Antarctic Soils Select Copiotroph-Dominated Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling
2.3. Soil Physicochemical Properties Analyses
2.4. DNA Extraction, PCR Amplification and Data Processing
2.5. Statistical Analysis
3. Results
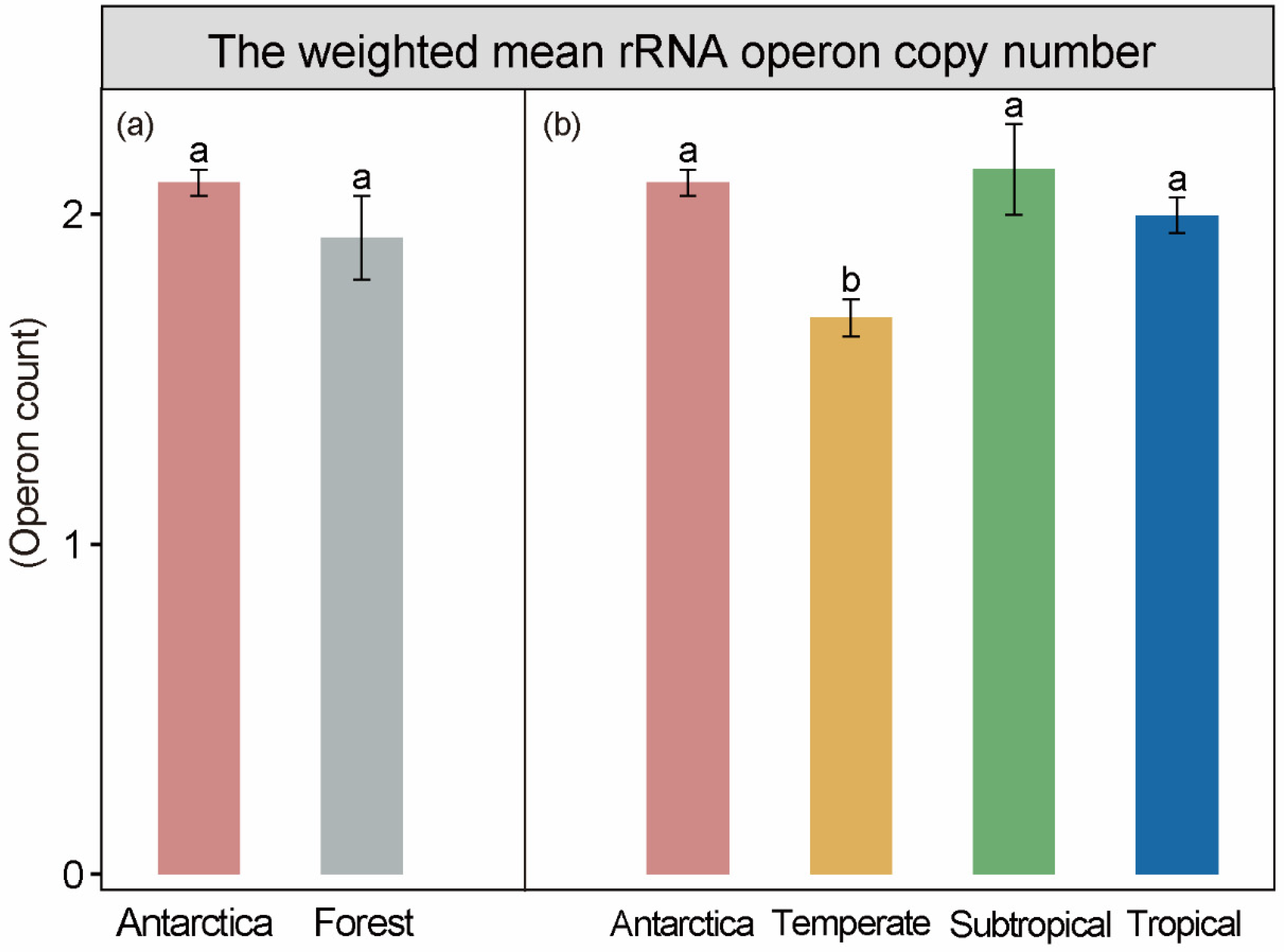
3.1. Life Strategies in Antarctic and Forest Soils
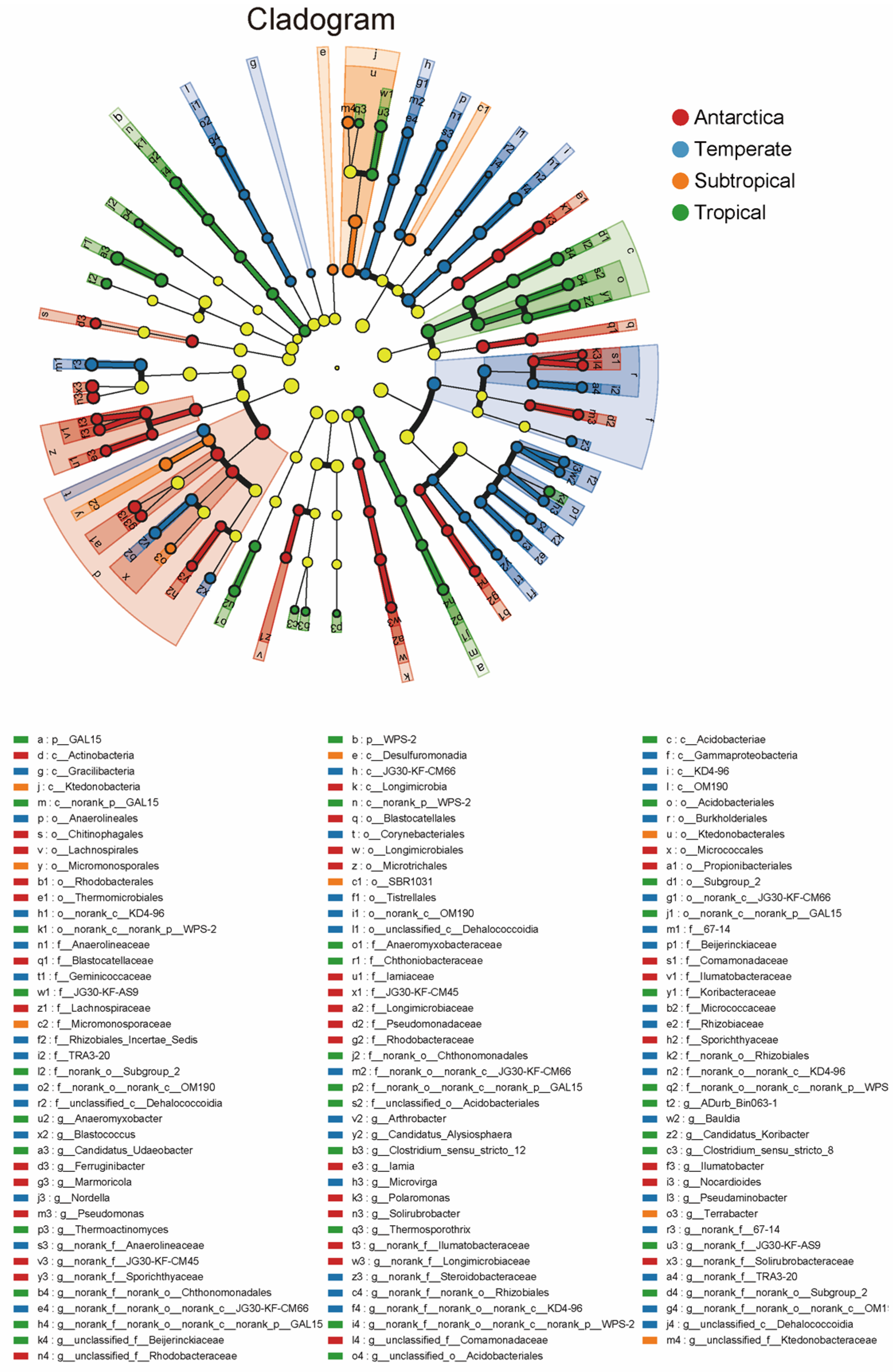
3.2. Bacterial Community Diversity and Composition in Antarctic and Forest Soils
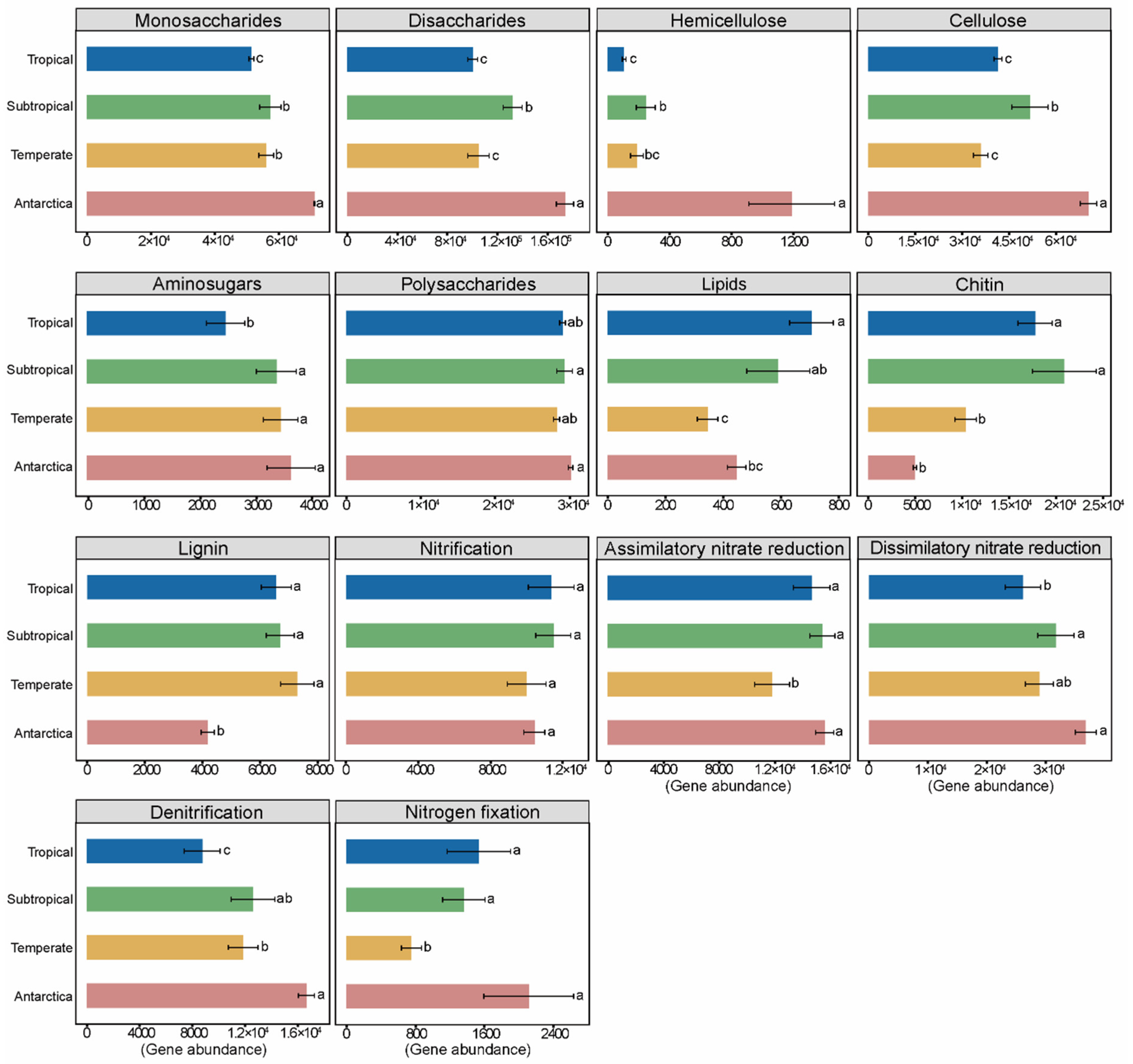
3.3. Bacterial Functional Potential of C and N Cycling in Antarctic and Forest Soils
4. Discussion
4.1. The Selection of Bacterial Life Strategies under Different Soil Conditions
4.2. Distinct Soil Conditions Shape Different Bacterial Structure and Functions: Based on Copiotrophs and Oligotrophs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, P.; Zhang, W.; Kuzyakov, Y.; Xiao, L.; Xiao, D.; Xu, L.; Chen, H.; Zhao, J.; Wang, K. Linking bacterial life strategies with soil organic matter accrual by karst vegetation restoration. Soil Biol. Biochem. 2023, 177, 108925. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, W.; Lu, X.; Gu, Y.; Wu, S.; Shen, Z.; Han, X.; Yang, G.; Ren, C. Adaptive pathways of soil microorganisms to stoichiometric imbalances regulate microbial respiration following afforestation in the Loess Plateau, China. Soil Biol. Biochem. 2020, 151, 108048. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, H.; Hu, Y.; Sun, Y.; Liu, Q.; Li, J.; Dong, Y. Shift in soil bacterial communities from K- to r-strategists facilitates adaptation to grassland degradation. Land Degrad. Dev. 2022, 33, 2076–2091. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, H.; Zheng, C.; Wu, X.; Zhao, Y.; Li, X.; Liu, H.; Dong, L.; Lu, Z.; Zhou, J.; et al. Bacteria life-history strategies and the linkage of soil C-N-P stoichiometry to microbial resource limitation differed in karst and non-karst plantation forests in southwest China. Catena 2023, 231, 107341. [Google Scholar] [CrossRef]
