, M.; Guo, M.; Wei, S.; Zhao, Y.; Jia, X. Effect of pH on MEC desulfurization performance and microbial mechanism of action. Chem. Ind. Eng. Prog. 2024, 43, 2219–2225. [Google Scholar] [CrossRef]
- Wu, Y.C.; Zeng, J.; Zhu, Q.H.; Zhang, Z.F.; Lin, X.G. pH is the primary determinant of the bacterial community structure in agricultural soils impacted by polycyclic aromatic hydrocarbon pollution. Sci. Rep. 2017, 7, 40093. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.M.; Kim, M.; Lai-Hoe, A.; Shukor, N.A.A.; Rahim, R.A.; Go, R.; Adams, J.M. pH dominates variation in tropical soil archaeal diversity and community structure. FEMS Microbiol. Ecol. 2013, 86, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.X.; An, L.R.; Zhang, S.G.; Feng, J.C.; Sun, D.X.; Yao, Y.Y.; Shen, T.L.; Yang, Y.C.; Zhang, M. The long-term effects of microplastics on soil organomineral complexes and bacterial communities from controlled-release fertilizer residual coating. J. Environ. Manag. 2022, 304, 114193. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, J.M.; Liang, Y.M.; Lan, G.Y. Soil bacterial community changes along elevation gradients in karst graben basin of Yunnan-Kweichow Plataau. Front. Microbiol. 2022, 13, 1054667. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, G.; Gu, Z.; Liu, X.; Shu, L. Effects of fire disturbance on soil bacterial community in Pinus tabuliformis forest. Chin. J. Ecol. 2023, 42, 1355–1364. [Google Scholar] [CrossRef]
- Xiang, X.; Shi, Y.; Yang, J.; Kong, J.; Lin, X.; Zhang, H.; Zeng, J.; Chu, H. Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest. Sci. Rep. 2014, 4, 3829. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.J.; Wang, T.; Xu, Y.D.; Deng, J.; Zhao, F.Z.; Yang, G.H.; Han, X.H.; Feng, Y.Z.; Ren, G.X. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For. Ecol. Manage. 2018, 409, 170–178. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zheng, J.Q.; Liu, X.; Yun, Q.; Hu, Y.L. Short-term impact of fire-deposited charcoal on soil microbial community abundance and composition in a subtropical plantation in China. Geoderma 2020, 359, 113992. [Google Scholar] [CrossRef]
- Sul, W.J.; Asuming-Brempong, S.; Wang, Q.; Tourlousse, D.M.; Penton, C.R.; Deng, Y.; Rodrigues, J.L.M.; Adiku, S.G.K.; Jones, J.W.; Zhou, J.Z.; et al. Tropical agricultural land management influences on soil microbial communities through its effect on soil organic carbon. Soil Biol. Biochem. 2013, 65, 33–38. [Google Scholar] [CrossRef]
- Zhao, J.G.; Li, Y.H.; Chen, X.R.; Li, Y. Effects of carbon sources on sludge performance and microbial community for 4-chlorophenol wastewater treatment in sequencing batch reactors. Bioresour. Technol. 2018, 255, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Sun, X.; Xiao, E.; Xu, Z.; Li, B.; Sun, W. Characterization of iron-metabolizing communities in soils contaminated by acid mine drainage from an abandoned coal mine in Southwest China. Environ. Sci. Pollut. Res. 2019, 26, 9585–9598. [Google Scholar] [CrossRef] [PubMed]


| Plantation Types | BP | UBP | BC | UBC |
|---|---|---|---|---|
| Tree age (year) | 16 | 15 | 15 | 16 |
| Density (each plant·hm−2) | 275 | 275 | 375 | 375 |
| Latitude and longitude | 25°30′33″ N 110°26′59″ E | 25°29′59″ N 110°25′56″ E | 25°30′01″ N 110°26′04″ E | 25°30′24″ N 110°25′07″ E |
| Altitude (m) | 254 | 221 | 233 | 212 |
| Slope degree (°) | 40 | 39 | 25 | 28 |
| Slope position | Middle | Middle | Middle | Middle |
| Sample | pH | SOC (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | CEC (cmol·kg−1) |
|---|---|---|---|---|---|---|
| BP | 5.31 ± 0.04 | 35.89 ± 1.43 | 234.50 ± 14.85 | 28.16 ± 1.92 | 51.22 ± 1.87 | 1.83 ± 0.05 |
| UBP | 4.71 ± 0.04 | 39.82 ± 1.09 | 156.33 ± 10.69 | 28.10 ± 2.15 | 34.29 ± 1.37 | 4.52 ± 0.64 |
| BC | 5.09 ± 0.06 | 42.47 ± 0.99 | 241.50 ± 4.95 | 37.18 ± 1.98 | 40.88 ± 1.82 | 1.16 ± 0.01 |
| UBC | 5.18 ± 0.05 | 34.95 ± 1.27 | 186.67 ± 4.04 | 21.06 ± 2.17 | 36.16 ± 0.60 | 2.51 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liu, X.; Huang, S.; Jia, J.; Wang, C.; Hu, L.; Li, K.; Deng, H. Effects of Wildfire on Soil CO2 Emission and Bacterial Community in Plantations. Microorganisms 2024, 12, 1666. https://doi.org/10.3390/microorganisms12081666
Yang Y, Liu X, Huang S, Jia J, Wang C, Hu L, Li K, Deng H. Effects of Wildfire on Soil CO2 Emission and Bacterial Community in Plantations. Microorganisms. 2024; 12(8):1666. https://doi.org/10.3390/microorganisms12081666
Chicago/Turabian StyleYang, Yu, Xuehui Liu, Shilin Huang, Jinchen Jia, Chuangye Wang, Lening Hu, Ke Li, and Hua Deng. 2024. "Effects of Wildfire on Soil CO2 Emission and Bacterial Community in Plantations" Microorganisms 12, no. 8: 1666. https://doi.org/10.3390/microorganisms12081666
APA StyleYang, Y., Liu, X., Huang, S., Jia, J., Wang, C., Hu, L., Li, K., & Deng, H. (2024). Effects of Wildfire on Soil CO2 Emission and Bacterial Community in Plantations. Microorganisms, 12(8), 1666. https://doi.org/10.3390/microorganisms12081666






