Effects of Long-Term Application of Nitrogen Fertilizer on Soil Acidification and Biological Properties in China: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Categorization
2.3. Meta-Analysis
2.4. Data Analysis
3. Results
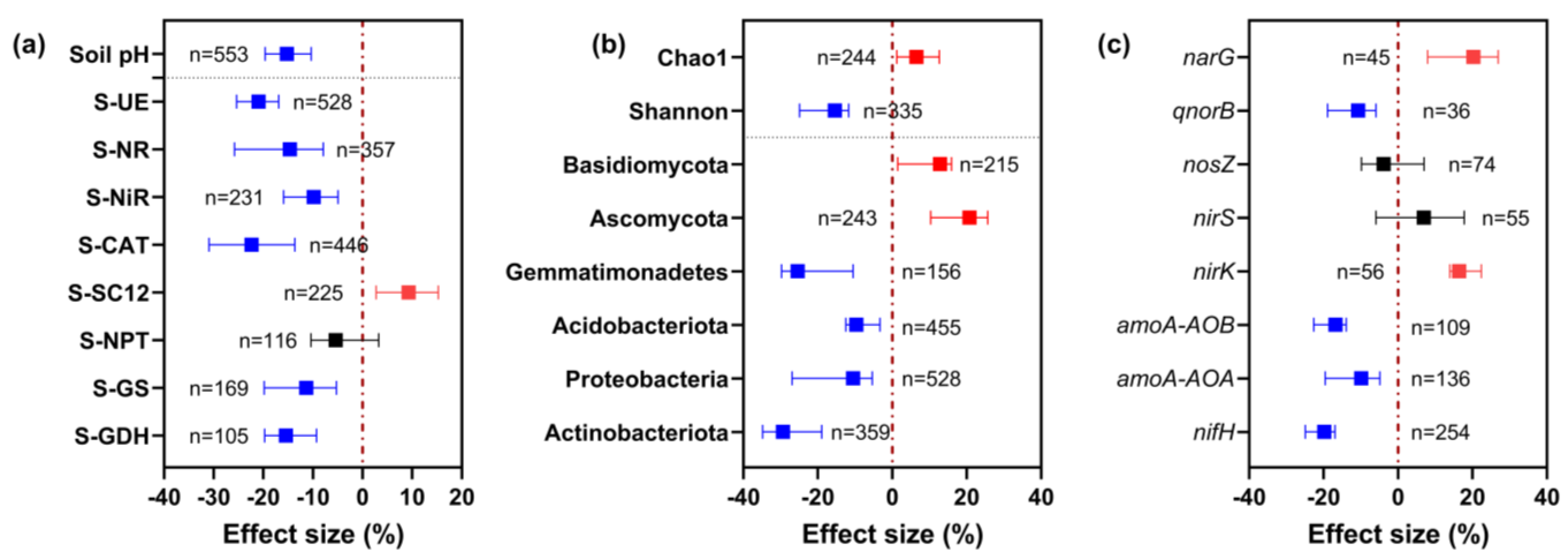
3.1. Overview of the Dataset
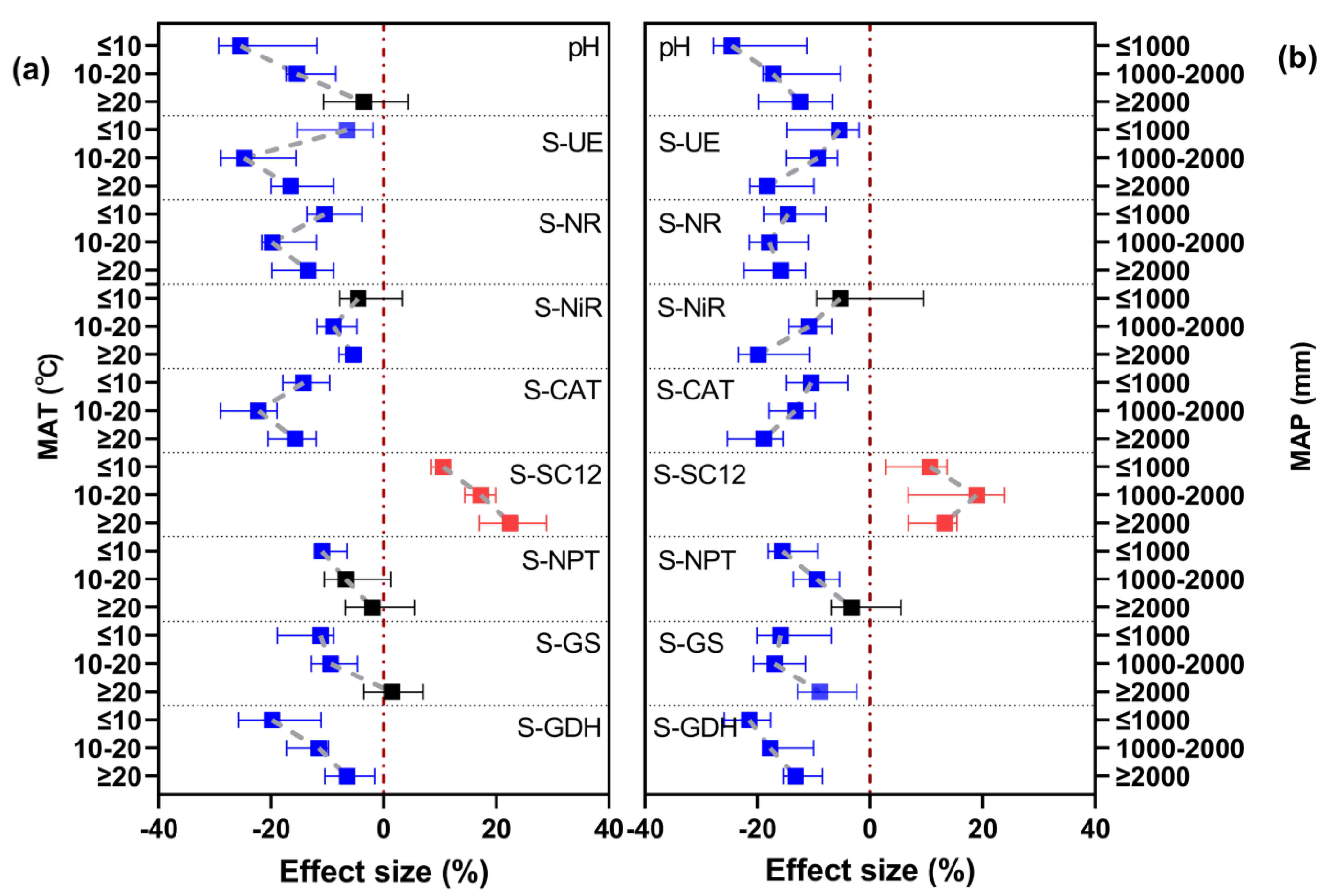
3.2. Effect of Climate Conditions on the Response of Soil PH and Enzyme Activities under Long-Term Nitrogen Fertilizer Application
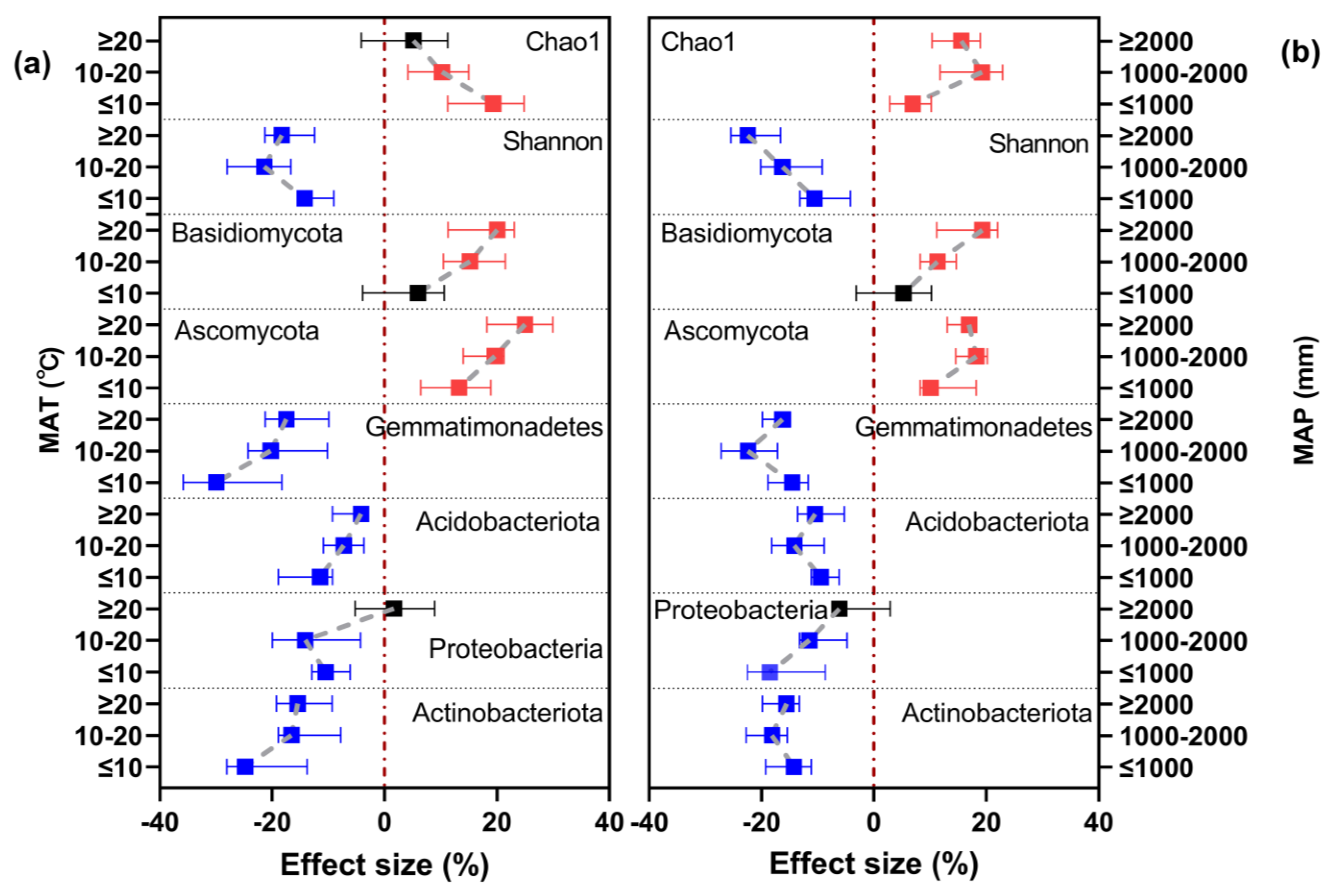
3.3. Effect of Climatic Conditions on the Response of Soil Microbial Community Alpha Diversity and Community Composition to Long-Term Nitrogen Fertilizer Application
3.4. Effect of Climatic Conditions on the Response of Functional Genes Related to Nitrogen Cycling under Long-Term Nitrogen Fertilizer Application
3.5. Importance of MAT and MAP for Soil PH and Biological Properties
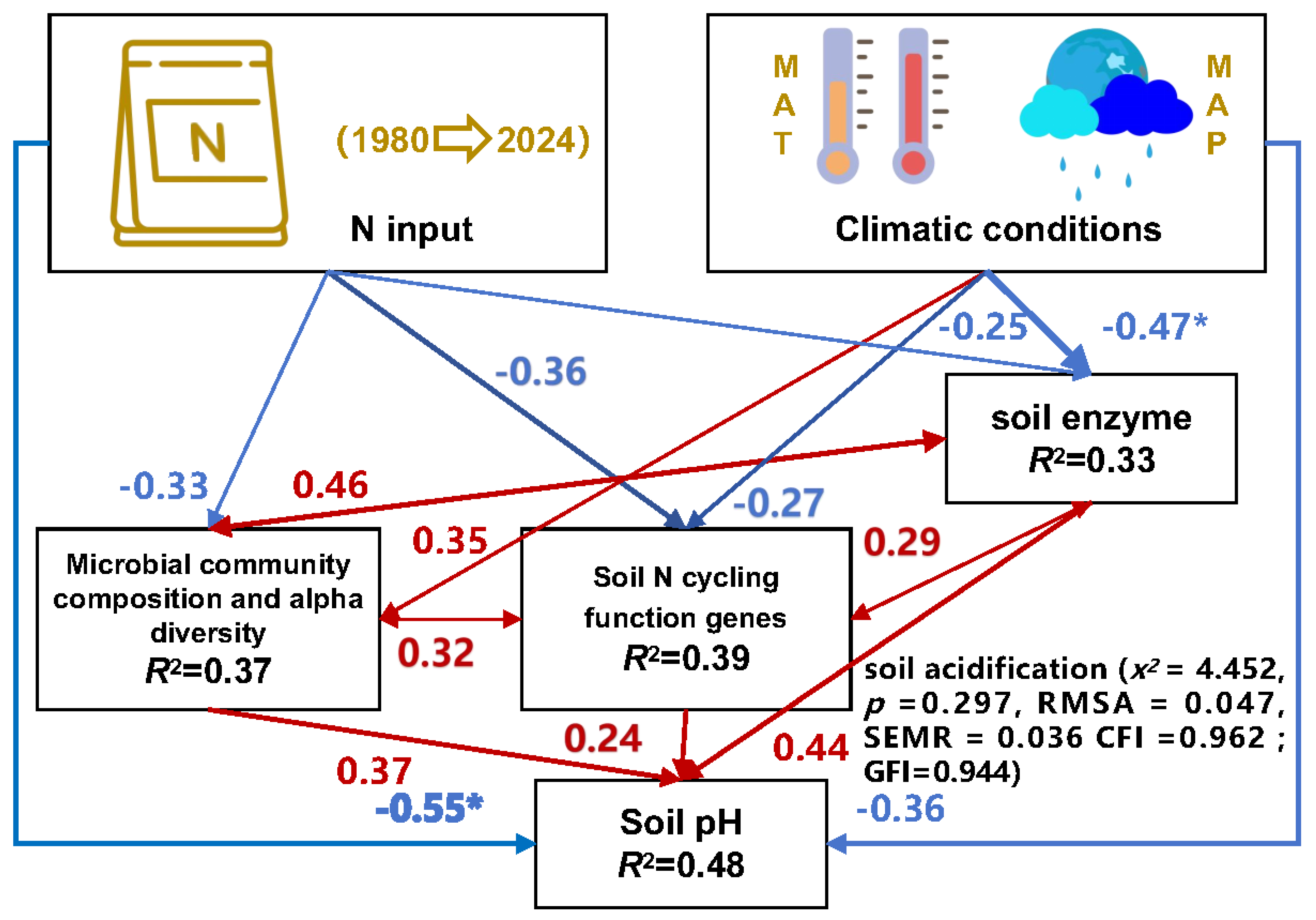
3.6. Structural Equation Modeling Analysis of the Pathways through which Long-Term Nitrogen Fertilizer Application and Climate Conditions Affect Soil Acidification
4. Discussion
4.1. Effects of Long-Term Nitrogen Application on Soil PH in China
4.2. Effects of Long-Term Nitrogen Application on Soil Enzyme Activities in China
4.3. Effects of Long-Term Nitrogen Application on the Structure and Function of Soil Microbial Communities in China
4.4. Climatic Conditions as a Major Influence on Soil Acidification and Changes in Biological Properties in China under Long-Term N Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Chen, Y.L.; Liu, K.; Dang, Y.C.; Li, G.L.; Wen, L.Y.; Cao, Y. Optimization and classification control of permanent basic farmland based on quality classification. Front. Environ. Sci. 2024, 12, 14. [Google Scholar] [CrossRef]