- Kearns, P.J.; Shade, A. Trait-based patterns of microbial dynamics in dormancy potential and heterotrophic strategy: Case studies of resource-based and post-press succession. ISME J. 2018, 12, 2575–2581. [Google Scholar] [CrossRef]
- Chen, Y.; Neilson, J.W.; Kushwaha, P.; Maier, R.M.; Barberan, A. Life-history strategies of soil microbial communities in an arid ecosystem. ISME J. 2021, 15, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Roller, B.R.K.; Stoddard, S.F.; Schmidt, T.M. Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nat. Microbiol. 2016, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Klappenbach, J.A.; Dunbar, J.M.; Schmidt, T.M. rRNA operon copy number reflects ecological strategies of bacteria. Appl. Microbiol. 2000, 66, 1328–1333. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.H.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Li, T.; Wang, C.; Dang, N.; Wang, R.; Jiang, Y.; Wang, H.; Li, H. N and P fertilization enhanced carbon decomposition function by shifting microbes towards an r-selected community in meadow grassland soils. Ecol. Indic. 2021, 132, 108306. [Google Scholar] [CrossRef]
- He, Q.; Wu, Y.; Wang, J.; Bing, H. Community rRNA operon copy number of soil bacteria decreases with soil depth and ecosystem succession in postglacial ecosystems. Appl. Soil Ecol. 2023, 186, 104817. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Global Change Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, P.; Wegner, C.-E.; Luo, Y.; Xiao, K.-Q.; Cui, Z.; Zhang, F.; Liesack, W.; Peng, J. Deciphering microbial mechanisms underlying soil organic carbon storage in a wheat-maize rotation system. Sci. Total Environ. 2021, 788, 147798. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Nemergut, D.R.; Knelman, J.E.; Ferrenberg, S.; Bilinski, T.; Melbourne, B.; Jiang, L.; Violle, C.; Darcy, J.L.; Prest, T.; Schmidt, S.K.; et al. Decreases in average bacterial community rRNA operon copy number during succession. ISME J. 2016, 10, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, W.; Grossart, H.-P.; Gadd, G.M.; Liu, W.; Yang, Y. The unique climate shapes distinct life-history traits of abundant bacteria in Tibetan Plateau grassland soil. Sci. Total Environ. 2024, 908, 168353. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S.X. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2022, 2, e66. [Google Scholar] [CrossRef]
