Abstract
Dietary fiber (DF) is an important nutrient component in pig’s diet that remarkably influences their growth and slaughter performance. The ability of pigs to digest DF depends on the microbial composition of the intestinal tract, particularly in the hindgut. However, studies on how DF alters the growth and slaughter performance of pigs by shaping the gut microbial composition and metabolites are still limited. Therefore, this study aimed to investigate the effects of DF on microbial composition, functions, and metabolites, ultimately altering host growth and slaughter performance using Durco × Bamei crossbred pigs supplemented with 0%, 10%, 17%, and 24% broad bean silage in the basic diet. We found that the final weight, average daily gain, fat, and lean meat weight significantly decreased with increasing DF. Pigs with the lowest slaughter rate and fat weight were observed in the 24% fiber-supplemented group. Gut microbial communities with the highest alpha diversity were formed in the 17% fiber group. The relative abundance of fiber-degrading bacteria, bile acid, and succinate-producing bacteria, including Prevotella sp., Bacteroides sp., Ruminococcus sp., and Parabacteroides sp., and functional pathways, including the butanoate metabolism and the tricarboxylic acid [TCA] cycle, significantly increased in the high-fiber groups. The concentrations of several bile acids significantly decreased in the fiber-supplemented groups, whereas the concentrations of succinate and long-chain fatty acids increased. Our results indicate that a high-fiber diet may alter the growth and slaughter performance of Durco × Bamei crossbred pigs by modulating the composition of Prevotella sp., Bacteroides sp., Ruminococcus sp., Parabacteroides sp., and metabolite pathways of bile acids and succinate.
1. Introduction
Many microorganisms inhabit the intestinal tract of pigs [1]. Many gut microbiota can ferment food resources and produce various metabolites that play important roles in host health [2,3], energy metabolism [4], growth and development [5], reproduction [6,7], and fat storage [8,9]. Gut microbial fermentation provides the host with a nitrogen source, energy, and essential amino acids to improve growth and health [10,11]. Additionally, microbial metabolites can act as signaling molecules to regulate host metabolic processes [12,13,14,15]. Gut bacteria participate in regulating bile acid synthesis and generating secondary bile acids that can activate the intestinal gluconeogenesis and Farnesoid X-receptor pathways in the gut of mice, ultimately improving the body weight and health of the host [12,16].
DF is widely used in pig diets because of its easy availability, low cost, and special nutrition [17]. Different sources and proportions of DF have distinct effects on the growth, slaughter performance, and meat quality of pigs [18,19,20]. Adding corn or wheat bran to the basic diet of weaned piglets can alter the gut microbial composition, resulting in increased butyrate production and improved piglet growth performance [20]. Supplementation with 19.1% total dietary fiber improved the utilization efficiency of food without altering the growth rate of finishing pigs [18]. However, many studies also indicated that diets with a high proportion of fiber may negatively affect average daily feed intake (ADFI) and nutrient digestibility in pigs [21,22,23]. A high proportion of DF in the diet can decrease the digestibility of energy and protein and reduce feed conversion rate and growth performance in pigs [19,24]. An appropriate fiber diet can contribute to the diversity of the gut microbial community and improve the health of pigs [18,20].
Digesting DF in pigs mainly depends on enzymes from their gut microbiota [25,26]. Short-chain fatty acids (SCFAs) are the major bacterial metabolites that ferment DF in the pig gut ecosystem [20]. SCFAs are important energy substrates that directly provide energy and improve pig growth performance [18,20]. Dietary supplementation with 5% corn bran or 5% wheat bran enhanced the abundance of butyrate-producing bacteria and butyrate production, contributing to the growth performance of weaned piglets [20]. Additionally, SCFAs can serve as signaling molecules that regulate metabolism via two major signaling pathways: G-protein-coupled receptors and histone deacetylases [27,28], thereby improving gut health and obesity [29,30,31,32]. The effects of DF on gut microbial metabolites include amino acid metabolites [33], carbohydrate metabolism [18], acid metabolites [34], and bile acids [35,36]. Although many studies have revealed that DF can significantly alter the gut microbial composition and metabolites and affect growth and slaughter performance in pigs, the underlying mechanism of the association between DF and growth and slaughter performance remains unclear. Therefore, this study aimed to investigate the effects of DF on microbial composition, functions, and metabolites, ultimately altering host growth and slaughter performance.
Bamei pigs are a native breed in Qinghai Province that is capable of digesting a high-fiber diet but has a slow growth rate [37]. Duroc pigs possess a high growth rate and lean meat rate but a low tolerance to a high-fiber diet [38]. Hybridization contributes to offspring inheriting good quality from their parents [38,39]. Therefore, we used Duroc × Bamei crossbred pigs as subjects. Broad bean straw is easily available in Qinghai province. Silage is an important technology used to ferment crop straw, which can prolong the storage period and improve the forage palatability, and it is widely used in animal husbandry [40]. Therefore, we used broad bean silage as a DF supplement in the basic diet of Duroc × Bamei crossbred pigs and employed metagenomic sequencing technology to uncover the effects of DF on gut microbial composition and functions and untargeted metabolomics analysis to detect differentiated metabolites among different diet groups. The effects of DF on the growth and slaughter performance of Duroc × Bamei crossbred pigs were also measured. The association between gut microbiota and metabolites and growth and slaughter performance was analyzed to uncover the potential mechanism of DF effect on growth and slaughter performance in Duroc × Bamei crossbred pigs. We expected that (1) DF would affect the growth and slaughter performance of Duroc × Bamei crossbred pigs by shaping the gut microbial composition, functions, and metabolites, and (2) high DF would reduce fat weight and promote the lean meat weight of Duroc × Bamei crossbred pigs.
2. Materials and Methods
2.1. Ethics Approval and Consent to Participate
All animal experimental procedures adhered to the relevant laws and institutional guidelines and were approved by the Institutional Animal Care and Use Committee of Qinghai University (approval number: NQH2019102).
2.2. Animals and Treatment
The experimental animals were the hybrid offspring of male Duroc and female Bamei pigs. Twenty-four pigs were selected and randomly divided into four groups; each group was housed in a separate pen and had ad libitum access to water and treatment diets. The initial body weight of all the pigs was approximately 25.5 kg. The entire process comprised a 7-day pre-trial period, followed by a 90-day experimental period. The experiment was conducted at the Qinghai Huzhu Bamei Breeding Farm.
2.3. Experimental Diet and Feeding Management
The experimental diet was formulated according to NRC2012 “Standard for fleshy growing finishing pigs” [41]. The composition and nutrients are listed in Table 1, and the nutrient content of the silage diet is listed in Table S1. Pigs in the control group were fed a basic diet, while the diets of the pigs in groups I, II, and III were supplemented with 10%, 17%, and 24% broad bean silage, respectively. The percentages of crude fiber for control groups (groups I, II, and III) were 2.4%, 4.2%, 5.5%, and 6.8%, respectively.

Table 1.
Ingredient composition and nutrient and energy levels of the experimental diets.
2.4. Data Collection and Sampling
After the experiment, growth performance, including final weight, average daily gain (ADG), and ADFI, was calculated. The slaughter performance was measured after slaughter. The contents of the cecum were collected and stored at −80 °C for subsequent intestinal microbial metagenomic sequencing and untargeted metabolomics analysis.
2.5. DNA Extraction, Library Construction, and Metagenomic Sequencing
Microbial DNA was extracted from cecal samples using a QIAamp DNA Stool Mini Kit (Qiagen 51504, Dusseldorf, Germany) according to the manufacturer’s instructions. The DNA was purified using QIAamp Mini Spin columns, following the standard protocol. The DNA concentration was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA).
DNA was extracted from the cecal contents using the E. Z. N. A. Soil DNA Kit (Omega BioTek, Norcross, GA, USA) according to the manufacturer’s instructions. DNA quality was checked using a 1% agarose gel and a NanoDrop 2000Q (Thermo Scientific, Waltham, MA, USA). Qualified DNA was randomly interrupted to approximately 400 bp using Covaris M220 (Gene Company Limited, Hong Kong, China). A paired-end library was constructed using NEXTFLEX Rapid DNASeq (BioScientific, Austin, TX, USA). Sequencing was performed using Illumina HiSeq 2000 at NovoGene Biological Information Technology Co., Ltd. (Beijing, China).
2.6. Sequencing Data Analysis
Raw sequencing data were analyzed on the free online Majorbio Cloud Platform (https://cloud.majorbio.com/page/task/index.html accessed on 9 July 2024). The paired-end reads were trimmed of adaptors, and low-quality reads (length < 50 bp, with a quality value < 20, or with N bases) were removed using fastp (https://github.com/OpenGene/fastp accessed on 9 July 2024, version 0.20.0). The metagenomic data were assembled using MEGAHIT (https://github.com/voutcn/megahit accessed on 9 July 2024, version 1.1.2). Contigs with a length ≥ 300 bp were selected as the final assembling result, and then the contigs were used for further gene prediction and annotation. Open reading frames from each assembled contig were predicted using Prodigal (https://github.com/hyattpd/Prodigal accessed on 9 July 2024, version 2.6.3). Predicted open reading frames with length ≥ 100 bp were retrieved and translated into amino acid sequences using the National Center for Biotechnology Information (NCBI) translation table (http://www.ncbi.nlm.nih.gov/Taxonomy/taxonomyhome.html/index.cgi?chapter=tgencodes#SG1 accessed on 9 July 2024). A non-redundant gene catalog was constructed using CD-HIT (http://www.bioinformatics.org/cd-hit/ accessed on 9 July 2024, version 4.6.1) with 90% sequence identity and 90% coverage. After quality control, reads were mapped to the non-redundant gene catalog with 95% identity using the Short Oligonucleotide Analysis Package aligner (https://github.com/ShujiaHuang/SOAPaligner accessed on 9 July 2024, version 2.21 release), and the gene abundance in each sample was evaluated. Representative sequences of the non-redundant gene catalog were aligned to the NCBI NR database with an e-value cutoff of 1 × 10−5 using Diamond (https://github.com/bbuchfink/diamond accessed on 9 July 2024, version 0.8.35) for taxonomic annotations. Kyoto Encyclopedia of Genes and Genomes annotation was performed using the Diamond software (https://github.com/bbuchfink/diamond accessed on 9 July 2024, version 0.8.35). Carbohydrate-active enzymes annotation was conducted using hmmscan (http://hmmer.org/download.html accessed on 9 July 2024, version 3.4) against the Carbohydrate-Active enZYmes (CAZy) database (http://www.cazy.org/) with an e-value cutoff of 1 × 10−5.