- Wang, F.W.; Gao, Y.; Li, X.; Luan, M.D.; Wang, X.Y.; Zhao, Y.W.; Zhou, X.H.; Du, G.Z.; Wang, P.; Ye, C.L.; et al. Changes in microbial composition explain the contrasting responses of glucose and lignin decomposition to soil acidification in an alpine grassland. Sci. Total Environ. 2024, 930, 8. [Google Scholar] [CrossRef]
- Lin, S.X.; Liu, Z.J.; Wang, Y.C.; Li, J.Y.; Wang, G.G.; Ye, J.H.; Wang, H.B.; He, H.B. Soil metagenomic analysis on changes of functional genes and microorganisms involved in nitrogen-cycle processes of acidified tea soils. Front. Plant Sci. 2022, 13, 13. [Google Scholar] [CrossRef]
- Liang, F.; Li, B.Z.; Vogt, R.D.; Mulder, J.; Song, H.; Chen, J.S.; Guo, J.H. Straw return exacerbates soil acidification in major Chinese croplands. Resour. Conserv. Recycl. 2023, 198, 8. [Google Scholar] [CrossRef]
- Zhu, Q.C.; de Vries, W.; Liu, X.J.; Hao, T.X.; Zeng, M.F.; Shen, J.B.; Zhang, F.S. Enhanced acidification in Chinese croplands as derived from element budgets in the period 1980–2010. Sci. Total Environ. 2018, 618, 1497–1505. [Google Scholar] [CrossRef]
- Jin, L.; Hua, K.K.; Zhan, L.C.; He, C.L.; Wang, D.Z.; Nagano, H.; Cheng, W.G.; Inubushi, K.; Guo, Z.B. Effect of Soil Acidification on Temperature Sensitivity of Soil Respiration. Agronomy 2024, 14, 16. [Google Scholar] [CrossRef]
- Fujii, K.; Hayakawa, C. Fluxes of dissolved organic matter and nitrate and their contribution to soil acidification across changing permafrost landscapes in northwestern Canada. Geoderma 2023, 430, 13. [Google Scholar] [CrossRef]
- Cui, T.T.; Zhang, J.B.; Luo, W.Q. The Quantity and Quality of Humic Substances following Different Land Uses in Karst Peak-Cluster Depression in Guangxi, China. Agriculture 2023, 13, 14. [Google Scholar] [CrossRef]
- Ma, C.; Tu, Q.; Zheng, S.M.; Deng, S.H.; Xia, Y.H.; Mao, W.Q.; Gao, W.; Hu, L.N.; Kuzyakov, Y.; Hu, Y.J.; et al. Soil acidification induced by intensive agricultural use depending on climate. J. Soils Sediments 2022, 22, 2604–2607. [Google Scholar] [CrossRef]