- Aislabie, J.M.; Chhour, K.-L.; Saul, D.J.; Miyauchi, S.; Ayton, J.; Paetzold, R.F.; Balks, M.R. Dominant bacteria in soils of Marble Point and Wright Valley, Victoria Land, Antarctica. Soil Boil. Biochem. 2006, 38, 3041–3056. [Google Scholar] [CrossRef]
- Martinez Alvarez, L.; Bolhuis, H.; Mau, G.K.; Kok-Gan, C.; Sing, C.C.; Mac Cormack, W.; Ruberto, L. Identification of key bacterial players during successful full-scale soil field bioremediation in Antarctica. Int. Biodeter. Biodegr. 2022, 168, 105354. [Google Scholar] [CrossRef]
- Jin, J.; Chen, X.; Xu, L.; Nie, Y.; Wang, X.; Huang, H.; Emslie, S.D.; Liu, X. Chronology and paleoclimatic implications of lacustrine sediments at Inexpressible Island, Ross Sea, Antarctica. Palaeogeogr. Palaeocl. 2021, 576, 110497. [Google Scholar] [CrossRef]
- Ding, M.; Bian, L.; Zhang, L.; Wang, Z.; Lu, C.; Sun, W.; Yuan, N.; Fu, L.; Xie, Z. Meteorological characteristics of Inexperssible island, Antarctica. Chin. J. Polar Res. 2015, 27, 344–350. [Google Scholar]
- Zhang, C.; Xue, S.; Liu, G.-B.; Song, Z.-L. A comparison of soil qualities of different revegetation types in the Loess Plateau, China. Plant and Soil 2011, 347, 163–178. [Google Scholar] [CrossRef]
- Wang, J.Y.; Ren, C.J.; Feng, X.X.; Zhang, L.; Doughty, R.; Zhao, F.Z. Temperature sensitivity of soil carbon decomposition due to shifts in soil extracellular enzymes after afforestation. Geoderma 2020, 374, 114426. [Google Scholar] [CrossRef]
- Stoddard, S.F.; Smith, B.J.; Hein, R.; Roller, B.R.K.; Schmidt, T.M. rrnDB: Improved tools for interpreting rRNA gene abundance in bacteria and archaea and a new foundation for future development. Nucleic Acids Res. 2015, 43, D593–D598. [Google Scholar] [CrossRef]
