Comparative Analysis of Bacterial Community Structures in Earthworm Skin, Gut, and Habitat Soil across Typical Temperate Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
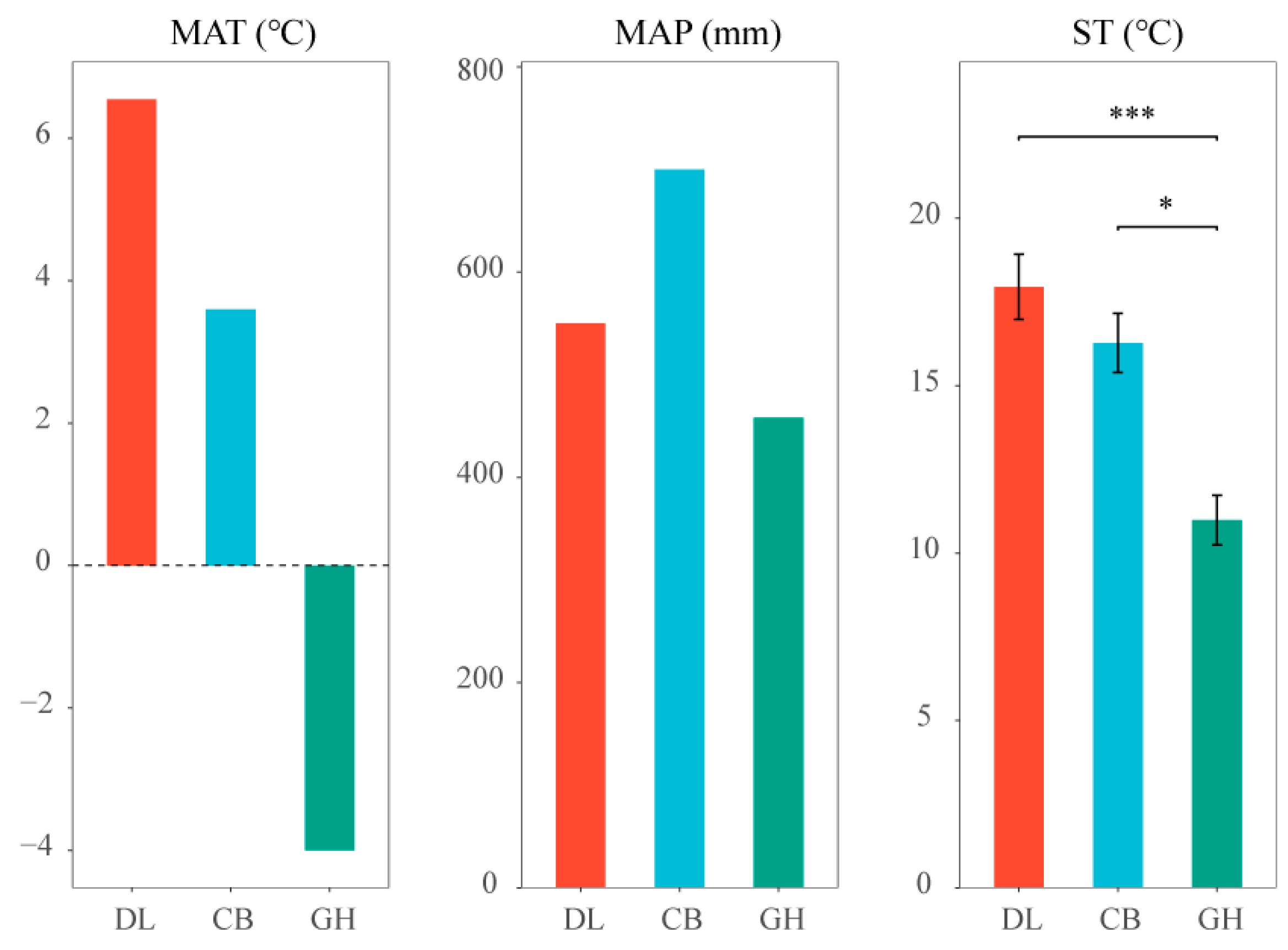
2.2.1. Study Site
2.2.2. Experiment Design
2.2.3. Bacterial Collection from Earthworm Skin
2.2.4. Earthworm Skin Mucus Collection
2.2.5. Earthworm Gut Collection
2.3. Physicochemical Properties of Soil and Skin Mucus
2.4. DNA Extraction of Bacterial Communities
2.5. Statistical Analyses
3. Results
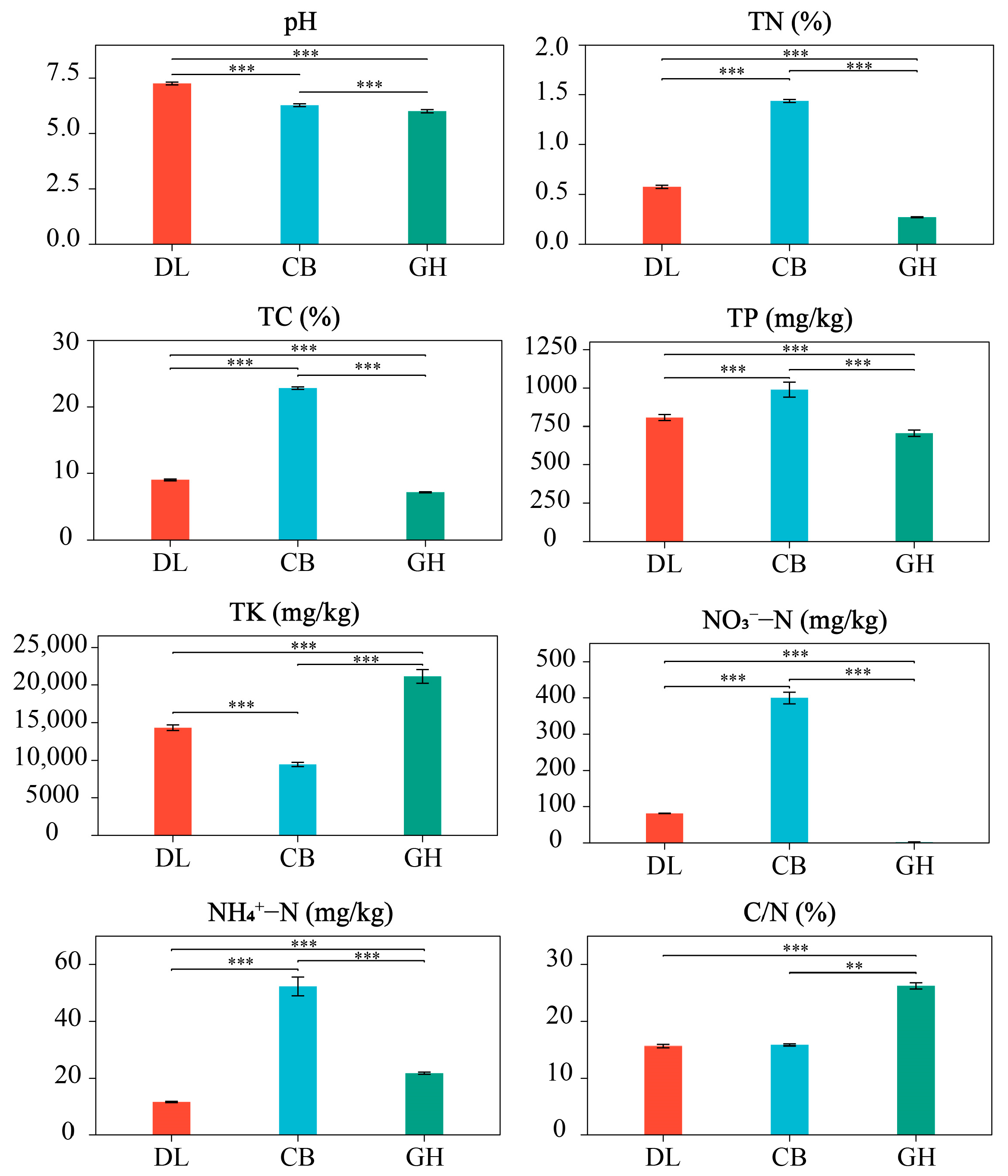
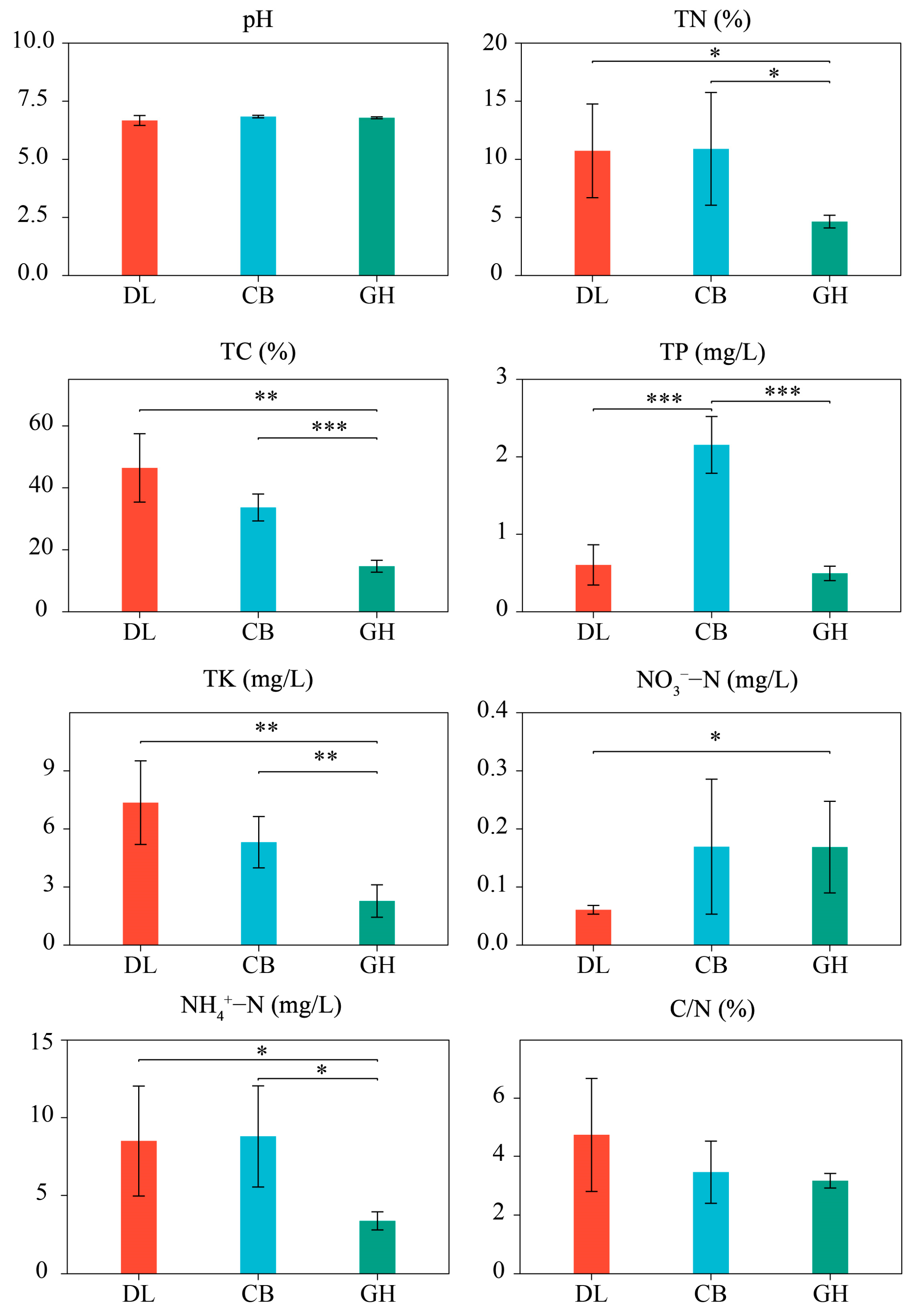
3.1. Physicochemical Properties of Earthworm Habitat Soils and Skin Mucus
3.2. Bacterial Community Diversity and Structure
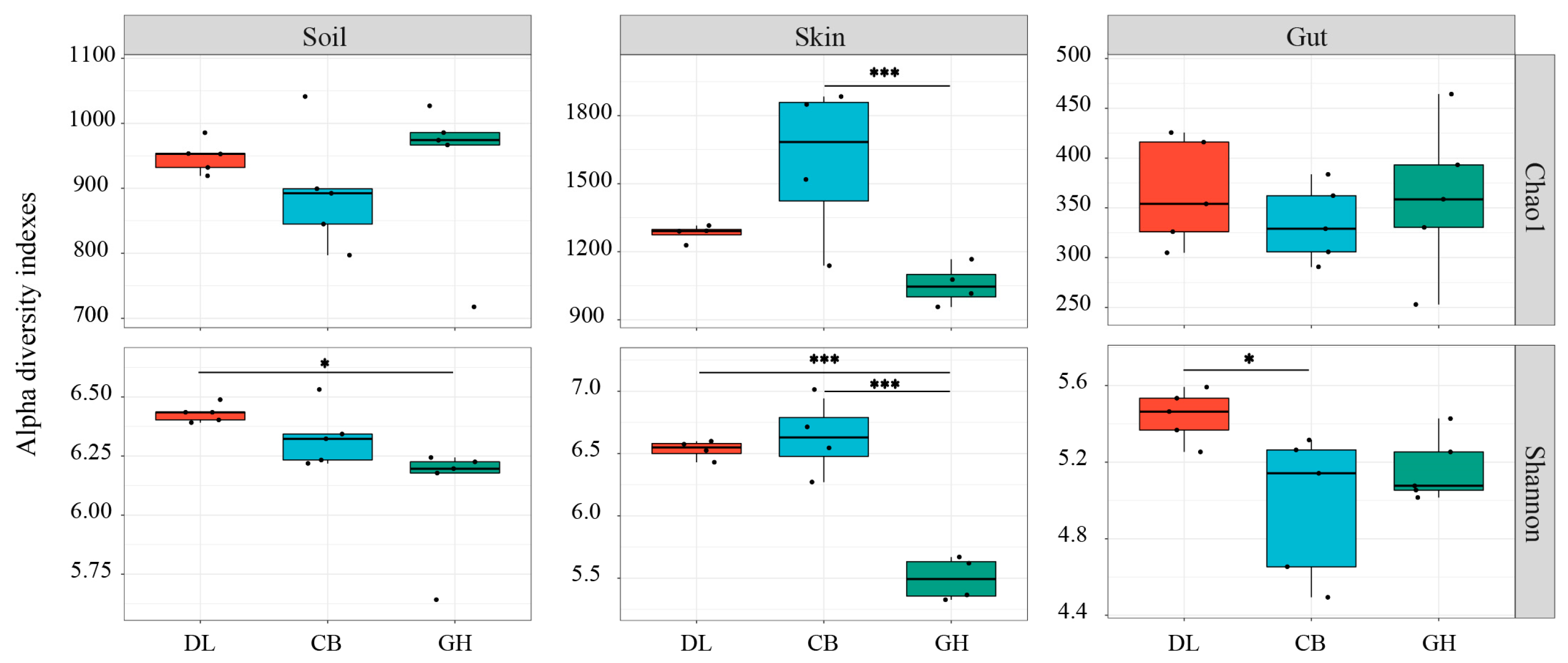
3.2.1. Bacterial Community Alpha Diversity
3.2.2. Bacterial Community Beta Diversity
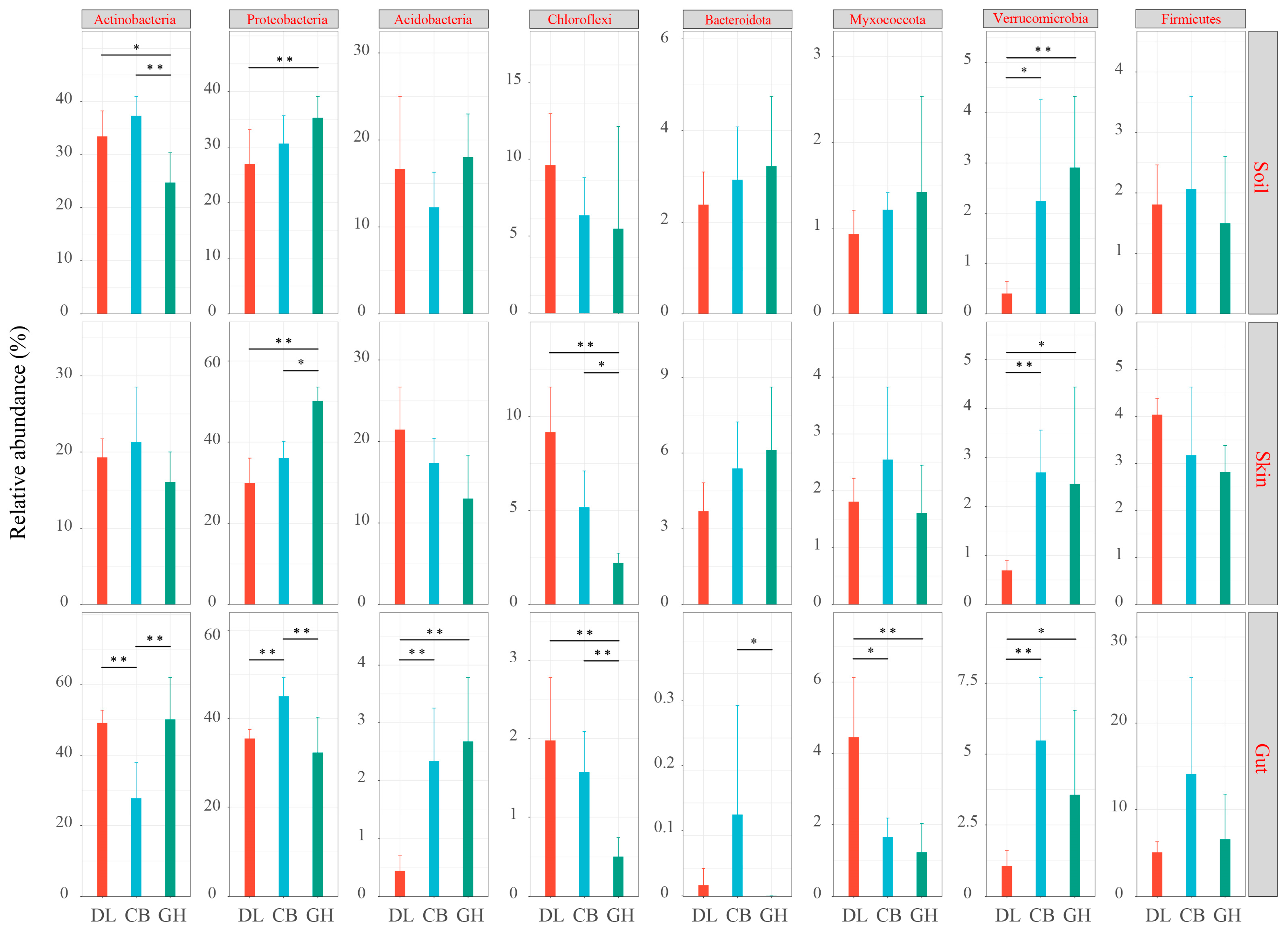
3.2.3. Bacterial Community Composition
3.3. Environmental Factors Influencing Bacterial Communities
4. Discussion
4.1. Effects of Temperate Forest Type Transition on Bacterial Community Diversity in Earthworm-Associated Microhabitats
4.2. Impact of Temperate Forest Type Transition on Bacterial Community Composition in Earthworm-Associated Microhabitats
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phillips, H.R.P.; Guerra, C.A.; Bartz, M.L.C.; Briones, M.J.I.; Brown, G.; Crowther, T.W.; Ferlian, O.; Gongalsky, K.B.; van den Hoogen, J.; Krebs, J.; et al. Global distribution of earthworm diversity. Science 2019, 366, 480–485. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Van Groenigen, J.W.; Van Groenigen, K.J.; Koopmans, G.F.; Stokkermans, L.; Vos, H.M.J.; Lubbers, I.M. How fertile are earthworm casts? A meta-analysis. Geoderma 2019, 338, 525–535. [Google Scholar] [CrossRef]
- Shuster, W.D.; Subler, S.; McCoy, E.L. Deep-burrowing earthworm additions changed the distribution of soil organic carbon in a chisel-tilled soil. Soil Biol. Biochem. 2001, 33, 983–996. [Google Scholar] [CrossRef]
- James, S.W. Soil, Nitrogen, Phosphorus, and Organic Matter Processing by Earthworms in Tallgrass Prairie. Ecology 1991, 72, 2101–2109. [Google Scholar] [CrossRef]
- Milleret, R.; Le Bayon, R.C.; Lamy, F.; Gobat, J.M.; Boivin, P. Impact of roots, mycorrhizas and earthworms on soil physical properties as assessed by shrinkage analysis. J. Hydrol. 2009, 373, 499–507. [Google Scholar] [CrossRef]
- West, J.R.; Herrick, B.M.; Whitman, T. Earthworm co-invasion by Amynthas tokioensis and Amynthas agrestis affects soil microaggregate bacterial communities. Appl. Soil Ecol. 2024, 195, 105224. [Google Scholar] [CrossRef]