2.7. Determination of the Metabolomic Profiles of Cecal Contents
A total of 24 cecal contents were analyzed using a liquid chromatography–mass spectrometry (LC-MS) platform (Thermo Fisher, Waltham, MA, USA). An amount of 100 mg cecal contents from each sample was ground in liquid nitrogen and resuspended in 200 µL pre-chilled ddH2O and 800 µL mixed liquids of methanol and acetonitrile (1:1) and then shaken for 30 s to blend. Thereafter, an ultrasound was performed in the ice bath for 60 min. Samples were incubated on ice for 5 min and then centrifuged at 15,000 rpm at 4 °C for 5 min. The supernatant was transferred into a new centrifuge tube and blow-dried by vacuum concentration. Thereafter, samples were redissolved using 100 µL acetonitrile–water solution (1:1, 4 °C) and then transferred into a fresh microcentrifuge tube (Eppendorf, Hamburg, Germany) with a 0.22 µm filter and centrifuged at 15,000 rpm at 4 °C for 10 min. The filtrate was subjected to LC-MS/MS analysis. More detailed methods for LC-MS procedures and metabolomic data processing have been described in the previous report [42].
2.8. Statistical Analysis
All results were presented as the mean ± SEM, and all the statistical analyses were performed using SPSS 20.0 and R software (version 4.2.1). The Kruskal–Wallis test was used to detect differences in the relative abundance of bacterial phylum, genus, functional pathways and CAZy, the alpha diversity, the ratio of feed to gain, the ratio of Firmicutes to Bacteroidetes, slaughter rate, fat percentage, lean meat percentage and cooked meat percentage between the control and treatment groups, and the median test was employed as post-hoc test. The rest of the growth and carcass performance between the control and treatment groups were compared by one-way ANOVA. Prior to one-way ANOVA statistical analyses, the data were examined for assumptions of normality and homogeneity of variance by Kolmogorov–Smirnov and Levene tests, respectively. The permutational multivariate analysis of variance (PERMANOVA) and analysis of similarities (ANOSIM) based on Bray–Curtis distance matrices (nested adonis function in R4.2.1, package “vegan”) were used to compare the differences in the gut microbial community and CAZy between four diet groups, respectively. Spearman’s correlation was used to analyze the correlation between bacterial genera, metabolic pathways, growth, and slaughter performance. Statistical significance was defined as p < 0.05.
3. Results
3.1. Effects of Dietary Fiber on Growth and Slaughter Performance
To determine the effects of DF supplementation on the growth and slaughter performance of pigs, several features were collected and compared among the four diet groups. The results showed that final body weight, carcass weight, ADG, and ADFI significantly (p < 0.05) decreased with an increase in DF, whereas the ratio of feed to gain (F/G) significantly (p < 0.05) increased in high-fiber diet groups (Table 2). The slaughter rate and fat weight showed no difference (p > 0.05) between the control group and groups I and II but significantly (p < 0.05) decreased in group III (Table 3). Lean meat weight, hind leg weight, and bone weight significantly (p < 0.05) decreased in high-fiber diet groups, whereas lean meat percentage, fat percentage, carcass length, back fat depth, skin thickness, and cooked meat percentage were not significantly (p > 0.05) different among the different diet groups (Table 3).

Table 2.
Effects of DF on the growth performance of Durco × Bamei crossbred pigs.

Table 3.
Effects of DF on slaughter performance of Durco × Bamei crossbred pigs.
3.2. Effects of Dietary Fiber on the Composition and Diversity of Gut Bacteria
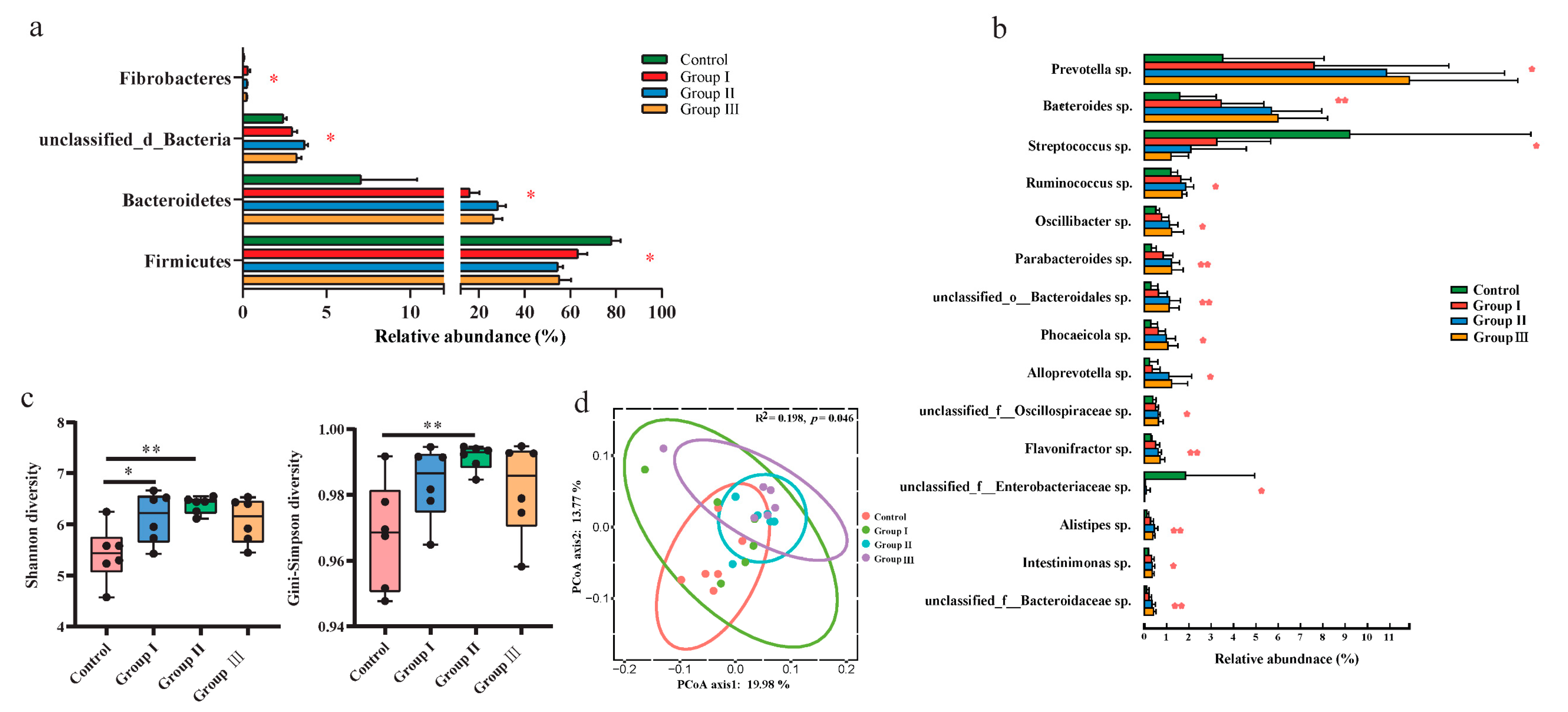
Metagenomic sequencing technology was used to explore the changes in gut bacteria among the different diet groups. The top two abundant bacteria phyla were Firmicutes and Proteobacteria in the control group, while it turned to Firmicutes and Bacteroidetes in groups I, II, and III (Figure S1a). The top three most abundant bacterial genera were Lactobacillus sp., Clostridium sp., and Streptococcus sp. in the control group; Lactobacillus sp., Prevotella sp., and Clostridium sp. were dominant in group I; and Lactobacillus sp., Prevotella sp., and Bacteroides sp. dominated groups II and III (Figure S1b). At the phylum level, the relative abundance of Firmicutes significantly (p < 0.05) increased with increasing DF, whereas that of Bacteroidetes and unclassified bacteria decreased (p < 0.05) (Figure 1a). The relative abundance of Fibrobacteres was also significantly (p < 0.05) decreased in the high-fiber diet groups (Figure 1a). Additionally, the ratio of Firmicutes to Bacteroidetes also significantly (p< 0.05) decreased with the increasing DF (Figure S2). The relative abundances of 13 bacterial genera, including Prevotella sp., Bacteroides sp., Ruminococcus sp., Oscillibacter sp., and Parabacteroides sp., significantly (p < 0.05) increased with an increase in DF, whereas the abundances of Streptococcus sp. and unclassified Enterobacteriaceae sp. decreased (p < 0.05) in the high-fiber diet groups (Figure 1b). The Shannon and Simpson indices significantly (p < 0.05) increased in groups I and II compared to the control group, whereas they did not significantly (p > 0.05) change with the continuous increase in DF (Figure 1c). Principal coordinate analysis (PCoA) also showed that the beta diversity of gut bacteria was significantly (p < 0.05) different among the four diet groups (Figure 1d).