- Ren, C.; Wang, J.; Bastida, F.; Delgado-Baquerizo, M.; Yang, Y.; Wang, J.; Zhong, Z.; Zhou, Z.; Zhang, S.; Guo, Y.; et al. Microbial traits determine soil C emission in response to fresh carbon inputs in forests across biomes. Global Change Bio. 2022, 28, 1516–1528. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Li, Y.; Xu, X.; He, L.; Wang, J.; Ren, C.; Guo, Y. Microbial functional genes driving the positive priming effect in forest soils along an elevation gradient. Soil Biol. Biochem. 2022, 165, 108498. [Google Scholar] [CrossRef]
- Lian, W.H.; Mohamad, O.A.A.; Dong, L.; Zhang, L.Y.; Wang, D.; Liu, L.; Han, M.X.; Li, S.; Wang, S.; Antunes, A.; et al. Culturomics- and metagenomics-based insights into the microbial community and function of rhizosphere soils in Sinai desert farming systems. Environ. Microbiome 2023, 18, 4. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.; Chen, S.; Jason Shi, Z.; Zhao, M.; Zhu, Z.; Yang, S.; Qu, Y.; Ma, Q.; He, Z.; et al. Microbial functional trait of rRNA operon copy numbers increases with organic levels in anaerobic digesters. ISME J. 2017, 11, 2874–2878. [Google Scholar] [CrossRef]
- Kleinteich, J.; Hildebrand, F.; Bahram, M.; Voigt, A.Y.; Wood, S.A.; Jungblut, A.D.; Küpper, F.C.; Quesada, A.; Camacho, A.; Pearce, D.A.; et al. Pole-to-Pole Connections: Similarities between Arctic and Antarctic Microbiomes and Their Vulnerability to Environmental Change. Front. Ecol. Evol. 2017, 5, 00137. [Google Scholar] [CrossRef]
- Cox, F.; Newsham, K.K.; Bol, R.; Dungait, J.A.J.; Robinson, C.H.; Casper, B. Not poles apart: Antarctic soil fungal communities show similarities to those of the distant Arctic. Ecol. Lett. 2016, 19, 528–536. [Google Scholar] [CrossRef]
- Leo, C.; Nardi, F.; Cucini, C.; Frati, F.; Convey, P.; Weedon, J.T.; Roelofs, D.; Carapelli, A. Evidence for strong environmental control on bacterial microbiomes of Antarctic springtails. Sci. Rep. 2021, 11, 2973. [Google Scholar] [CrossRef]
