Advancing Bacillus licheniformis as a Superior Expression Platform through Promoter Engineering
Abstract
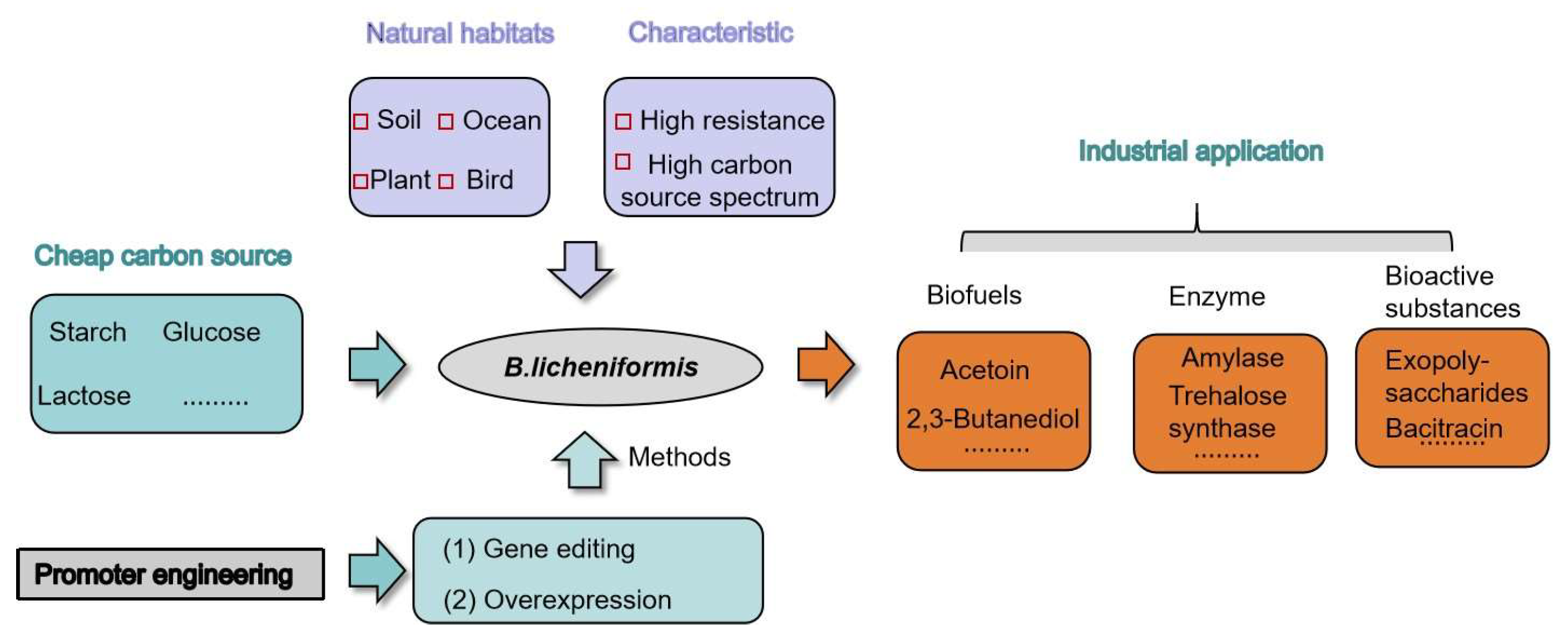
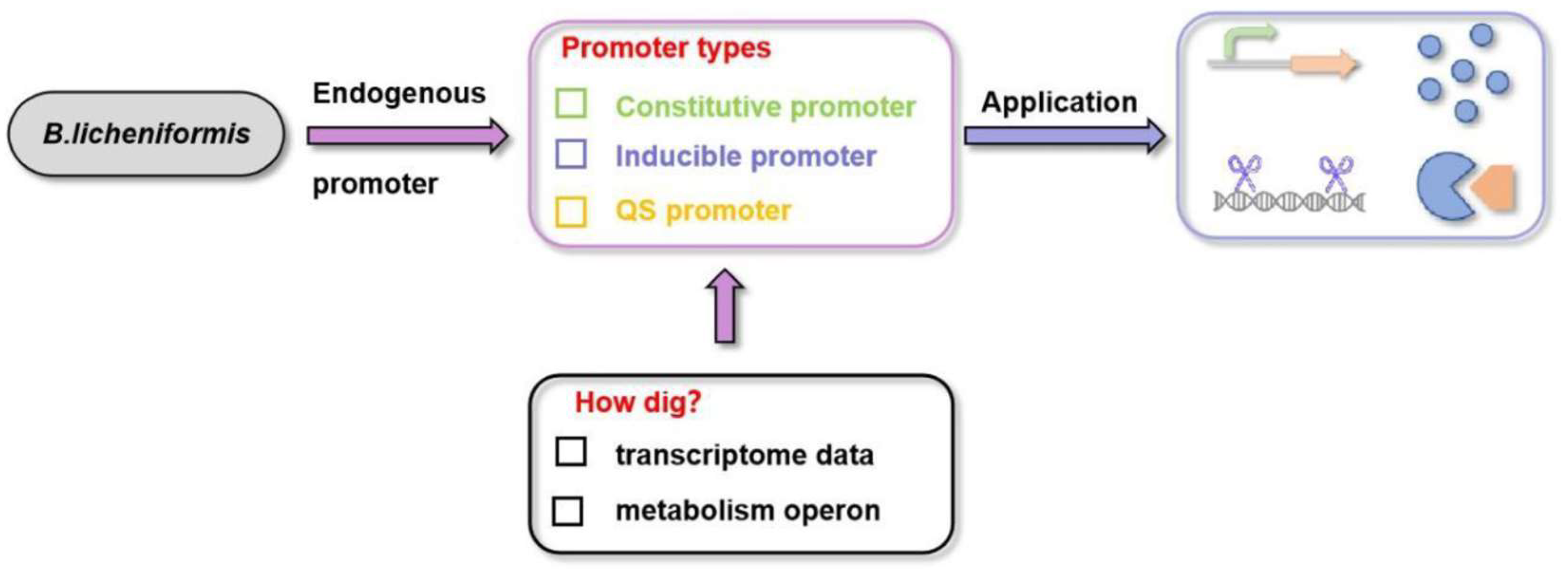
:1. Introduction
2. Constitutive Promoters
2.1. Endogenous Strong Constitutive Promoters
2.1.1. PbacA Derived from Bacitracin Synthase Operon
2.1.2. PalsSD Derived from alsSD Operon
2.2. Heterologous Strong Constitutive Promoters
2.2.1. P43
2.2.2. PShuttle-09
3. Inducible Promoters
3.1. Sugar-Inducible Promoters
3.1.1. Xylose-Inducible Promoter
3.1.2. Acetoin/2,3-Butanediol-Inducible Promoter
3.1.3. Mannitol-Inducible Promoter
3.1.4. Trehalose-Inducible Promoter
3.1.5. Rhamnose-Inducible Promoter
3.1.6. Mannose-Inducible Promoter
3.1.7. Lactose-Inducible Promoter
3.1.8. IPTG-Inducible Promoter
3.2. Nitrogen-Inducible Promoters
Ammonia-Inducible Promoter
3.3. Antibiotic-Inducible Promoter
Tetracycline-Inducible Promoter
3.4. Auto-Inducible Phosphate-Controlled Promoter
3.5. Environmental-Inducible Promoters
3.5.1. Salt-Inducible Promoter
3.5.2. pH-Inducible Promoter
3.5.3. Temperature-Inducible Promoter
4. Quorum Sensing Promoter
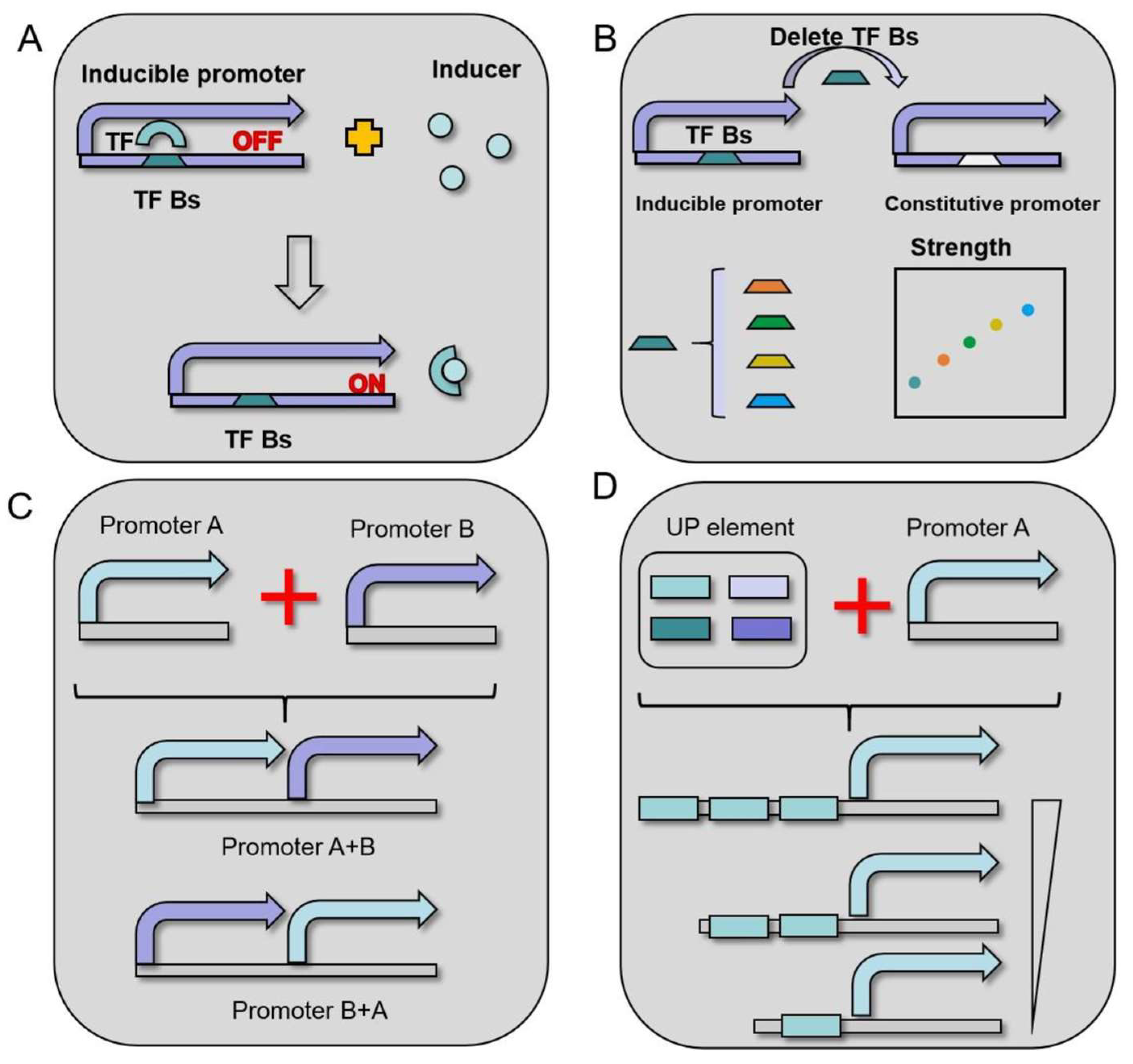
5. Promoter Engineering in B. licheniformis
5.1. Hybrid Promoter Engineering
5.2. Transcription Factor-Inducible Promoter Engineering
5.3. RBS Engineering
6. Concluding Remarks and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Alrumman, S.A.; Mostafa, Y.S.; Al-Izran, K.A.; Alfaifi, M.Y.; Taha, T.H.; Elbehairi, S.E. Production and anticancer activity of an L-Asparaginase from Bacillus licheniformis isolated from the red sea, saudi arabia. Sci. Rep. 2019, 9, 3756. [Google Scholar] [CrossRef] [PubMed]
- Sotnychuk, N.M.; Cutshaw, L.R.; Tuhela, L.; Beckmann, C. Prevalence of feather-degrading Bacillus spp. on the plumage of birds in Australia. Emu Austral Ornithol. 2019, 120, 65–73. [Google Scholar] [CrossRef]
- Othoum, G.; Bougouffa, S.; Razali, R.; Bokhari, A.; Alamoudi, S.; Antunes, A.; Gao, X.; Hoehndorf, R.; Arold, S.T.; Gojobori, T.; et al. In silico exploration of red sea Bacillus genomes for natural product biosynthetic gene clusters. BMC Genom. 2018, 19, 382. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Zhu, M.; Wan, H.; Chen, Z.; Yang, N.; Duan, J.; Wei, Z.; Hu, T.; Liu, F. Effects of plant growth promoting rhizobacteria (PGPR) strain Bacillus licheniformis with biochar amendment on potato growth and water use efficiency under reduced irrigation regime. Agronomy 2022, 12, 1031. [Google Scholar] [CrossRef]