- Ganault, P.; Nahmani, J.; Capowiez, Y.; Fromin, N.; Shihan, A.; Bertrand, I.; Buatois, B.; Milcu, A. Earthworms and plants can decrease soil greenhouse gas emissions by modulating soil moisture fluctuations and soil macroporosity in a mesocosm experiment. PLoS ONE 2024, 19, e0289859. [Google Scholar] [CrossRef]
- Puga-Freitas, R.; Barot, S.; Taconnat, L.; Renou, J.-P.; Blouin, M. Signal Molecules Mediate the Impact of the Earthworm Aporrectodea caliginosa on Growth, Development and Defence of the Plant Arabidopsis thaliana. PLoS ONE 2012, 7, e49504. [Google Scholar] [CrossRef]
- Wang, H.-T.; Liang, Z.-Z.; Ding, J.; Li, G.; Fu, S.-L.; Zhu, D. Deciphering roles of microbiota in arsenic biotransformation from the earthworm gut and skin. J. Hazard. Mater. 2023, 446, 130707. [Google Scholar] [CrossRef]
- Mirleau, P.; Jouni, F.; Chappat, J.; Mazzia, C.; Sanchez-Hernandez, J.C.; Capowiez, Y.; Rault, M. Effect of an organophosphorus insecticide, soil texture and earthworm species on the turnover of soil, gut and cast microbiota during the earthworm’s gut transit. Soil Biol. Biochem. 2024, 190, 109293. [Google Scholar] [CrossRef]
- Jin, B.-J.; Liu, X.-P.; Roux, X.L.; Bi, Q.-F.; Li, K.-J.; Wu, C.-Y.; Sun, C.-L.; Zhu, Y.-G.; Lin, X.-Y. Biochar addition regulates soil and earthworm gut microbiome and multifunctionality. Soil Biol. Biochem. 2022, 173, 108810. [Google Scholar] [CrossRef]
- Song, J.; Li, T.; Zheng, Z.; Fu, W.; Long, Z.; Shi, N.; Han, Y.; Zhang, L.; Yu, Y.; Fang, H. Carbendazim shapes microbiome and enhances resistome in the earthworm gut. Microbiome 2022, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, W.; Jiang, Y.; Dai, W.; Li, P.; Yao, D.; Wang, J.; Shi, Y.; Cui, Z.; Cao, H.; et al. Variations in bacterial taxonomic profiles and potential functions in response to the gut transit of earthworms (Eisenia fetida) feeding on cow manure. Sci. Total Environ. 2021, 787, 147392. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Schrader, S.; Tebbe, C.C. Legacy effects of earthworms on soil microbial abundance, diversity, and community dynamics. Soil Biol. Biochem. 2024, 190, 109294. [Google Scholar] [CrossRef]
- Pelosi, C.; Taschen, E.; Redecker, D.; Blouin, M. Earthworms as conveyors of mycorrhizal fungi in soils. Soil Biol. Biochem. 2024, 189, 109283. [Google Scholar] [CrossRef]
- Fourcade, Y.; Vercauteren, M. Predicted changes in the functional structure of earthworm assemblages in France driven by climate change. Divers. Distrib. 2022, 28, 1050–1066. [Google Scholar] [CrossRef]