- Goldfarb, K.C.; Karaoz, U.; Hanson, C.A.; Santee, C.A.; Bradford, M.A.; Treseder, K.K.; Wallenstein, M.D.; Brodie, E.L. Differential Growth Responses of Soil Bacterial Taxa to Carbon Substrates of Varying Chemical Recalcitrance. Front. Microbiol. 2011, 2, 94. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; Wang, Z.; Semenov, M.V.; Ye, J.; Yin, L.; Wang, X.; Kravchenko, I.; Semenov, V.; Kuzyakov, Y.; et al. Linkages between the temperature sensitivity of soil respiration and microbial life strategy are dependent on sampling season. Soil Biol. Biochem. 2022, 172, 108758. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, Z.; Xu, X.; Liu, S.; Jones, D.L.; Kuzyakov, Y.; Shibistova, O.; Wu, J.; Ge, T. Carbon and nitrogen recycling from microbial necromass to cope with C:N stoichiometric imbalance by priming. Soil Biol. Biochem. 2020, 142, 107720. [Google Scholar] [CrossRef]
- Hopkins, D.W.; Sparrow, A.D.; Elberling, B.; Gregorich, E.G.; Novis, P.M.; Greenfield, L.G.; Tilston, E.L. Carbon, nitrogen and temperature controls on microbial activity in soils from an Antarctic dry valley. Soil Biol. Biochem. 2006, 38, 3130–3140. [Google Scholar] [CrossRef]
- Barrett, J.E.; Virginia, R.A.; Parsons, A.N.; Wall, D.H. Potential Soil Organic Matter Turnover in Taylor Valley, Antarctica. Arct. Antarct. Alp. Res. 2005, 37, 108–117. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Global Change Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef]
- Chen, H.; Jing, Q.; Liu, X.; Zhou, X.; Fang, C.; Li, B.; Zhou, S.; Nie, M. Microbial respiratory thermal adaptation is regulated by r-/K-strategy dominance. Ecol. Lett. 2022, 25, 2489–2499. [Google Scholar] [CrossRef]
- Cary, S.C.; McDonald, I.R.; Barrett, J.E.; Cowan, D.A. On the rocks: The microbiology of Antarctic Dry Valley soils. Nat. Rev. Microbiol. 2010, 8, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Global Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, C.; Yao, H.; Yang, E.; An, S. The accumulation of microbial necromass carbon from litter to mineral soil and its contribution to soil organic carbon sequestration. Catena 2021, 207, 105622. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Y.; Li, N.; Yao, H.; Yang, E.; Soromotin, A.V.; Kuzyakov, Y.; Cheptsov, V.; Yang, Y.; An, S. Initial soil formation by biocrusts: Nitrogen demand and clay protection control microbial necromass accrual and recycling. Soil Biol. Biochem. 2022, 167, 108607. [Google Scholar] [CrossRef]