- de, O. Nunes, P.S.; de Medeiros, F.H.V.; de Oliveira, T.S.; de Almeida Zago, J.R.; Bettiol, W. Bacillus subtilis and Bacillus licheniformis promote tomato growth. Braz. J. Microbiol. 2023, 54, 397–406. [Google Scholar]
- Liu, Q.; Liu, Y.; Kang, Z.; Xiao, D.; Gao, C.; Xu, P.; Ma, C. 2,3-Butanediol catabolism in Pseudomonas aeruginosa PAO1. Environ. Microbiol. 2018, 20, 3927–3940. [Google Scholar] [CrossRef] [PubMed]
- Effantin, G.; Rivasseau, C.; Gromova, M.; Bligny, R.; Hugouvieux-Cotte-Pattat, N. Massive production of butanediol during plant infection by phytopathogenic bacteria of the genera Dickeya and Pectobacterium. Mol. Microbiol. 2011, 82, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, K.; Zhang, L.; Ding, Z.; Gu, Z.; Shi, G. Development of an inducible secretory expression system in Bacillus licheniformis based on an engineered xylose operon. J. Agric. Food Chem. 2018, 66, 9456–9464. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, J.; Liang, Y.; Li, L.; Wang, Y.; Pi, L.; Xing, P.; Nomura, C.T.; Chen, S.; Zhu, C.; et al. Engineering the Tat-secretion pathway of Bacillus licheniformis for the secretion of cytoplasmic enzyme arginase. Appl. Microbiol. Biotech. 2024, 108, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Niu, D.; McHunu, N.P.; Zhang, M.; Singh, S.; Wang, Z. Secretory expression of amylosucrase in Bacillus licheniformis through twin-arginine translocation pathway. J. Ind. Microbiol. Biot. 2024, 51, kuae004. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, X.; Yu, W.; Wen, Z.; Chen, S. Enhancement of acetoin production from Bacillus licheniformis by 2,3-butanediol conversion strategy: Metabolic engineering and fermentation control. Process Biochem. 2017, 57, 35–42. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, H.; Chen, S.; Qi, G. Production of optically pure 2,3-butanediol from miscanthus floridulus hydrolysate using engineered Bacillus licheniformis strains. World J. Microb. Biot. 2018, 34, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Y.; Liu, D.; Lv, R. The effect of carbon source and temperature on the formation and growth of Bacillus licheniformis and Bacillus cereus biofilms. LWT Food Sci. Technol. 2023, 186, 115239. [Google Scholar] [CrossRef]