- Jimma, T.B.; Chemura, A.; Spillane, C.; Demissie, T.; Abera, W.; Ture, K.; Terefe, T.; Solomon, D.; Gleixner, S. Coupled Impacts of Soil Acidification and Climate Change on Future Crop Suitability in Ethiopia. Sustainability 2024, 16, 17. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef]
- Alkorta, I.; Epelde, L.; Garbisu, C. Environmental parameters altered by climate change affect the activity of soil microorganisms involved in bioremediation. FEMS Microbiol. Lett. 2017, 364, 9. [Google Scholar] [CrossRef]
- Shi, R.Y.; Ni, N.; Wang, R.H.; Nkoh, J.N.; Pan, X.Y.; Dong, G.; Xu, R.K.; Cui, X.M.; Li, J.Y. Dissolved biochar fractions and solid biochar particles inhibit soil acidification induced by nitrification through different mechanisms. Sci. Total Environ. 2023, 874, 8. [Google Scholar] [CrossRef] [PubMed]
- Filimon, M.N.; Roman, D.L.; Bordean, D.M.; Isvoran, A. Impact of the Herbicide Oxyfluorfen on the Activities of Some Enzymes Found in Soil and on the Populations of Soil Microorganisms. Agronomy 2021, 11, 19. [Google Scholar] [CrossRef]
- Wade, J.; Li, C.Y.; Vollbracht, K.; Hooper, D.G.; Wills, S.A.; Margenot, A.J. Prescribed pH for soil β-glucosidase and phosphomonoesterase do not reflect pH optima. Geoderma 2021, 401, 11. [Google Scholar] [CrossRef]
- Gu, S.S.; Wu, S.L.; Zeng, W.A.I.; Deng, Y.; Luo, G.W.; Li, P.F.; Yang, Y.S.; Wang, Z.Q.; Hu, Q.L.; Tan, L. High-elevation-induced decrease in soil pH weakens ecosystem multifunctionality by influencing soil microbiomes. Environ. Res. 2024, 257, 14. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.Q.; Zhang, Y.L.; Jiang, N.; Chen, Z.H.; Chen, L.J. Neutral soil pH conditions favor the inhibition of phenol on hydrolase activities and soil organic carbon mineralization. Eur. J. Soil Biol. 2024, 121, 10. [Google Scholar] [CrossRef]
- Waheed, A.; Li, C.; Muhammad, M.; Ahmad, M.; Khan, K.A.; Ghramh, H.A.; Wang, Z.W.; Zhang, D.Y. Sustainable Potato Growth under Straw Mulching Practices. Sustainability 2023, 15, 16. [Google Scholar] [CrossRef]