- van de Logt, R.; van der Sluijs, T.; van Eekeren, N. Lumbricus terrestris abundance in grasslands on sandy soils in relation to soil texture, hydrology and earthworm community. Eur. J. Soil Biol. 2023, 119, 103545. [Google Scholar] [CrossRef]
- Gabriac, Q.; Ganault, P.; Barois, I.; Aranda-Delgado, E.; Cimetière, E.; Cortet, J.; Gautier, M.; Hedde, M.; Marchán, D.F.; Pimentel Reyes, J.C.; et al. Environmental drivers of earthworm communities along an elevational gradient in the French Alps. Eur. J. Soil Biol. 2023, 116, 103477. [Google Scholar] [CrossRef]
- Zuo, J.; Muys, B.; Berg, M.P.; Hefting, M.M.; van Logtestijn, R.S.P.; van Hal, J.; Cornelissen, J.H.C. Earthworms are not just “earth” worms: Multiple drivers to large diversity in deadwood. For. Ecol. Manag. 2023, 530, 120746. [Google Scholar] [CrossRef]
- Singh, J.; Cameron, E.; Reitz, T.; Schädler, M.; Eisenhauer, N. Grassland management effects on earthworm communities under ambient and future climatic conditions. Eur. J. Soil Sci. 2021, 72, 343–355. [Google Scholar] [CrossRef]
- Tian, J.; He, N.; Hale, L.; Niu, S.; Yu, G.; Liu, Y.; Blagodatskaya, E.; Kuzyakov, Y.; Gao, Q.; Zhou, J. Soil organic matter availability and climate drive latitudinal patterns in bacterial diversity from tropical to cold temperate forests. Funct. Ecol. 2018, 32, 61–70. [Google Scholar] [CrossRef]
- Kim, G.; Jo, H.; Kim, H.-S.; Kwon, M.; Son, Y. Earthworm effects on soil biogeochemistry in temperate forests focusing on stable isotope tracing: A review. Appl. Biol. Chem. 2022, 65, 88. [Google Scholar] [CrossRef]
- Jang, J.; Xiong, X.; Liu, C.; Yoo, K.; Ishii, S. Invasive earthworms alter forest soil microbiomes and nitrogen cycling. Soil Biol. Biochem. 2022, 171, 108724. [Google Scholar] [CrossRef]
- Córdova, S.C.; Olk, D.C.; Dietzel, R.N.; Mueller, K.E.; Archontouilis, S.V.; Castellano, M.J. Plant litter quality affects the accumulation rate, composition, and stability of mineral-associated soil organic matter. Soil Biol. Biochem. 2018, 125, 115–124. [Google Scholar] [CrossRef]
- Quideau, S.A.; Chadwick, O.A.; Benesi, A.; Graham, R.C.; Anderson, M.A. A direct link between forest vegetation type and soil organic matter composition. Geoderma 2001, 104, 41–60. [Google Scholar] [CrossRef]
- Ganin, G.N.; Atopkin, D.M. Molecular differentiation of epigeic and anceic forms of Drawida ghilarovi Gates, 1969 (Moniligastridae, Clitellata) in the Russian Far East: Sequence data of two mitochondrial genes. Eur. J. Soil Biol. 2018, 86, 1–7. [Google Scholar] [CrossRef]