- Klaewkla, M.; Pichyangkura, R.; Charoenwongpaiboon, T.; Wangpaiboon, K.; Chunsrivirot, S. Computational design of oligosaccharide producing levansucrase from Bacillus licheniformis RN-01 to improve its thermostability for production of levan-type fructooligosaccharides from sucrose. Int. J. Biol. Macromol. 2020, 160, 252–263. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.; Kang, J.; Wang, S.; Zha, Z.; Zhan, Y.; Wang, Z.; Li, J.; Cai, D.; Chen, S. Engineering Bacillus licheniformis as industrial chassis for efficient bioproduction from starch. Bioresour. Technol. 2024, 406, 131061. [Google Scholar] [CrossRef]
- Dutschei, T.; Zühlke, M.-K.; Welsch, N.; Eisenack, T.; Hilkmann, M.; Krull, J.; Stühle, C.; Brott, S.; Dürwald, A.; Reisky, L.; et al. Metabolic engineering enables Bacillus licheniformis to grow on the marine polysaccharide ulvan. Microb. Cell Fact. 2022, 21, 207. [Google Scholar] [CrossRef]
- Han, X.; Liu, J.; Wu, Y.; Yang, Y.; Tao, F.; Xu, P. Activating a dormant metabolic pathway for high-temperature l-alanine production in Bacillus licheniformis. iScience 2023, 26, 106397. [Google Scholar] [CrossRef]
- Han, X.; Liu, J.; Tian, S.; Tao, F.; Xu, P. Microbial cell factories for bio-based biodegradable plastics production. iScience 2022, 25, 105462. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Xu, Y.; Zhang, Y.; Tao, G.; Zhang, L.; Shi, G. Transcriptome analysis of Bacillus licheniformis for improving bacitracin production. ACS Synth. Biol. 2022, 11, 1325–1335. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. Efficient genome editing in Bacillus licheniformis mediated by a conditional CRISPR/Cas9 system. Microorganisms 2020, 8, 754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, F.; Zhang, L.; Ding, Z.; Shi, G.; Li, Y. A new mechanism of carbon metabolism and acetic acid balance regulated by CcpA. Microorganisms 2023, 11, 2303. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Hu, S.; Zhang, Y.; Xiang, Z.; Zhu, A.; Chen, S. Transcription factor AbrB regulates ROS generation and clearance in Bacillus licheniformis. Microbiol. Res. 2024, 287, 127843. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; Ma, X.; Xu, S.; Zhang, L.; Ding, Z.; Shi, G. Design of a sorbitol-activated nitrogen metabolism-dependent regulatory system for redirection of carbon metabolism flow in Bacillus licheniformis. Nucleic Acids Res. 2023, 51, 11952–11966. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; He, P.; Zhang, Y.; Jiang, M.; Wang, Q.; Yang, S.; Chen, S. Transcription factor DegU-mediated multi-pathway regulation on lichenysin biosynthesis in Bacillus licheniformis. Metab. Eng. 2022, 74, 108–120. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Shi, G.; Li, Y. Recent biotechnological advances and future prospective of Bacillus licheniformis as microbial cell factories. Syst. Microbiol. Biomanufacturing 2023, 3, 521–532. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Wan, X.; Huang, H.; Li, J.; Nomura, C.T.; Wang, C.; Chen, S. Optimization of inexpensive agricultural by-products as raw materials for bacitracin production in Bacillus licheniformis DW2. Appl. Biochem. Biotech. 2017, 183, 1146–1157. [Google Scholar] [CrossRef]
- Konz, D.; Klens, A.; Schorgendorfer, K.; Marahiel, M.A. The bacitracin biosynthesis operon of Bacillus licheniformis ATCC 10716: Molecular characterization of three multi-modular peptide synthetases. Chem. Biol. 1997, 4, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cai, D.; Wang, Z.; He, Z.; Chen, S. Development of an efficient genome editing tool in Bacillus licheniformis using CRISPR-Cas9 nickase. Appl. Environ. Microbiol. 2018, 84, e02608-17. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, C.; Sheng, B.; Cai, D.; Wang, Q.; Wen, Z.; Chen, S. Improvement of glycerol catabolism in Bacillus licheniformis for production of poly-γ-glutamic acid. Appl. Microbiol. Biotech. 2017, 101, 7155–7164. [Google Scholar] [CrossRef]
- Shi, J.; Zhan, Y.; Zhou, M.; He, M.; Wang, Q.; Li, X.; Wen, Z.; Chen, S. High-level production of short branched-chain fatty acids from waste materials by genetically modified Bacillus licheniformis. Bioresour. Technol. 2019, 271, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Ge, Y.; Cao, M.; Guo, X.; Liu, P.; Gao, C.; Xu, P.; Ma, C. Metabolic engineering of Bacillus licheniformis for production of acetoin. Front Bioeng. Biotechnol. 