- He, Z.J.; Cao, H.X.; Qi, C.; Hu, Q.Y.; Liang, J.P.; Li, Z.J. Straw management in paddy fields can reduce greenhouse gas emissions: A global meta-analysis. Field Crop. Res. 2024, 306, 15. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Wang, Y.L.; Lou, Z.X.; Hsu, L.F.; Chen, D.; Piao, R.Z.; Zhao, H.Y.; Cui, Z.J. Meta-Analysis of Factors Affecting C-N Fractions and Yield of Paddy Soils by Total Straw Return and N Fertilizer Application. Agronomy 2022, 12, 21. [Google Scholar] [CrossRef]
- Dang, C.R.; Kong, F.L.; Li, Y.; Jiang, Z.X.; Xi, M. Soil inorganic carbon dynamic change mediated by anthropogenic activities: An integrated study using meta-analysis and random forest model. Sci. Total Environ. 2022, 835, 11. [Google Scholar] [CrossRef] [PubMed]
- Franco, H.H.S.; Guimaraes, R.M.L.; Tormena, C.A.; Cherubin, M.R.; Favilla, H.S. Global applications of the Visual Evaluation of Soil Structure method: A systematic review and meta-analysis. Soil Tillage Res. 2019, 190, 61–69. [Google Scholar] [CrossRef]
- Fan, X.P.; Yin, C.; Yan, G.C.; Cui, P.Y.; Shen, Q.; Wang, Q.; Chen, H.; Zhang, N.; Ye, M.J.; Zhao, Y.H.; et al. The contrasting effects of N-(n-butyl) thiophosphoric triamide (NBPT) on N2O emissions in arable soils differing in pH are underlain by complex microbial mechanisms. Sci. Total Environ. 2018, 642, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.; Wang, Z.F.; Huang, H.; Zhang, C.; Shang, X.; Dahlgren, R.A.; Zhang, M.H.; Xia, F. Stimulation of N2O emission by conservation tillage management in agricultural lands: A meta-analysis. Soil Tillage Res. 2018, 182, 86–93. [Google Scholar] [CrossRef]