- Ferlian, O.; Goldmann, K.; Bonkowski, M.; Dumack, K.; Wubet, T.; Eisenhauer, N. Invasive earthworms shift soil microbial community structure in northern North American forest ecosystems. iScience 2024, 27, 108889. [Google Scholar] [CrossRef]
- Niu, G.; Liu, T.; Zhao, Z.; Zhang, X.; Guan, H.; He, X.; Lu, X. Subtropical forest macro-decomposers rapidly transfer litter carbon and nitrogen into soil mineral-associated organic matter. For. Ecosyst. 2024, 11, 100172. [Google Scholar] [CrossRef]
- Ross Ashley, A.; Doxey Andrew, C.; Neufeld Josh, D. The Skin Microbiome of Cohabiting Couples. mSystems 2017, 2, 4. [Google Scholar] [CrossRef]
- Ross, A.A.; Müller, K.M.; Weese, J.S.; Neufeld, J.D. Comprehensive skin microbiome analysis reveals the uniqueness of human skin and evidence for phylosymbiosis within the class Mammalia. Proc. Natl. Acad. Sci. USA 2018, 115, E5786–E5795. [Google Scholar] [CrossRef] [PubMed]
- Ogai, K.; Nagase, S.; Mukai, K.; Iuchi, T.; Mori, Y.; Matsue, M.; Sugitani, K.; Sugama, J.; Okamoto, S. A Comparison of Techniques for Collecting Skin Microbiome Samples: Swabbing Versus Tape-Stripping. Front. Microbiol. 2018, 9, 2362. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, L.; Aspe, N.M.; Han, A.C.; Chen, M.; Li, M.; Zhang, S.; Wu, D. Seasonal dynamics of gut microbiome: A study of multi-kingdom microbiota of earthworm gut in an urban park. Appl. Soil Ecol. 2024, 195, 105259. [Google Scholar] [CrossRef]
- Shutenko, G.S.; Kelleher, B.P.; Simpson, A.J.; Soong, R.; Mobarhan, Y.L.; Schmidt, O. Evidence for substantial acetate presence in cutaneous earthworm mucus. J. Soils Sediments 2020, 20, 3627–3632. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, F.; Li, H. Effects of earthworm mucus and amino acids on cadmium subcellular distribution and chemical forms in tomato seedlings. Bioresour. Technol. 2009, 100, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Jacques, R.; Dan, B.; Andreas, R. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar]
- Guhra, T.; Stolze, K.; Schweizer, S.; Totsche, K.U. Earthworm mucus contributes to the formation of organo-mineral associations in soil. Soil Biol. Biochem. 2020, 145, 107785. [Google Scholar] [CrossRef]
- Huan, H.; Wang, X.; Chu, Z.; Yu, X.; Fan, T.; Li, G.; Xu, X.; Zhen, Q.; Sun, L.; Dong, Z.; et al. Compositional changes and ecological characteristics of earthworm mucus under different electrical stimuli. Sci. Rep. 2023, 13, 2332. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z.; et al. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef] [PubMed]