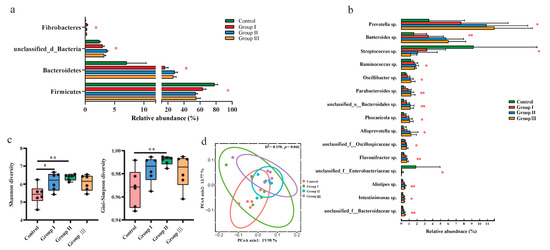
Figure 1.
Comparison of the gut microbial composition and diversity among the four diet groups. The effects of DF on the relative abundance of the dominant bacterial phyla (a), genus (b), and alpha diversity of the community (c). Different letters indicate significant differences between the treatments (p < 0.05). The PCoA plot of ASV-level and permutational multivariate analysis of variance based on Bray–Curtis distances between samples from four diet groups (d). p values: * p < 0.05; ** p < 0.01.
3.3. Effects of Dietary Fiber on the Function of the Microbial Community
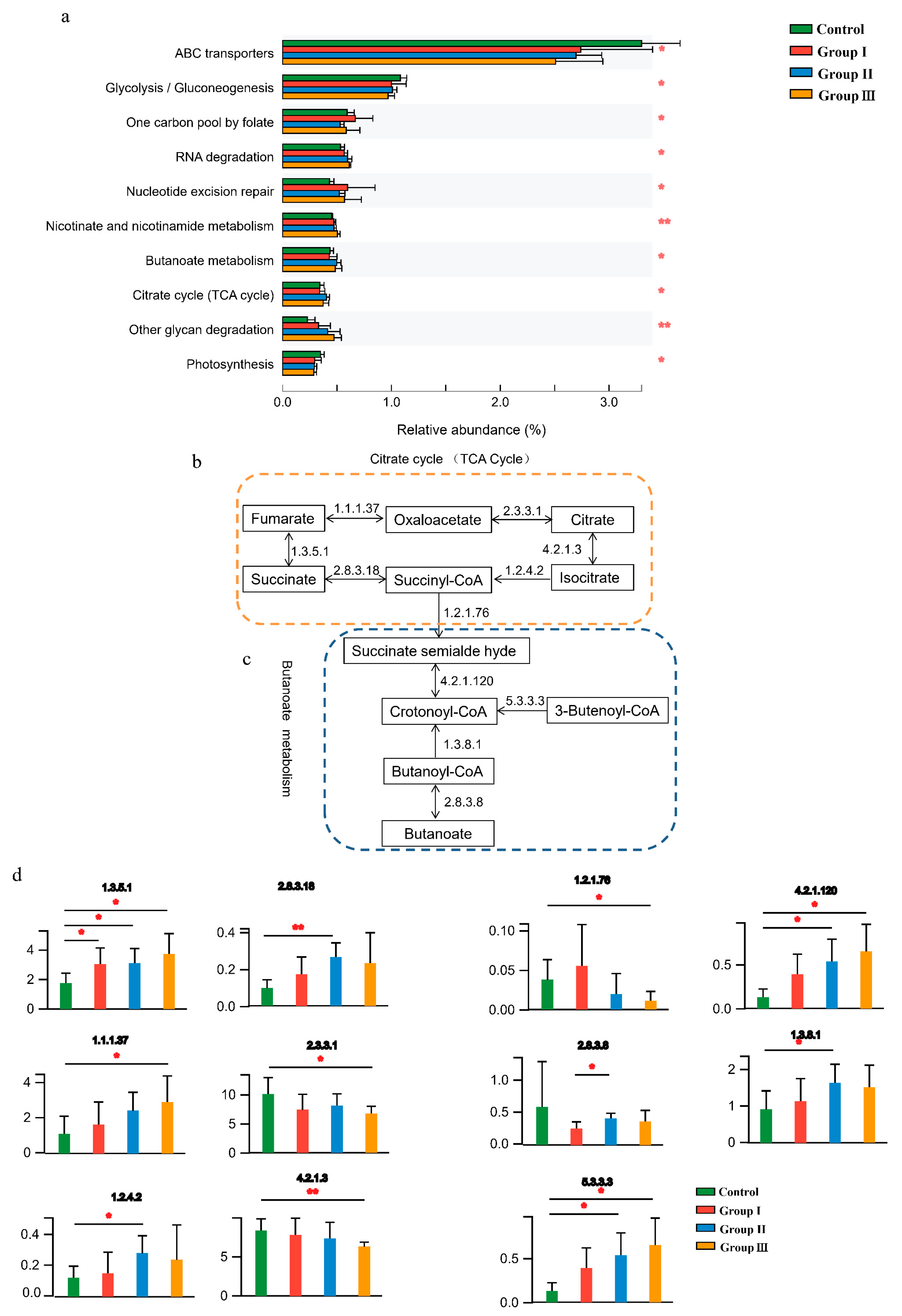
Gut microbial functions were evaluated based on the KEGG and CAZy databases. In total, 51 functional pathways were significantly (p < 0.05) different among the four diet groups (Table S2), indicating that DF fiber significantly affected the bacterial functions of the crossbred pigs. Of the top 10 abundant functional pathways, the abundance of ABC transporters, glycolysis/gluconeogenesis, and photosynthesis significantly decreased with an increase in DF, whereas RNA degradation, nicotinate and nicotinamide metabolism, butanoate metabolism, tricarboxylic acid cycle (TCA cycle), and other glycan degradation pathways significantly (p < 0.05) increased in the high-fiber diet (Figure 2a). Additionally, the combination of butanoate metabolism and the TCA cycle was closely associated with the production and consumption of succinate (Figure 2b,c), and the abundance of enzymes involved in the production and consumption of succinate significantly (p < 0.05) changed with increasing DF (Figure 2d).
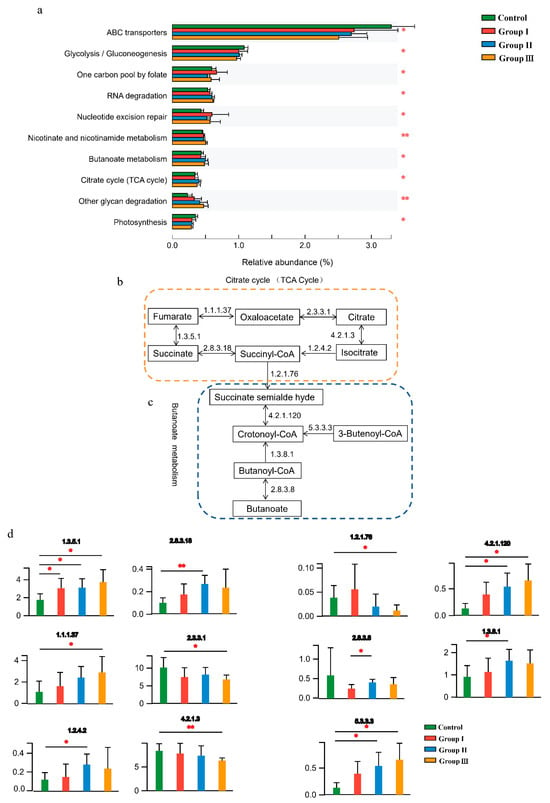
Figure 2.
Effect of DF on the gut microbial functions of the crossbred pigs. The shifts of the top 10 abundant function pathways in four diet groups (a). The processes of succinate accumulation and consumption in the TCA cycle (b) and butanoate metabolism (c). The shifts of enzymes involved in metabolizing succinate among the four diet groups (d). Different letters indicate significant differences between the treatments (p < 0.05). p values: * p < 0.05; ** p < 0.01.