- van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, R.; Wang, X.; Xu, X.; Ai, C.; He, P.; Liang, G.; Zhou, W.; Zhu, P. Effect of high soil C/N ratio and nitrogen limitation caused by the long-term combined organic-inorganic fertilization on the soil microbial community structure and its dominated SOC decomposition. J. Environ. Manag. 2022, 303, 114155. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, M.; Lu, P. Effects of pyrene on the structure and metabolic function of soil microbial communities. Environ. Pollut. 2022, 305, 119301. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nature Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]

| Antarctica | Temperate | Subtropical | Tropical | |
|---|---|---|---|---|
| pH | 7.98 ± 0.31 a | 6.47 ± 0.49 b | 6.11 ± 0.21 b | 5.69 ± 0.28 b |
| SOC (g/kg) | 2.93 ± 0.12 c | 56.46 ± 11.11 a | 39.20 ± 4.70 b | 27.06 ± 3.01 bc |
| MBC (mg/kg) | 97.84 ± 0.56 b | 522.04 ± 101.12 a | 273.28 ± 33.61 b | 254.69 ± 25.14 b |
| NH4+-N (mg/kg) | 3.89 ± 1.53 b | 25.80 ± 3.11 a | 29.19 ± 3.28 a | 24.25 ± 0.34 a |
| NO3−-N (mg/kg) | 1.70 ± 0.71 c | 12.50 ± 3.17 ab | 16.45 ± 2.58 a | 10.36 ± 1.70 b |
| C/N | 13.67 ± 0.17 b | 28.45 ± 3.35 a | 15.04 ± 1.40 b | 13.25 ± 0.82 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, X.; Wang, J.; He, L.; Ren, C.; Wang, J.; Guo, Y.; Wang, N.; Zhao, F. Antarctic Soils Select Copiotroph-Dominated Bacteria. Microorganisms 2024, 12, 1689. https://doi.org/10.3390/microorganisms12081689
Zhang L, Zhao X, Wang J, He L, Ren C, Wang J, Guo Y, Wang N, Zhao F. Antarctic Soils Select Copiotroph-Dominated Bacteria. Microorganisms. 2024; 12(8):1689. https://doi.org/10.3390/microorganisms12081689
Chicago/Turabian StyleZhang, Lujie, Xue Zhao, Jieying Wang, Liyuan He, Chengjie Ren, Jun Wang, Yaoxin Guo, Ninglian Wang, and Fazhu Zhao. 2024. "Antarctic Soils Select Copiotroph-Dominated Bacteria" Microorganisms 12, no. 8: 1689. https://doi.org/10.3390/microorganisms12081689