- Thakuria, D.; Schmidt, O.; Finan, D.; Egan, D.; Doohan, F.M. Gut wall bacteria of earthworms: A natural selection process. ISME J. 2010, 4, 357–366. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, B.-J.; Bi, Q.-F.; Li, K.-J.; Sun, C.-L.; Lin, X.-Y.; Zhu, Y.-G. Variations of earthworm gut bacterial community composition and metabolic functions in coastal upland soil along a 700-year reclamation chronosequence. Sci. Total Environ. 2022, 804, 149994. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.P.; Schmidt, O. The feeding ecology of earthworms—A review. Pedobiologia 2007, 50, 463–477. [Google Scholar] [CrossRef]
- Astaykina, A.; Streletskii, R.; Maslov, M.; Krasnov, G.; Gorbatov, V. Effects of Three Pesticides on the Earthworm Lumbricus terrestris Gut Microbiota. Front. Microbiol. 2022, 13, 853535. [Google Scholar] [CrossRef] [PubMed]
- Pass, D.A.; Morgan, A.J.; Read, D.S.; Field, D.; Weightman, A.J.; Kille, P. The effect of anthropogenic arsenic contamination on the earthworm microbiome. Environ. Microbiol. 2015, 17, 1884–1896. [Google Scholar] [CrossRef]
- Liu, D.; Lian, B.; Wang, B.; Jiang, G. Degradation of Potassium Rock by Earthworms and Responses of Bacterial Communities in Its Gut and Surrounding Substrates after Being Fed with Mineral. PLoS ONE 2011, 6, e28803. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Zeitler, K.; Feldhahn, L.; Neu, T.R.; Weber, T.; Krištůfek, V.; Wubet, T.; Herrmann, S.; Buscot, F.; Tarkka, M.T. Detection and quantification of a mycorrhization helper bacterium and a mycorrhizal fungus in plant-soil microcosms at different levels of complexity. BMC Microbiol. 2013, 13, 205. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolařík, M.; Štursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Žifčáková, L.; Šnajdr, J.; Rídl, J.; Vlček, Č.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chao, H.; Zheng, X.; Deng, S.; Ye, M.; Hu, F. Ecological role of earthworm intestinal bacteria in terrestrial environments: A review. Sci. Total Environ. 2020, 740, 140008. [Google Scholar] [CrossRef]
- Qiu, M.; Wu, Z.; Song, J.; Zheng, C.; Zhan, X.; Shan, M.; Cui, M.; Chen, L.; Zhang, L.; Yu, Y.; et al. Chlorothalonil drives the antibiotic resistome in earthworm guts. J. Hazard. Mater. 2024, 463, 132831. [Google Scholar] [CrossRef]
- Knapp, B.A.; Podmirseg, S.M.; Seeber, J.; Meyer, E.; Insam, H. Diet-related composition of the gut microbiota of Lumbricus rubellus as revealed by a molecular fingerprinting technique and cloning. Soil Biol. Biochem. 2009, 41, 2299–2307. [Google Scholar] [CrossRef]
- Ma, L.; Xie, Y.; Han, Z.; Giesy, J.P.; Zhang, X. Responses of earthworms and microbial communities in their guts to Triclosan. Chemosphere 2017, 168, 1194–1202. [Google Scholar] [CrossRef]