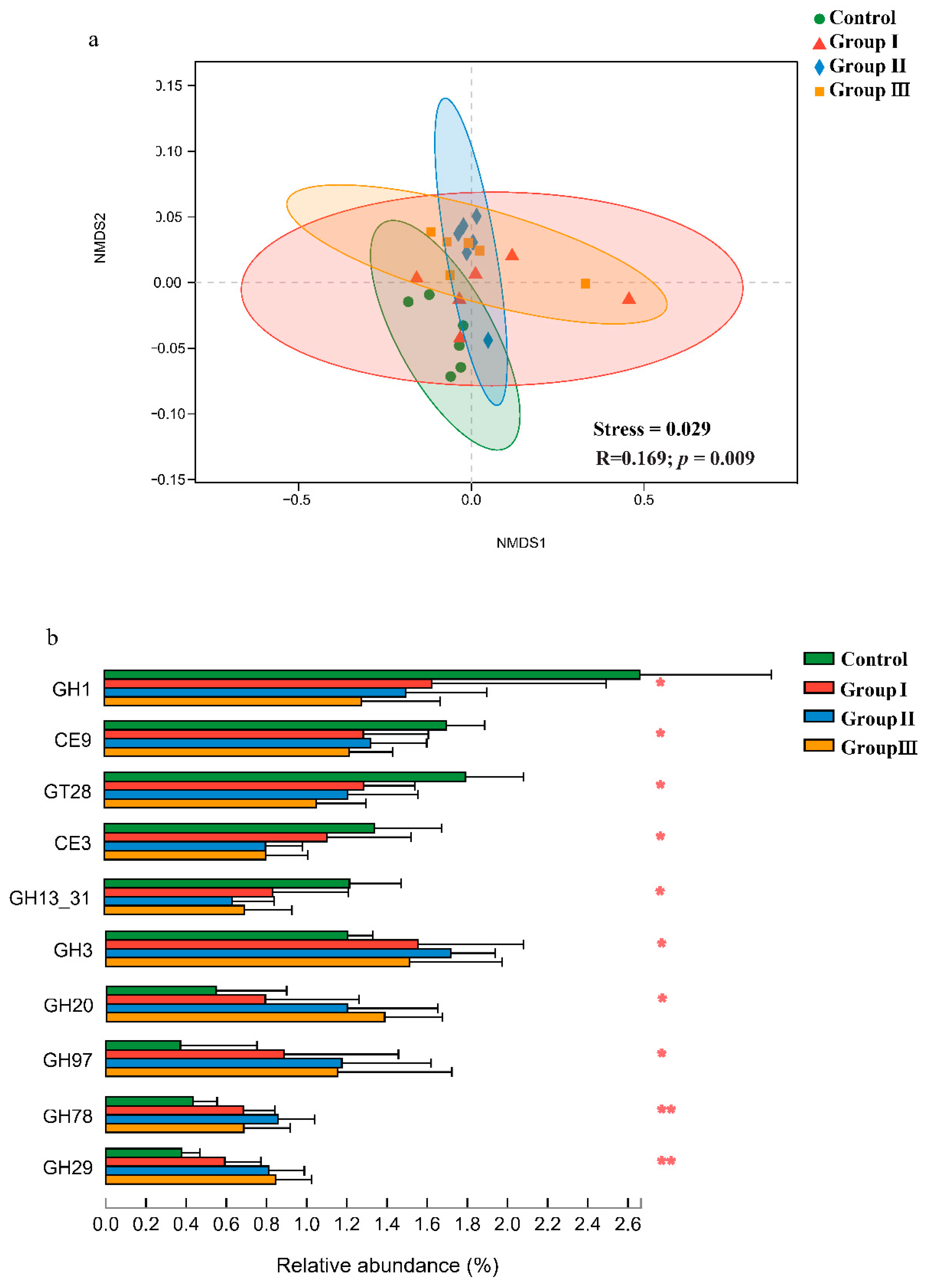
The non-metric multidimensional scaling (NMDS) plots and similarity analysis based on Bray–Curtis distance showed that the CAZy composition of the gut microbiota significantly (p < 0.05) differed among the four diet groups (Figure 3a). Furthermore, significant (p < 0.05) differences in many CAZy families were found among the four diet groups (Figure 3b). The relative abundances of the GH3, GH20, GH97, GH78, and GH29 families significantly (p < 0.05) increased in the high-fiber diet groups, while the relative abundances of the GH1, CE9, GT28, CE3, and GH13_31 families decreased (Figure 3b).
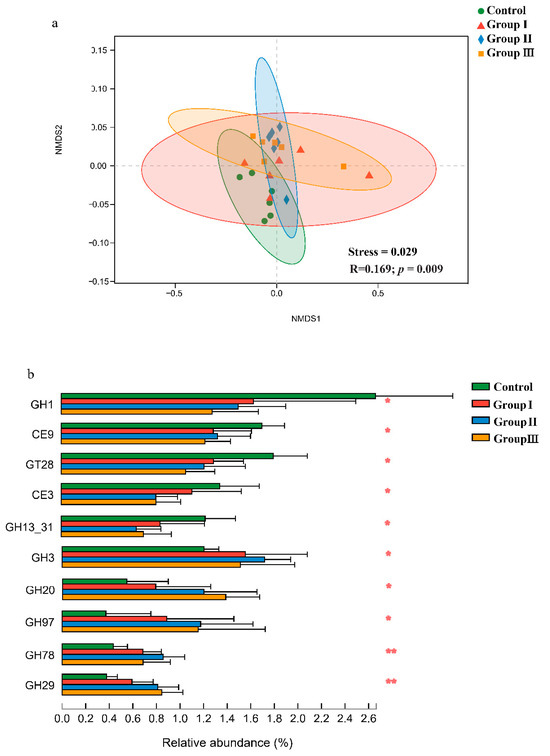
Figure 3.
Effect of DF on the Carbohydrate-Active enZymes (CAZy) in the crossbred pigs. Non-metric multidimensional scaling analysis and analysis of similarities of CAZy in the crossbred pigs from four diet groups (a). The shifts of CAZy families in four diet groups (b). p values: * p < 0.05; ** p < 0.01.
3.4. Effects of Dietary Fiber on Microbial Metabolite
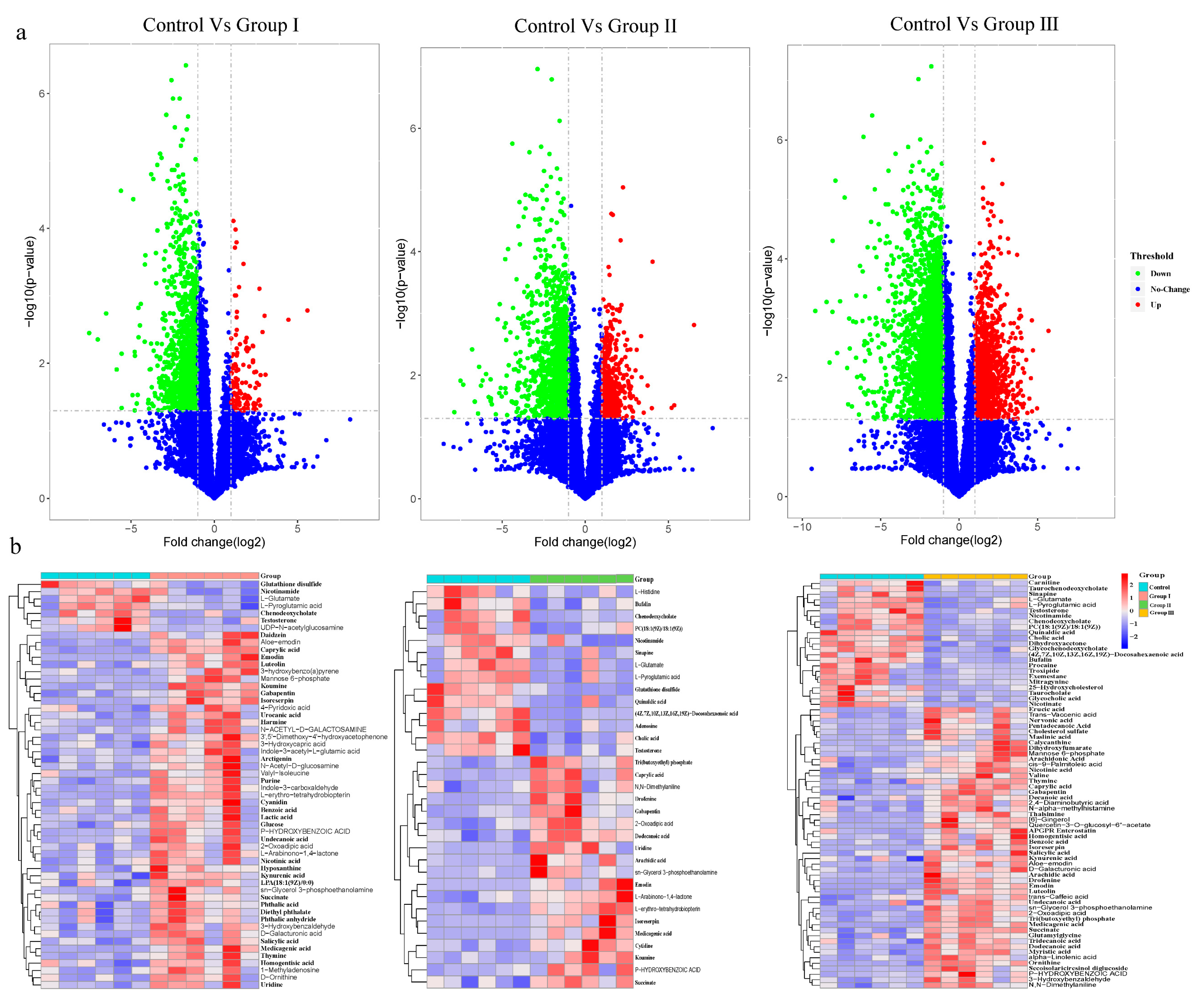
To evaluate the effect of DF on microbial metabolites, LC-MS/MS analyses were used to detect metabolites in cecal contents. Based on the variable importance for projection (VIP) obtained from the Orthogonal Projections to Latent Structures Discriminant Analysis model, the impact and interpretation of each metabolite were measured, and different metabolites were explored with biological significance. In this study, metabolites with both multidimensional statistical analysis (VIP > 1) and univariate statistical analysis p value < 0.05 were significantly different. The results showed that 59, 34, and 78 metabolites were significantly different between the control group and groups I, II, and III, respectively (Figure 4). Among the altered metabolites, many were bile acids, amino acids, polypeptides, and long-chain fatty acids (Figure 4b). The bile acids, including cholic acid, chenodeoxycholate, taurochenodeoxycholate, glycochenodeoxycholate, taurocholate and glycocholic acid, and amino acids and polypeptides, including the glutamate, pyroglutamic acid, glutathione disulfide, and nicotinamide, were enriched in the control group, while some long-chain fatty acids, including caprylic acid, undecanoic acid, arachidonic acid, erucic acid, pentadecanoic acid, decanoic acid, tridecanoic acid, and alpha-linolenic acid, were enriched in DF adding diet groups (Figure 4b). Additionally, the abundance of succinate was significantly increased in all DF-adding groups (Figure 4b).