- Philibert, A.; Loyce, C.; Makowski, D. Assessment of the quality of meta-analysis in agronomy. Agric. Ecosyst. Environ. 2012, 148, 72–82. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Yan, X.J.; Chen, X.H.; Ma, C.C.; Cai, Y.Y.; Cui, Z.L.; Chen, X.P.; Wu, L.Q.; Zhang, F.S. What are the key factors affecting maize yield response to and agronomic efficiency of phosphorus fertilizer in China? Field Crop. Res. 2021, 270, 12. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Dong, A.H.; Liu, F.L.; Niu, W.Q.; Siddique, K.H.M. Effect of film mulching on crop yield and water use efficiency in drip irrigation systems: A meta-analysis. Soil Tillage Res. 2022, 221, 11. [Google Scholar] [CrossRef]
- Feijoó, C.; Hegoburu, C.; Messetta, M.L.; Guerra-López, J.; Rigacci, L.; Anselmo, J.; Di Franco, L.; Marcé, R. Acidification and increase of phosphorus levels in Pampean streams after 12 years of agricultural intensification. Aquat. Sci. 2023, 85, 14. [Google Scholar] [CrossRef]
- Lu, X.Q.; Zhang, X.Y.; Zhan, N.; Wang, Z.; Li, S.F. Factors contributing to soil acidification in the past two decades in China. Environ. Earth Sci. 2023, 82, 12. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Fu, W.G.; Xu, C.; Zhang, H.; Xu, X.J.; Ma, H.B.; Wang, J.D.; Zhang, Y.C. Soil Acidification Can Be Improved under Different Long-Term Fertilization Regimes in a Sweetpotato-Wheat Rotation System. Plants 2024, 13, 13. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ye, C.; Su, Y.W.; Peng, W.C.; Lu, R.; Liu, Y.X.; Huang, H.C.; He, X.H.; Yang, M.; Zhu, S.S. Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 10. [Google Scholar] [CrossRef]
- Zhu, X.; Ros, G.H.; Xu, M.; Xu, D.; Cai, Z.; Sun, N.; Duan, Y.; de Vries, W. The contribution of natural and anthropogenic causes to soil acidification rates under different fertilization practices and site conditions in southern China. Sci. Total Environ. 2024, 934, 172986. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.Y.; Li, J.Y.; Ni, N.; Xu, R.K. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Gálvez, S.; Lancien, M.; Hodges, M. Are isocitrate dehydrogenases and 2-oxoglutarate involved in the regulation of glutamate synthesis? Trends Plant Sci. 1999, 4, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.C.; Zhou, Y.; Chen, P.; Zhang, X.N.; Du, Q.; Yang, H.; Wang, X.C.; Yang, F.; Xiao, T.; Li, L.; et al. Maize-legume intercropping promote N uptake through changing the root spatial distribution, legume nodulation capacity, and soil N availability. J. Integr. Agric. 2022, 21, 1755–1771. [Google Scholar]
- Lu, M.; Zhao, J.X.; Lu, Z.R.; Li, M.J.; Yang, J.F.; Fullen, M.; Li, Y.M.; Fan, M.P. Maize-soybean intercropping increases soil nutrient availability and aggregate stability. Plant Soil 2023. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Zheng, M.H.; Jiang, L.F.; Luo, Y.Q. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Xu, Z.H.; Teng, Y.; Christie, P.; Wang, J.; Ren, W.J.; Luo, Y.M.; Li, Z.G. Non-target effects of repeated chlorothalonil application on soil nitrogen cycling: The key functional gene study. Sci. Total Environ. 2016, 543, 636–643. [Google Scholar] [CrossRef]
- Wang, W.B.; Chen, D.S.; Sun, X.M.; Zhang, Q.; Koide, R.T.; Insam, H.; Zhang, S.G. Impacts of mixed litter on the structure and functional pathway of microbial community in litter decomposition. Appl. Soil Ecol. 2019, 144, 72–82. [Google Scholar] [CrossRef]
- Cui, H.Y.; Vitousek, P.M.; Reed, S.C.; Sokoya, B.; Bamigboye, A.R.; Mukherjee, A.; Peñaloza-Bojacá, G.F.P.; Teixido, A.L.; Trivedi, P.; He, J.Z.; et al. Environmental filtering controls soil biodiversity in wet tropical ecosystems. Soil Biol. Biochem. 2022, 166, 9. [Google Scholar] [CrossRef]
- Gandois, L.; Perrin, A.S.; Probst, A. Impact of nitrogenous fertiliser-induced proton release on cultivated soils with contrasting carbonate contents: A column experiment. Geochim. Cosmochim. Acta 2011, 75, 1185–1198. [Google Scholar] [CrossRef]