- Meier Anja, B.; Hunger, S.; Drake Harold, L. Differential Engagement of Fermentative Taxa in Gut Contents of the Earthworm Lumbricus terrestris. Appl. Environ. Microbiol. 2018, 84, e01851-17. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Kong, L.; Zhang, H.; Sun, M.; Ye, M.; Huang, D.; Zhang, Z.; Sun, D.; Zhang, S.; Yuan, Y.; et al. Metaphire guillelmi gut as hospitable micro-environment for the potential transmission of antibiotic resistance genes. Sci. Total Environ. 2019, 669, 353–361. [Google Scholar] [CrossRef]
- Chang, X.; Sun, Y.; Zhao, L.; Li, X.; Yang, S.; Weng, L.; Li, Y. Exposure to fomesafen alters the gut microbiota and the physiology of the earthworm Pheretima guillelmi. Chemosphere 2021, 284, 131290. [Google Scholar] [CrossRef]
- Wang, H.-T.; Ding, J.; Xiong, C.; Zhu, D.; Li, G.; Jia, X.-Y.; Zhu, Y.-G.; Xue, X.-M. Exposure to microplastics lowers arsenic accumulation and alters gut bacterial communities of earthworm Metaphire californica. Environ. Pollut. 2019, 251, 110–116. [Google Scholar] [CrossRef]
- Eilers, K.G.; Debenport, S.; Anderson, S.; Fierer, N. Digging deeper to find unique microbial communities: The strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol. Biochem. 2012, 50, 58–65. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Jeanbille, M.; Buée, M.; Bach, C.; Cébron, A.; Frey-Klett, P.; Turpault, M.P.; Uroz, S. Soil Parameters Drive the Structure, Diversity and Metabolic Potentials of the Bacterial Communities Across Temperate Beech Forest Soil Sequences. Microb. Ecol. 2016, 71, 482–493. [Google Scholar] [CrossRef]
- García-Fraile, P.; Benada, O.; Cajthaml, T.; Baldrian, P.; Lladó, S. Terracidiphilus gabretensis gen. nov., sp. nov., an Abundant and Active Forest Soil Acidobacterium Important in Organic Matter Transformation. Appl. Environ. Microbiol. 2016, 82, 560–569. [Google Scholar] [CrossRef]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef]
- Szoboszlay, M.; Dohrmann, A.B.; Poeplau, C.; Don, A.; Tebbe, C.C. Impact of land-use change and soil organic carbon quality on microbial diversity in soils across Europe. FEMS Microbiol. Ecol. 2017, 93, fix146. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wang, J.; Lv, W.; Xu, S.; Zhu, H. Nitrogen fertilization alters the effects of earthworms on soil physicochemical properties and bacterial community structure. Appl. Soil Ecol. 2020, 150, 103478. [Google Scholar] [CrossRef]