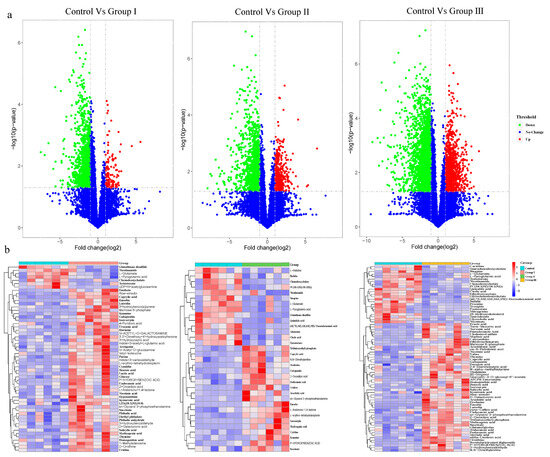
Figure 4.
Effect of DF on the microbial metabolites in crossbred pigs. Volcano plots show the differentiation in the concentration of microbial metabolites between the control and treatment groups (a). The heatmaps show significantly altered metabolites between the control and treatment groups (b).
3.5. Correlation among Gut Microbiota, Microbial Metabolites, Host Growth, and Slaughter Performance
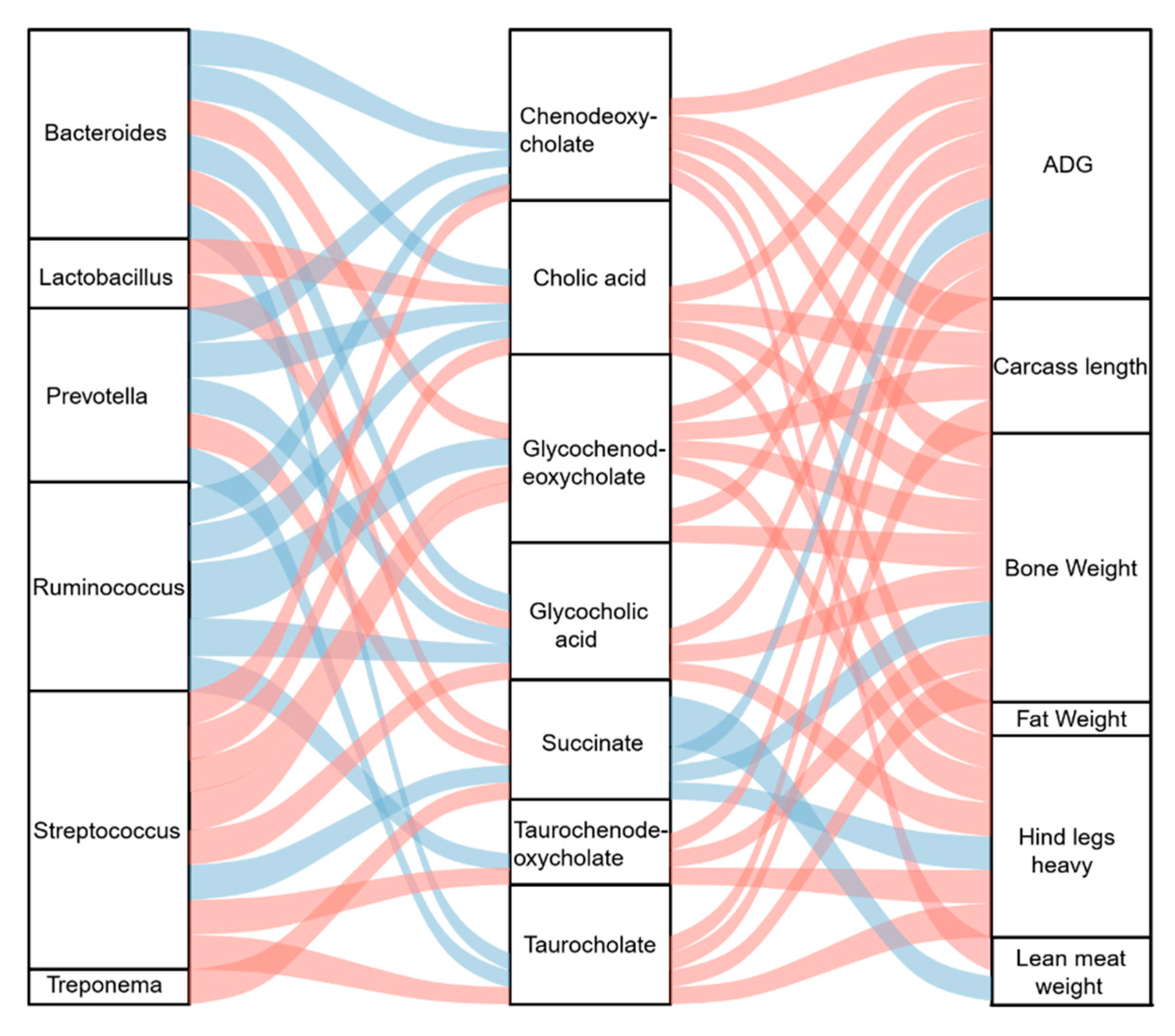
To determine the potential mechanism underlying the effects of DF on the growth and slaughter performance of crossbred pigs, Spearman’s correlation analysis was performed between microbial genera and metabolites and a correlation analysis between microbial metabolites and host growth and slaughter performance. The relative abundances of Bacteroides sp. and Prevotella sp. were significantly (p < 0.05) negatively associated with the concentrations of chenodeoxycholate, cholic acid, glycocholic acid, and taurocholate, whereas Lactobacillus sp. was significantly (p < 0.05) positively associated with cholic acid and glycocholic acid (Figure 5). The relative abundance of Ruminococcus sp. was significantly (p < 0.05) negatively associated with all the significantly altered bile acids, except for taurocholate, whereas Streptococcus sp. was significantly (p < 0.05) positively correlated with all the altered bile acids (Figure 5). Succinate concentration was significantly (p < 0.05) positively associated with the relative abundance of Bacteroides, Prevotella sp., and Treponema sp. and significantly (p < 0.05) negatively associated with Streptococcus sp. (Figure 5). Of the altered bile acids, all were significantly (p < 0.05) positively associated with ADG, bone weight, and hind leg weight (Figure 5). Additionally, lean meat weight was significantly (p < 0.05) positively associated with chenodeoxycholate (Figure 5). The carcass lengths of the pigs were significantly (p < 0.05) positively correlated with altered bile acid levels, except for glycocholic acid and taurochenodeoxycholate (Figure 5). The succinate concentration was significantly (p < 0.05) negatively associated with ADG, bone weight, lean meat weight, and hind leg weight (Figure 5).
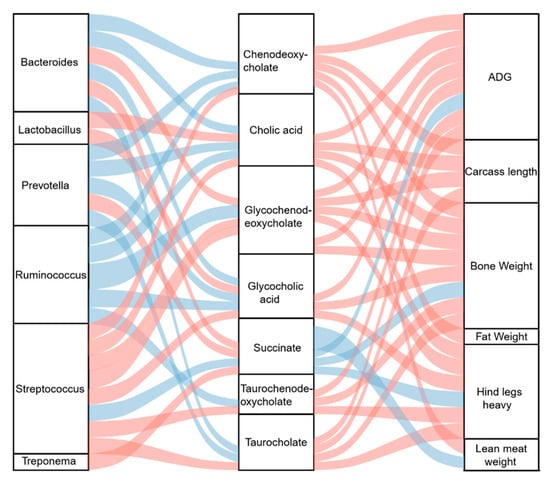
Figure 5.
Sankey plot showing the Spearman correlation between gut bacteria, microbial metabolites, and growth and slaughter performances of the crossbred pigs. The blue lines represent a negative correlation. The red lines represent a positive correlation. The thickness of the lines represents the absolute values of the correlation coefficient.
4. Discussion
We found that the ADG and final weight of crossbred pigs significantly decreased in groups fed with 5.5% and 6.8% crude fiber in the diet compared to the control group, whereas they were not different in the group fed with 4.2% crude fiber (Table 2). These results indicate that Duroc × Bamei crossbred pigs could tolerate a diet with a low level of crude fiber, that is, a diet containing 10% broad bean silage. Similarly, previous studies also suggested that DF contributed to the decrease in ADG and the final weight of finishing pigs [24,43]. Additionally, another previous study showed that purebred Bamei pigs could tolerate a diet with over 5.1% crude fiber, which was higher than that of wild boars [37]. Our results suggest that crossbreeding with Western-breed pigs could reduce the tolerance of their offspring to DF compared with that of their maternal individuals [37,44]. Similar characteristics were also found in different local breeds of pigs, including the Tunchang pig [45], Jiaxing pig [46], and wild boar [37,47].
Both the final body weight and ADFI significantly decreased in the high-fiber diet groups (Table 2). However, the effect of DF on ADFI in pigs has been contradictory in different studies [17,48]. For example, adding 3.72% total fiber to the diet had no effect on the ADFI of Duroc and Taoyuan pigs [48], whereas studies on Mashen and Duroc × Landrace × Yorkshire pigs showed that ADFI increased with an increase in DF [17]. Therefore, the effects of DF on ADFI vary according to the pig breed and the DF resources or physicochemical properties of the fiber [22,49,50].