2020, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Wei, X.; Qiu, Y.; Chen, Y.; Chen, J.; Wen, Z.; Chen, S. High-level expression of nattokinase in Bacillus licheniformis by manipulating signal peptide and signal peptidase. J. Appl. Microbiol. 2016, 121, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xiao, F.; Wang, H.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. CcpA mutants influence selective carbon source utilization by changing interactions with target genes in Bacillus licheniformis. Syst. Microbiol. Biomanufacturing 2021, 2, 193–207. [Google Scholar] [CrossRef]
- Driessen, A.; Yang, M.; Zhang, W.; Ji, S.; Cao, P.; Chen, Y.; Zhao, X. Generation of an artificial double promoter for protein expression in Bacillus subtilis through a promoter trap system. PLoS ONE 2013, 8, e56321. [Google Scholar]
- Dahi, M.K.; Schmiedel, D.; Hillen, W. Glucose and glucose-6-phosphate interaction with xyl repressor proteins from Bacillus-spp may contribute to regulation of xylose utilization. J. Bacteriol. 1995, 177, 5467–5472. [Google Scholar]
- Li, Y.; Liu, X.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. Transcriptional changes in the xylose operon in Bacillus licheniformis and their use in fermentation optimization. Int. J. Mol. Sci. 2019, 20, 4615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gu, Z.; Zhang, L.; Ding, Z.; Shi, G. Inducible expression of trehalose synthase in Bacillus licheniformis. Protein Expr. Purif. 2017, 130, 115–122. [Google Scholar] [CrossRef]
- Qiu, Y.; Xiao, F.; Wei, X.; Wen, Z.; Chen, S. Improvement of lichenysin production in Bacillus licheniformis by replacement of native promoter of lichenysin biosynthesis operon and medium optimization. Appl. Microbiol. Biotech. 2014, 98, 8895–8903. [Google Scholar] [CrossRef]
- Thanh, T.N.; Jürgen, B.; Bauch, M.; Liebeke, M.; Lalk, M.; Ehrenreich, A.; Evers, S.; Maurer, K.-H.; Antelmann, H.; Ernst, F.; et al. Regulation of acetoin and 2,3-butanediol utilization in Bacillus licheniformis. Appl. Microbiol. Biotech. 2010, 87, 2227–2235. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhao, X.; Wen, J.; Huang, M.; Zhang, J.; Song, F. Transcription in the acetoin catabolic pathway is regulated by AcoR and CcpA in Bacillus thuringiensis. Microbiol. Res. 2020, 235, 126438. [Google Scholar] [CrossRef]