- Morrow, J.L.; Sa, P.T.; Beattie, G.A.C.; Milham, P.J.; Riegler, M.; Spooner-Hart, R.N.; Holford, P. Additions of sugar and nitrogenous fertiliser affect plant nitrogen status and soil microbial communities. Appl. Soil Ecol. 2019, 139, 47–55. [Google Scholar] [CrossRef]
- Hu, X.J.; Gu, H.D.; Liu, J.J.; Wei, D.; Zhu, P.; Cui, X.A.; Zhou, B.K.; Chen, X.L.; Jin, J.; Liu, X.B.; et al. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 10. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Liu, T.Q.; Ding, H.N.; Li, C.F.; Tan, W.F.; Yu, M.; Liu, J.; Cao, C.G. Effects of nitrogen fertilizer on soil microbial residues and their contribution to soil organic carbon and total nitrogen in a rice-wheat system. Appl. Soil Ecol. 2023, 181, 9. [Google Scholar] [CrossRef]
- Wang, T.T.; Cao, X.X.; Chen, M.M.; Lou, Y.H.; Wang, H.; Yang, Q.G.; Pan, H.; Zhuge, Y.P. Effects of Soil Acidification on Bacterial and Fungal Communities in the Jiaodong Peninsula, Northern China. Agronomy 2022, 12, 11. [Google Scholar] [CrossRef]
- Tang, Z.X.; Sun, X.L.; Luo, Z.K.; He, N.P.; Sun, O.J. Effects of temperature, soil substrate, and microbial community on carbon mineralization across three climatically contrasting forest sites. Ecol. Evol. 2018, 8, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.B.; Liu, C.A.; Hua, K.K.; Wang, D.Z.; Wu, P.P.; Wan, S.X.; He, C.L.; Zhan, L.C.; Wu, J. Changing soil available substrate primarily caused by fertilization management contributed more to soil respiration temperature sensitivity than microbial community thermal adaptation. Sci. Total Environ. 2024, 912, 13. [Google Scholar] [CrossRef]
- Ballhausen, M.B.; Hewitt, R.; Rillig, M.C. Mimicking climate warming effects on Alaskan soil microbial communities via gradual temperature increase. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef]