Firmicutes were the most abundant gut bacterial phylum in the crossbred pigs, followed by Bacteroidetes, Proteobacteria, and Actinobacteria (Figure S1a). Firmicutes and Bacteroidetes also predominate in the cecal contents of different pig breeds, although their relative abundances vary [18,51,52]. Furthermore, the abundances of other phyla vary [18,51,52]. For example, Proteobacteria were hardly found in the cecum of Laiwu pigs, as were Spirochaetes [52]. Of the top three abundant bacterial genera, Lactobacillus sp. was the most abundant in the cecum, followed by Clostridium sp. and Streptococcus sp. in the control group (Figure S1b), and similar results were found in Suhuai pigs, with the second most abundant genus being Ruminococcaceae UCG-005 sp. [18]. The dominant genera in the ceca of Duroc × Landrace × Large White and Laiwu pigs were significantly different [51,52]. Many factors, including breed [42], age [53], and diet [20], can explain these differences. Additionally, the top abundant bacterial phyla and genera in the crossbred pigs were slightly different from the purebred Bamei pigs [54], as Firmicutes, Bacteroidetes, and Spirochaetes were the top three abundant bacterial phyla, and unidentified Clostridiales sp., Terrisporobacter sp., and Streptococcus sp. were the top three abundant genera in the gut of Bamei pigs [54]. This difference is probably attributable to crossbreeding, which could alter the gut microbiota of the offspring [55]. Lactobacillus sp. is a lactic acid bacterium that enhances the growth performance of pigs [56,57,58]. The high abundance of Lactobacillus sp. in the crossbred pigs suggests a higher growth performance than in purebred Bamei pigs.
DF significantly affects the composition and structure of the gut microbiota [18]. We found that the ratio of Firmicutes to Bacteroidetes was significantly decreased in the high-fiber diet groups (Figure S2). A previous study in humans also found that lean individuals harbored a lower ratio of Firmicutes to Bacteroidetes than overweight individuals [59]. A decrease in the Firmicutes to Bacteroidetes ratio indicates a decreased capacity to harvest energy from the diet [60]. DF digestion is closely associated with the gut microbiota in mammals [61]. In our results, the relative abundance of fiber-degrading bacteria, including Prevotella sp. [62], Bacteroides sp. [63,64], Ruminococcus sp. [65,66], Oscillibacter sp. [67], and Alloprevotella sp. [68], significantly increased in the high-fiber diet groups (Figure 1b). Similar results have been reported in pigs of different breeds, such as Duroc × (Landrace × Yorkshire) crossbred [46], Suihua [18], and Tibetan pigs [68]. However, the altered fiber-degrading bacteria varied among different studies, which may be attributed to the differences in breeds and resources of DF [46]. Additionally, we found that the relative abundance of two animal pathogens, Streptococcus sp. [69,70] and unclassified Enterobacteriaceae sp. [71], significantly decreased in high-fiber diet groups (Figure 1b), while the two probiotics, Parabacteroides sp. [72] and Phocaeicola sp. [73], significantly increased in high-fiber diet groups (Figure 1b), indicating that adding appropriate DF in diet could promote pigs’ health. In agreement with previous studies [18], we also found that adding appropriate DF to the diet significantly increased the alpha diversity of gut bacteria (Figure 1c), whereas an excess fiber diet decreased the diversity of gut bacteria. The diversity of the gut microbial community is positively associated with host health [74].
We detected 461 CAZymes in the gut bacterial communities of crossbred pigs, of which 237 belonged to 130 GH families (Table S3). The abundance of CAZymes in Duroc × Bamei crossbred pigs is higher than that in Duroc × Landrace × Large White and finishing pigs [75,76], indicating that crossbred pigs may be more tolerant to a high-fiber diet than other breeds. Although the total number of CAZymes from GH families was lower than that in cow and buffalo rumens, the diversity of the GH family was higher in crossbred pigs [77], indicating that Duroc × Bamei crossbred pigs may tolerate a high-fiber diet compared to some ruminants. We also found that the structure of CAZyme was significantly different among the four diet groups (Figure 3a). Similar results were found in Duroc × Landrace × Large White pigs and humans, which showed that adding different sources of DF affected the abundance of various CAZymes [75,78]. Of the detected GH family, five CAZymes were upregulated in the high-fiber diet groups, four (GH 29, GH 97, GH 20, and GH3) CAZymes belonged to oligosaccharide-degrading enzymes, and one (GH 78) belonged to debranching enzymes, while two CAZymes (GH 1 and GH13_31) belonging to oligosaccharide-degrading enzymes were downregulated in the high-fiber diet groups (Figure 3b). Previous studies also showed that abundant CAZymes from the GH 3, GH 20, GH 78, and GH 29 families were detected in the rumen bacteria of cows and buffalos and the gut microbiome of giant pandas [61,77]. Additionally, adding 8% raw potato starch to the piglet diet significantly enhanced the abundance of GH 97 [75]. These results suggest that, to some extent, these CAZymes converge in the gut microbiota of mammals.
The concentrations of many bile acid metabolites significantly decreased in all fiber-supplemented diet groups (Figure 4b), indicating that DF suppressed bile acid metabolites in Duroc × Bamei crossbred pigs. A similar result was found in a previous study in humans [36]. The gut microbiota plays many roles in the metabolism of bile acids, including the production of secondary bile acids, deconjugation of conjugated bile acids, and dehydrogenation of unconjugated bile acids [79]. Conjugated bile acids (free bile acid conjugate with glycine or taurine) were deconjugated by bacteria with bile salt hydrolase (BSH) activity (for example, Lactobacilli sp., Bifidobacteria sp., Clostridium sp., and Bacteroides sp.), which could prevent active reuptake from the small intestine [16]. Two bacterial genera belonging to Firmicutes, Clostridium sp. (clusters XIVa and XI), Eubacterium sp., and Parabacteroides sp., can produce secondary bile acids [12,16]. Another major microbial biotransformation of bile acids is generating oxo- (or keto-) bile acids by bacteria using hydroxysteroid dehydrogenases present in Actinobacteria, Proteobacteria, Firmicutes, and Bacteroidetes [16]. In this study, we found that the relative abundance of bacterial phyla, including Firmicutes and Bacteroidetes, and bacterial genera, including Parabacteroides sp. and Bacteroides sp., significantly decreased or increased with an increase in DF (Figure 1a,b), suggesting that DF affects the metabolism or concentration of bile acids in crossbred pigs by shaping the gut microbiota. Additionally, we found that the relative abundance of Prevotella sp. and Ruminococcus sp. was significantly negatively associated with several conjugated bile acids (Figure 5), suggesting that these two bacterial genera might be involved in the metabolism of bile acids in Duroc × Bamei crossbred pigs.
Bile acids are endocrine molecules that facilitate the absorption of fat-soluble nutrients and regulate numerous metabolic processes, including glucose, lipid, and energy homeostasis [15,80,81,82]. A shift in the composition of the bile acid pool is closely associated with variations in host weight gain, physiology [12,36,82], and bile acid metabolic bacteria [12,79,82]. Furthermore, an increase in the abundance of the BSH-expressing bacteria Escherichia coli and secondary bile acid-producing bacteria Parabacteroides distasonis reduced host weight gain in mice [12,82]. Similarly, we found that the relative abundances of two bacterial genera with BSH, Bacteroides sp. and Parabacteroides sp., significantly increased in the high-fiber diet groups (Figure 1b), which might contribute to the reduction in ADG and fat weight in crossbred pigs. Additionally, DF can adsorb bile acids, which are disadvantageous for the host in digesting dietary fat [83]. Supporting this, we detected higher concentrations of several fatty acids in all fiber-supplemented groups than in the control group (Figure 4b). Therefore, a reduction in dietary fat intake may contribute to losing weight [59].
We detected a significant increase in the concentration of succinate in all fiber-supplemented groups (Figure 4b). Similar results were found in a previous study in mice [14]. Succinate is an important intermediate in the TCA cycle that plays a crucial role in host metabolism [12,13,14,30,84]. Succinate is produced by gut bacteria, including Prevotella sp., Bacteroides sp., and Parabacteroides sp. [12,85]. We found significant positive associations between the concentration of succinate and the relative abundances of Bacteroides sp. and Prevotella sp. (Figure 5). Additionally, the relative abundance of Treponema sp. was positively correlated with the concentration of succinate (Figure 5), indicating that it is a potential succinate-producing bacterium in crossbred pigs. Previous studies have shown that succinate serves as a glucose signaling to activate intestinal gluconeogenesis, resulting in improved plasma glucose and body weight in overweight mice [12,14,86]. We found a significant negative association between succinate concentration and ADG, lean meat weight, bone weight, and hind leg weight (Figure 5). Therefore, our results may indicate that a high-fiber diet altered the growth and slaughter performance of Durco × Bamei crossbred pigs by shaping the composition of the gut microbiota and microbiota-produced succinate.
5. Conclusions
This study investigated the effects of a fiber diet on the growth and slaughter performance, gut microbiota composition, and microbial metabolites of Durco × Bamei crossbred pigs. Multi-omics technology revealed that a high-fiber diet significantly increased the relative abundance of many bacterial genera, including Prevotella sp., Bacteroides sp., and Ruminococcus sp., which could generate or metabolize bile acids and succinate that alters the ADG, lean meat weight, fat weight, bone weight, hind leg weight, slaughter rate, and carcass length of crossbred pigs. This study provides evidence on how a high-fiber diet affects gut bacteria and metabolism, resulting in variations in the growth and slaughter performance of Durco × Bamei crossbred pigs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms12081674/s1, Figure S1. The relative abundance of the top five abundant bacterial phylum (a) and top 10 abundant bacterial genera (b) in four diet groups. Figure S2. The effects of DF on the ratio of Firmicute to Bacteroidetes. Different letters indicate significant differences between the treatments (p < 0.05). Table S1. Nutrient and energy contents of the broad bean silage. Table S2. Inventory of 51 functional pathways significantly changed among four diet groups. Table S3. Inventory of putative CAZy family identified in the crossbred pig gut microbiome.