- Lv, M.; Fu, S.; Shao, Y.; Lin, Y.; Wu, J.; Zhang, W. Earthworm Pontoscolex corethrurus stimulated soil CO2 emission by enhancing substrate availability rather than changing microbiota community structure. Sci. Total Environ. 2020, 717, 137227. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.S.; Knowles, M.E.; Juillerat, J.I.; Görres, J.H.; Cogbill, C.V.; Wilmot, S.; D’Agati, K. Interaction of land use history, earthworms, soil chemistry and tree species on soil carbon distribution in managed forests in Vermont, USA. For. Ecol. Manag. 2021, 489, 119049. [Google Scholar] [CrossRef]
- Price-Christenson, G.J.; Johnston, M.R.; Herrick, B.M.; Yannarell, A.C. Influence of invasive earthworms (Amynthas spp.) on Wisconsin forest soil microbial communities and soil chemistry. Soil Biol. Biochem. 2020, 149, 107955. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Ma, Y.; Guo, L.; Sun, J.; Tong, J. Earthworm epidermal mucus: Rheological behavior reveals drag-reducing characteristics in soil. Soil Tillage Res. 2016, 158, 57–66. [Google Scholar] [CrossRef]
- Pan, X.; Song, W.; Zhang, D. Earthworms (Eisenia foetida, Savigny) mucus as complexing ligand for imidacloprid. Biol. Fertil. Soils 2010, 46, 845–850. [Google Scholar] [CrossRef]
- Hoang, D.T.T.; Bauke, S.L.; Kuzyakov, Y.; Pausch, J. Rolling in the deep: Priming effects in earthworm biopores in topsoil and subsoil. Soil Biol. Biochem. 2017, 114, 59–71. [Google Scholar] [CrossRef]
- Huang, K.; Xia, H. Role of earthworms’ mucus in vermicomposting system: Biodegradation tests based on humification and microbial activity. Sci. Total Environ. 2018, 610–611, 703–708. [Google Scholar] [CrossRef]
- Duan, Z.; Huang, K.; Huang, W.; Wang, B.; Shi, J.; Xia, H.; Li, F. Bacterial dispersal enhances the elimination of active fecal coliforms during vermicomposting of fruit and vegetable wastes: The overlooked role of earthworm mucus. J. Hazard. Mater. 2024, 471, 134280. [Google Scholar] [CrossRef]
- Singleton, D.R.; Hendrix, P.F.; Coleman, D.C.; Whitman, W.B. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta). Soil Biol. Biochem. 2003, 35, 1547–1555. [Google Scholar] [CrossRef]
- Egert, M.; Marhan, S.; Wagner, B.; Scheu, S.; Friedrich, M.W. Molecular profiling of 16S rRNA genes reveals diet-related differences of microbial communities in soil, gut, and casts of Lumbricus terrestris L. (Oligochaeta: Lumbricidae). FEMS Microbiol. Ecol. 2004, 48, 187–197. [Google Scholar] [CrossRef]
- Furlong Michelle, A.; Singleton David, R.; Coleman David, C.; Whitman William, B. Molecular and Culture-Based Analyses of Prokaryotic Communities from an Agricultural Soil and the Burrows and Casts of the Earthworm Lumbricus rubellus. Appl. Environ. Microbiol. 2002, 68, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Guhra, T.; Stolze, K.; Totsche, K.U. Pathways of biogenically excreted organic matter into soil aggregates. Soil Biol. Biochem. 2022, 164, 108483. [Google Scholar] [CrossRef]