- Barreiro, A.; Lombao, A.; Martín, A.; Cancelo-Gonzalez, J.; Carballas, T.; Díaz-Raviña, M. Soil Heating at High Temperatures and Different Water Content: Effects on the Soil Microorganisms. Geosciences 2020, 10, 17. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Su, Y.G. Effects of increasing precipitation on soil microbial community composition and soil respiration in a temperate desert, Northwestern China. Soil Biol. Biochem. 2015, 83, 52–56. [Google Scholar] [CrossRef]
- Yu, H.Y.; Li, L.; Ma, Q.H.; Liu, X.D.; Li, Y.B.; Wang, Y.H.; Zhou, G.S.; Xu, Z.Z. Soil microbial responses to large changes in precipitation with nitrogen deposition in an arid ecosystem. Ecology 2023, 104, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Du, D.L.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Liu, Y.C.; Tian, H.M.; Li, J.R.; Wang, H.; Liu, S.R.; Liu, X.J. Reduced precipitation neutralizes the positive impact of soil warming on soil microbial community in a temperate oak forest. Sci. Total Environ. 2022, 806, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Response of soil microbial communities to altered precipitation: A global synthesis. Glob. Ecol. Biogeogr. 2018, 27, 1121–1136. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, Z.; Jiang, B.; Baoyin, B.; Cui, Z.; Wang, H.; Li, Q.; Cui, J. Effects of Long-Term Application of Nitrogen Fertilizer on Soil Acidification and Biological Properties in China: A Meta-Analysis. Microorganisms 2024, 12, 1683. https://doi.org/10.3390/microorganisms12081683
Zhang L, Zhao Z, Jiang B, Baoyin B, Cui Z, Wang H, Li Q, Cui J. Effects of Long-Term Application of Nitrogen Fertilizer on Soil Acidification and Biological Properties in China: A Meta-Analysis. Microorganisms. 2024; 12(8):1683. https://doi.org/10.3390/microorganisms12081683
Chicago/Turabian StyleZhang, Liqiang, Zehang Zhao, Bailing Jiang, Bate Baoyin, Zhengguo Cui, Hongyu Wang, Qiuzhu Li, and Jinhu Cui. 2024. "Effects of Long-Term Application of Nitrogen Fertilizer on Soil Acidification and Biological Properties in China: A Meta-Analysis" Microorganisms 12, no. 8: 1683. https://doi.org/10.3390/microorganisms12081683