Author Contributions
G.W. and Y.Z. designed this study; L.W., J.Z., Y.M., F.X., G.W. and L.Z. performed the research and collected the samples; X.T., L.Z., S.R. and Y.Z. produced the initial draft of this manuscript; Y.Z. and G.W. revised this manuscript. All the authors contributed to this manuscript and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research and Demonstration of Reproduction of High-quality Pig Breeds and Industrialization Technology in Qinghai Province (No. 2023-NK-141) and the Second Tibetan Plateau Scientific Expedition and Research Program (No. 2019 QZKK0501).
Data Availability Statement
The metagenomic sequences data have been uploaded in the National Center for Bio-technology Information database using accession number of PRJNA1146790.
Acknowledgments
The affiliation, Technical Service Center of Bamei Pig Breeding of Qinghai Huzhu Tu Autonomous County, provided the place for us to carry out the experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J.; et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W. Intestinal microbiota in various animals. Integr. Zool. 2022, 17, 331–332. [Google Scholar] [CrossRef]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Ronan, V.; Yeasin, R.; Claud, E.C. Childhood Development and the Microbiome-The Intestinal Microbiota in Maintenance of Health and Development of Disease During Childhood Development. Gastroenterology 2021, 160, 495–506. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Su, J.; Li, H.; Xiong, J.; Fu, K.; Wang, Z.; Yuan, X.; Shi, Z.; Miao, X.; et al. Altitude-dependent metabolite biomarkers reveal the mechanism of plateau pika adaptation to high altitudes. Integr. Zool. 2023, 18, 1041–1055. [Google Scholar] [CrossRef]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Shi, Y.; Wang, J.; Niu, Z.; Wei, L.; Tian, H.; Yu, F.; Gao, L. The intestinal microbiota and metabolic profiles of Strauchbufo raddei underwent adaptive changes during hibernation. Integr. Zool. 2023, 19, 612–630. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Ma, Y.; Han, X.; Fang, J.; Jiang, H. Role of dietary amino acids and microbial metabolites in the regulation of pig intestinal health. Anim. Nutr. 2022, 9, 1–6. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e225. [Google Scholar] [CrossRef]
- Mills, E.; O’Neill, L.A. Succinate: A metabolic signal in inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Wahlström, A.; Marschall, H.U. Role of Bile Acids in Metabolic Control. Trends Endocrinol. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Niu, J.; Cui, Z.; Zhao, X.; Li, W.; Zhang, Y.; Yang, Y.; Gao, P.; Guo, X.; et al. Impacts of dietary fiber level on growth performance, apparent digestibility, intestinal development, and colonic microbiota and metabolome of pigs. J. Anim. Sci. 2023, 101, skad174. [Google Scholar] [CrossRef]
- Pu, G.; Li, P.; Du, T.; Niu, Q.; Fan, L.; Wang, H.; Liu, H.; Li, K.; Niu, P.; Wu, C.; et al. Adding Appropriate Fiber in Diet Increases Diversity and Metabolic Capacity of Distal Gut Microbiota Without Altering Fiber Digestibility and Growth Rate of Finishing Pig. Front. Microbiol. 2020, 11, 533. [Google Scholar] [CrossRef]
- Coble, K.F.; DeRouchey, J.M.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; Woodworth, J.C. Effects of withdrawing high-fiber ingredients before marketing on finishing pig growth performance, carcass characteristics, and intestinal weights. J. Anim. Sci. 2018, 96, 168–180. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, P.; Wu, Y.; Guo, P.; Liu, L.; Ma, N.; Levesque, C.; Chen, Y.; Zhao, J.; Zhang, J.; et al. Dietary Fiber Increases Butyrate-Producing Bacteria and Improves the Growth Performance of Weaned Piglets. J. Agric. Food Chem. 2018, 66, 7995–8004. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Emmans, G.C. The voluntary feed intake of pigs given feeds based on wheat bran, dried citrus pulp and grass meal, in relation to measurements of feed bulk. Br. J. Nutr. 1995, 73, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, D.; Liu, L.; Zang, J.; Duan, Q.; Yang, W.; Zhang, L. The effects of dietary fiber level on nutrient digestibility in growing pigs. J. Anim. Sci. Biotechnol. 2013, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.H.; MMNyachoti, C.M. Effect of dietary wheat bran inclusion on nutrient and energy digestibility and microbial metabolites in weaned pigs. Livest. Sci. 2017, 203, 110–113. [Google Scholar] [CrossRef]
- Gutierrez, N.A.; Kerr, B.J.; Patience, J.F. Effect of insoluble-low fermentable fiber from corn-ethanol distillation origin on energy, fiber, and amino acid digestibility, hindgut degradability of fiber, and growth performance of pigs. J. Anim. Sci. 2013, 91, 5314–5325. [Google Scholar] [CrossRef]
- Ziemer, C.J.; Kerr, B.J.; Weber, T.E.; Arcidiacono, S.; Morrison, M.; Ragauskas, A. Effects of feeding fiber-fermenting bacteria to pigs on nutrient digestion, fecal output, and plasma energy metabolites. J. Anim. Sci. 2012, 90, 4020–4027. [Google Scholar] [CrossRef]
- Pandey, S.; Kim, E.S.; Cho, J.H.; Song, M.; Doo, H.; Kim, S.; Keum, G.B.; Kwak, J.; Ryu, S.; Choi, Y.; et al. Swine gut microbiome associated with non-digestible carbohydrate utilization. Front. Vet. Sci. 2023, 10, 1231072. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; García-Espitia, M.; Ramírez-Sánchez, D.; García-Mena, J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Ma, S.; Ye, J.; Zhang, H.; Li, Y.; Sair, A.T.; Pan, J.; Liu, X.; Li, X.; et al. High-Dietary Fiber Intake Alleviates Antenatal Obesity-Induced Postpartum Depression: Roles of Gut Microbiota and Microbial Metabolite Short-chain Fatty Acid Involved. J. Agric. Food Chem. 2020, 68, 13697–13710. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Mao, X.; Chen, D. Short chain fatty acids could prevent fat deposition in pigs via regulating related hormones and genes. Food Funct. 2020, 11, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Tanes, C.; Bittinger, K.; Gao, Y.; Friedman, E.S.; Nessel, L.; Paladhi, U.R.; Chau, L.; Panfen, E.; Fischbach, M.A.; Braun, J.; et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe 2021, 29, 394–407.e395. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Alam, M.J.; Marques, F.Z.; Mackay, C.R. A major mechanism for immunomodulation: Dietary fibres and acid metabolites. Semin. Immunol. 2023, 66, 101737. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chu, Q.; Ma, S.; Ma, H.; Song, H. Dietary Fiber and Its Potential Role in Obesity: A Focus on Modulating the Gut Microbiota. J. Agric. Food Chem. 2023, 71, 14853–14869. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Zhang, Z.; Riva, A.; Armet, A.M.; Perez-Muñoz, M.E.; Nguyen, N.K.; Krysa, J.A.; Seethaler, B.; Zhao, Y.Y.; Cole, J.; et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 2022, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Gun, S.; Fu, Y.; Ma, D. Study on the digestive capacity and structure of the digestive tract of wild boar. J. Anim. Husb. Vet. Med. 2007, 26, 11–13. [Google Scholar] [CrossRef]
- Cassady, J.P.; Young, L.D.; Leymaster, K.A. Heterosis and recombination effects on pig growth and carcass traits. J. Anim. Sci. 2002, 80, 2286–2302. [Google Scholar] [CrossRef]
- Iversen, M.W.; Nordbø, Ø.; Gjerlaug-Enger, E.; Grindflek, E.; Lopes, M.S.; Meuwissen, T. Effects of heterozygosity on performance of purebred and crossbred pigs. Genet. Sel. Evol. 2019, 51, 8. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Silage and animal health. Nat. Toxins 1999, 7, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Shawk, D.; Nemechek, K.N.; Goodband, R.D.; Woodworth, J.C.; Tokach, S.S.; Dritz, J.M. National Research Council (NRC): Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- Yan, S.; Zhu, C.; Yu, T.; Huang, W.; Huang, J.; Kong, Q.; Shi, J.; Chen, Z.; Liu, Q.; Wang, S.; et al. Studying the Differences of Bacterial Metabolome and Microbiome in the Colon between Landrace and Meihua Piglets. Front. Microbiol. 2017, 8, 1812. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.T.; Patience, J.F.; Romoser, M.R.; Johnson, C.D.; Ross, J.W.; Gabler, N.K. Evaluation of increased fiber, decreased amino acids, or decreased electrolyte balance as dietary approaches to slow finishing pig growth rates. J. Anim. Sci. 2021, 99, skab164. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Jia, J.; Zhang, L.; Chen, Q.; Zhang, X.; Sun, W.; Ma, C.; Xu, F.; Zhan, S.; Ma, L.; et al. Jejunal inflammatory cytokines, barrier proteins and microbiome-metabolome responses to early supplementary feeding of Bamei suckling piglets. BMC Microbiol. 