- Zhu, B.-K.; Fang, Y.-M.; Zhu, D.; Christie, P.; Ke, X.; Zhu, Y.-G. Exposure to nanoplastics disturbs the gut microbiome in the soil oligochaete Enchytraeus crypticus. Environ. Pollut. 2018, 239, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Horn Marcus, A.; Schramm, A.; Drake Harold, L. The Earthworm Gut: An Ideal Habitat for Ingested N2O-Producing Microorganisms. Appl. Environ. Microbiol. 2003, 69, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, J.; Horn Marcus, A.; Matthies, C.; Gößner, A.; Schramm, A.; Drake Harold, L. N2O-Producing Microorganisms in the Gut of the Earthworm Aporrectodea caliginosa Are Indicative of Ingested Soil Bacteria. Appl. Environ. Microbiol. 2003, 69, 1655–1661. [Google Scholar] [CrossRef]
- Horn Marcus, A.; Drake Harold, L.; Schramm, A. Nitrous Oxide Reductase Genes (nosZ) of Denitrifying Microbial Populations in Soil and the Earthworm Gut Are Phylogenetically Similar. Appl. Environ. Microbiol. 2006, 72, 1019–1026. [Google Scholar] [CrossRef]

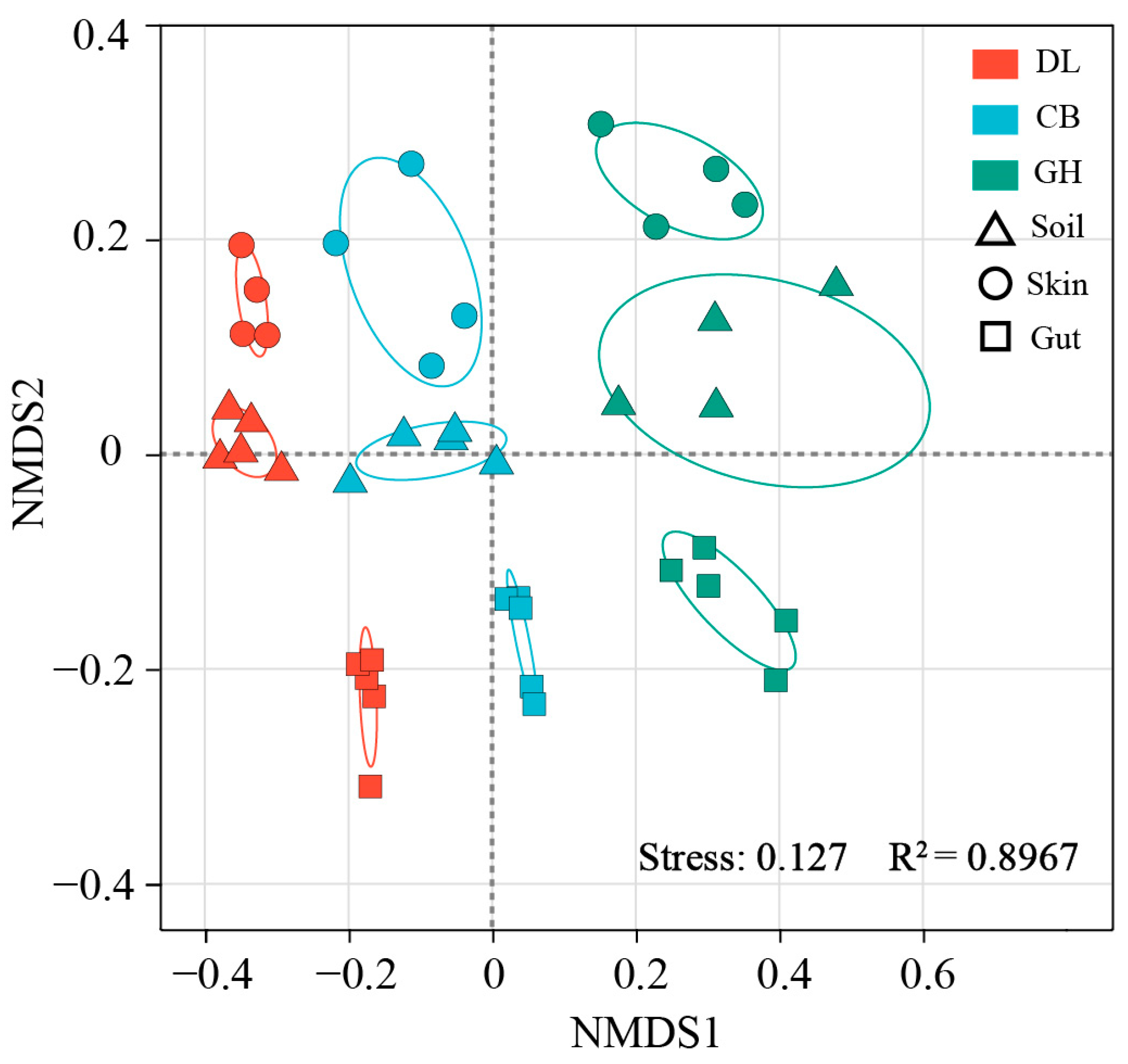


| Group | Process | ANOSIM | PERMANOVA | |
|---|---|---|---|---|
| R | R2 | F | ||
| Soil | DL vs. CB | 0.952 ** | 0.38007 | 4.9046 ** |
| DL vs. GH | 0.996 ** | 0.40547 | 5.456 ** | |
| CB vs. GH | 0.832 ** | 0.32696 | 3.8864 ** | |
| Skin | DL vs. CB | 0.776 * | 0.32961 | 2.9501 ** |
| DL vs. GH | 1 * | 0.43064 | 5.1244 * | |
| CB vs. GH | 0.896 * | 0.3221 | 2.8508 * | |
| Gut | DL vs. CB | 1 ** | 0.59679 | 11.841 ** |
| DL vs. GH | 1 ** | 0.50856 | 8.2787 ** | |
| CB vs. GH | 1 ** | 0.47142 | 7.1348 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Yuan, N.; Zhou, J.; Ni, H. Comparative Analysis of Bacterial Community Structures in Earthworm Skin, Gut, and Habitat Soil across Typical Temperate Forests. Microorganisms 2024, 12, 1673. https://doi.org/10.3390/microorganisms12081673
Wang K, Yuan N, Zhou J, Ni H. Comparative Analysis of Bacterial Community Structures in Earthworm Skin, Gut, and Habitat Soil across Typical Temperate Forests. Microorganisms. 2024; 12(8):1673. https://doi.org/10.3390/microorganisms12081673
Chicago/Turabian StyleWang, Kang, Ning Yuan, Jia Zhou, and Hongwei Ni. 2024. "Comparative Analysis of Bacterial Community Structures in Earthworm Skin, Gut, and Habitat Soil across Typical Temperate Forests" Microorganisms 12, no. 8: 1673. https://doi.org/10.3390/microorganisms12081673