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Y.; Li, H.; Xie, Y.; Huang, G.; Peng, C.; Zhao, P.; Wang, Z. Hybridization alters the gut microbial and metabolic profile concurrent with modifying intestinal functions in Tunchang pigs. Front. Microbiol. 2023, 14, 1159653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Q.; Liu, L.; Chen, Y.; Jin, A.; Liu, G.; Li, K.; Li, D.; Lai, C. Comparative digestibility of nutrients and amino acids in high-fiber diets fed to crossbred barrows of Duroc boars crossed with Berkshire×Jiaxing and Landrace×Yorkshire. Asian-Australas. J. Anim. Sci. 2018, 31, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, L.M.G.; Schokker, D.; Bergsma, R.; Jansman, A.J.M.; Molist, F.; Calus, M.P.L. Prediction of nutrient digestibility in grower-finisher pigs based on faecal microbiota composition. J. Anim. Breed. Genet. 2020, 137, 23–35. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Kong, X.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; Yu, J.; Luo, J.; Yan, H.; et al. Effects of Dietary Fiber on Growth Performance, Nutrient Digestibility and Intestinal Health in Different Pig Breeds. Animals 2022, 12, 3298. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.S.; van den Borne, J.J.; Gerrits, W.J.; Kemp, B.; Bolhuis, J.E. Effects of dietary fibers with different physicochemical properties on feeding motivation in adult female pigs. Physiol. Behav. 2012, 107, 218–230. [Google Scholar] [CrossRef]
- Chen, H.; Mao, X.B.; Che, L.Q.; Yu, B.; He, J.; Yu, J.; Han, G.Q.; Huang, Z.Q.; Zheng, P.; Chen, D.W. Impact of fiber types on gut microbiota, gut environment and gut function in fattening pigs. Anim. Feed Sci. Technol. 2014, 195, 101–111. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.; Lee, Y.K.; Xie, J.; Zhang, H. Spatial Heterogeneity and Co-occurrence of Mucosal and Luminal Microbiome across Swine Intestinal Tract. Front. Microbiol. 2018, 9, 48. [Google Scholar] [CrossRef]
- Yang, H.; Huang, X.; Fang, S.; Xin, W.; Huang, L.; Chen, C. Uncovering the composition of microbial community structure and metagenomics among three gut locations in pigs with distinct fatness. Sci. Rep. 2016, 6, 27427. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, G.; Song, L.; Chai, M.; Wang, Y.; Shui, S.; Zhang, H.; Sha, Y.; Yao, Y. Effects of Dietary Protein Levels on Bamei Pig Intestinal Colony Compositional Traits. BioMed Res. Int. 2020, 2020, 2610431. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zeng, B.; Zhang, S.; Guo, W.; Li, F.; Zhao, J.; Li, Y. Hybridization altered the gut microbiota of pigs. Front. Microbiol. 2023, 14, 1177947. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dun, Y.; Li, S.; Zhang, D.; Peng, N.; Zhao, S.; Liang, Y. Dietary Enterococcus faecalis LAB31 improves growth performance, reduces diarrhea, and increases fecal Lactobacillus number of weaned piglets. PLoS ONE 2015, 10, e0116635. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Huang, K.; Zhang, M.; Wang, J.; Pan, X. Compound Lactobacillus sp. administration ameliorates stress and body growth through gut microbiota optimization on weaning piglets. Appl. Microbiol. Biotechnol. 2020, 104, 6749–6765. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.; Tiezzi, F.; Howard, J.; Huang, Y.J.; Gray, K.A.; Schillebeeckx, C.; McNulty, N.P.; Maltecca, C. Gut microbiome composition differences among breeds impact feed efficiency in swine. Microbiome 2020, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, Q.; Dai, J.; Zhang, S.; Wei, F. Evidence of cellulose metabolism by the giant panda gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 17714–17719. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Chassard, C.; Lawson, P.A.; Bernalier-Donadille, A. Bacteroides cellulosilyticus sp. nov., a cellulolytic bacterium from the human gut microbial community. Int. J. Syst. Evol. Microbiol. 2007, 57, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Chassard, C.; Delmas, E.; Lawson, P.A.; Bernalier-Donadille, A. Bacteroides xylanisolvens sp. nov., a xylan-degrading bacterium isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 1008–1013. [Google Scholar] [CrossRef]
- Chassard, C.; Delmas, E.; Robert, C.; Lawson, P.A.; Bernalier-Donadille, A. Ruminococcus champanellensis sp. nov., a cellulose-degrading bacterium from human gut microbiota. Int. J. Syst. Evol. Microbiol. 2012, 62, 138–143. [Google Scholar] [CrossRef]
- Domingo, M.C.; Huletsky, A.; Boissinot, M.; Bernard, K.A.; Picard, F.J.; Bergeron, M.G. Ruminococcus gauvreauii sp. nov., a glycopeptide-resistant species isolated from a human faecal specimen. Int. J. Syst. Evol. Microbiol. 2008, 58, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Kumar, S.; Lee, J.H.; Chang, D.H.; Kim, D.S.; Choi, S.H.; Rhee, M.S.; Lee, D.W.; Yoon, M.H.; Kim, B.C. Genome sequence of Oscillibacter ruminantium strain GH1, isolated from rumen of Korean native cattle. J. Bacteriol. 2012, 194, 6362. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Sun, G.; Duan, J.; Luo, C.; Yangji, C.; Zhong, R.; Chen, L.; Zhu, Y.; Wangdui, B.; Zhang, H. Alterations in gut microbiota improve SCFA production and fiber utilization in Tibetan pigs fed alfalfa diet. Front. Microbiol. 2022, 13, 969524. [Google Scholar] [CrossRef] [PubMed]
- Andam, C.P.; Hanage, W.P. Mechanisms of genome evolution of Streptococcus. Infect. Genet. Evol. 2015, 33, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Issa, E.; Salloum, T.; Tokajian, S. From Normal Flora to Brain Abscesses: A Review of Streptococcus intermedius. Front. Microbiol. 2020, 11, 826. [Google Scholar] [CrossRef]
- Mizrahi, A.; Delerue, T.; Morel, H.; Le Monnier, A.; Carbonnelle, E.; Pilmis, B.; Zahar, J.R. Infections caused by naturally AmpC-producing Enterobacteriaceae: Can we use third-generation cephalosporins? A narrative review. Int. J. Antimicrob. Agents 2020, 55, 105834. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, Y.; Wu, H.; Meng, Q.; Liu, S.; Chai, S.; Hao, L.; Zhou, Z. Dynamic changes in the yak rumen eukaryotic community and metabolome characteristics in response to feed type. Front. Vet. Sci. 2022, 9, 1027967. [Google Scholar] [CrossRef]
- Sun, Z.; Jiang, X.; Wang, B.; Tian, F.; Zhang, H.; Yu, L. Novel Phocaeicola Strain Ameliorates Dextran Sulfate Sodium-induced Colitis in Mice. Curr. Microbiol. 2022, 79, 393. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.T.; Spector, T.D. Red Wine Consumption Associated With Increased Gut Microbiota α-Diversity in 3 Independent Cohorts. Gastroenterology 2020, 158, 270–272.e272. [Google Scholar] [CrossRef]
- Xu, J.; Xu, R.; Jia, M.; Su, Y.; Zhu, W. Metatranscriptomic analysis of colonic microbiota’s functional response to different dietary fibers in growing pigs. Anim. Microbiome 2021, 3, 45. [Google Scholar] [CrossRef]
- Holman, D.B.; Gzyl, K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol. Ecol. 2019, 95, fiz072. [Google Scholar] [CrossRef]
- Bohra, V.; Tikariha, H.; Purohit, H.J.; Dafale, N.A. Unique pool of carbohydrate-degrading enzymes in novel bacteria assembled from cow and buffalo rumen metagenomes. Appl. Microbiol. Biotechnol. 2022, 106, 4643–4654. [Google Scholar] [CrossRef]
- Delannoy-Bruno, O.; Desai, C.; Castillo, J.J.; Couture, G.; Barve, R.A.; Lombard, V.; Henrissat, B.; Cheng, J.; Han, N.; Hayashi, D.K.; et al. An approach for evaluating the effects of dietary fiber polysaccharides on the human gut microbiome and plasma proteome. Proc. Natl. Acad. Sci. USA 2022, 119, e2123411119. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef]
- Broeders, E.P.; Nascimento, E.B.; Havekes, B.; Brans, B.; Roumans, K.H.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef]
- Yao, L.; Seaton, S.C.; Ndousse-Fetter, S.; Adhikari, A.A.; DiBenedetto, N.; Mina, A.I.; Banks, A.S.; Bry, L.; Devlin, A.S. A selective gut bacterial bile salt hydrolase alters host metabolism. eLife 2018, 7, e37182. [Google Scholar] [CrossRef]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Surano, I.; Silvestri, A.; Vitale, M.; Panteca, E.; Zohar, Y.; Rescigno, M.; Sannino, A. Biomimetic cellulose-based superabsorbent hydrogels for treating obesity. Sci. Rep. 2021, 11, 21394. [Google Scholar] [CrossRef]
- Chinopoulos, C. Succinate in ischemia: Where does it come from? Int. J. Biochem. Cell Biol. 2019, 115, 105580. [Google Scholar] [CrossRef]
- Rubic, T.; Lametschwandtner, G.; Jost, S.; Hinteregger, S.; Kund, J.; Carballido-Perrig, N.; Schwärzler, C.; Junt, T.; Voshol, H.; Meingassner, J.G.; et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nat. Immunol. 2008, 9, 1261–1269. [Google Scholar] [CrossRef]
- de Vadder, F.; Mithieux, G. Gut-brain signaling in energy homeostasis: The unexpected role of microbiota-derived succinate. J. Endocrinol. 2018, 236, R105–R108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).