- Xiao, F.; Li, Y.; Zhang, Y.; Wang, H.; Zhang, L.; Ding, Z.; Gu, Z.; Xu, S.; Shi, G. Construction of a novel sugar alcohol-inducible expression system in Bacillus licheniformis. Appl. Microbiol. Biotech. 2020, 104, 5409–5425. [Google Scholar] [CrossRef]
- Lee, H.K.; Woo, S.; Baek, D.; Min, M.; Jung, G.Y.; Lim, H.G. Direct and robust citramalate production from brown macroalgae using fast-growing Vibrio sp. dhg. Bioresour. Technol. 2024, 394, 130304. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Zhong, C.; Wang, Y. Integrating the marine carbon resource mannitol into biomanufacturing. Trends Biotechnol. 2023, 41, 745–749. [Google Scholar] [CrossRef]
- Xiao, F.; Li, Y.; Zhang, Y.; Wang, H.; Zhang, L.; Ding, Z.; Gu, Z.; Xu, S.; Shi, G. A new CcpA binding site plays a bidirectional role in carbon catabolism in Bacillus licheniformis. iScience 2021, 24, 102400. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Ma, X.; Luo, C.; Li, D.; Shi, G.; Li, Y. Construction of a bacteriophage-derived recombinase system in Bacillus licheniformis for gene deletion. AMB Express 2023, 13, 89. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Xiao, F.; Zhang, Y.; Zhang, L.; Ding, Z.; Gu, Z.; Xu, S.; Shi, G. Transcriptional modulation of the global regulator CodY using a conditional CRISPRi system in Bacillus licheniformis. Syst. Microbiol. Biomanufacturing 2024, 4, 953–964. [Google Scholar] [CrossRef]
- Li, S.; Xiao, F.; He, H.; Zhang, Y.; Liu, P.; Xu, Y.; Zhang, L.; Xu, S.; Ding, Z.; Shi, G.; et al. Multilevel regulation of lactose operon in Bacillus licheniformis: Coordination of LacR, CcpA and TnrA regulators. Food Biosci. 2024, 61, 104811. [Google Scholar] [CrossRef]
- Gomaa, L.; Loscar, M.E.; Zein, H.S.; Abdel-Ghaffa, N.; Abdelhadi, A.A.; Abdelaal, A.S.; Abdallah, N.A. Boosting isoprene production via heterologous expression of the Kudzu isoprene synthase gene (kIspS) into Bacillus spp. cell factory. AMB Expr. 2017, 7, 161. [Google Scholar] [CrossRef]
- Shen, P.; Niu, D.; Permaul, K.; Tian, K.; Singh, S.; Wang, Z. Exploitation of ammonia-inducible promoters for enzyme overexpression in Bacillus licheniformis. J. Ind. Micro. Biotech. 2021, 48, kuab037. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Arsov, A.; Gergov, E.; Petrova, P.; Petrov, K. Influence of pH on Inulin conversion to 2,3-Butanediol by Bacillus licheniformis 24: A gene expression assay. Int. J. Mol. Sci. 2023, 24, 14065. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, Z.; Cai, D.; Chen, Y.; Wang, H.; Wei, X.; Li, S.; Chen, S. High-level production of α-amylase by manipulating the expression of alanine racamase in Bacillus licheniformis. Biotechnol. Lett. 2017, 39, 1389–1394. [Google Scholar] [CrossRef]
- Trung, N.T.; Hung, N.M.; Thuan, N.H.; Canh, N.X.; Schweder, T.; Jürgen, B. An auto-inducible phosphate-controlled expression system of Bacillus licheniformis. BMC Biotechnol. 2019, 19, 3. [Google Scholar] [CrossRef]
- Borgi, M.A.; Boudebbouze, S.; Mkaouar, H.; Maguin, E.; Rhimi, M. Bacillus phytases: Current status and future prospects. Bioengineered 2015, 6, 233–236. [Google Scholar] [CrossRef]
- Schroeter, R.; Hoffmann, T.; Voigt, B.; Meyer, H.; Bleisteiner, M.; Muntel, J.; Jürgen, B.; Albrecht, D.; Becher, D.; Lalk, M.; et al. Stress responses of the industrial workhorse Bacillus licheniformis to osmotic challenges. PLoS ONE 2013, 8, e80956. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.; Bleisteiner, M.; Sappa, P.K.; Steil, L.; Mäder, U.; Völker, U.; Bremer, E. Erhard Bremer. Synthesis of the compatible solute proline by Bacillus subtilis: Point mutations rendering the osmotically controlled proHJ promoter hyperactive. Environ. Microbiol. 2017, 19, 3700–3720. [Google Scholar] [CrossRef]
- Li, B.; Cai, D.; Hu, S.; Zhu, A.; He, Z.; Chen, S. Enhanced synthesis of poly gamma glutamic acid by increasing the intracellular reactive oxygen species in the Bacillus licheniformis Δ1-pyrroline-5-carboxylate dehydrogenase gene ycgN-deficient strain. Appl. Microbiol. Biotechnol. 2018, 102, 10127–10137. [Google Scholar] [CrossRef] [PubMed]
- Freitals, P.A.F.; Carvalho, H.H.; Costa, J.H.; Miranda, R.S.; Saraiva, K.D.C.; Oliverira, F.D.B.; Coelho, D.G.; Prisco, J.T.; Gomes-Filho, E. Salt acclimation in sorghum plants by exogenous proline: Physiological and biochemical changes and regulation of proline metabolism. Plant Cell Rep. 2019, 38, 403–416. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, G.; Gou, X.Y.; Xing, F.; Li, S.; Han, Y.C.; Wang, L.; Song, J.M.; Shu, C.C.; Chen, S.W.; et al. Comprehensive transcriptome and improved genome annotation of Bacillus licheniformis WX-02. FEBS Lett. 2015, 589, 2372–2381. [Google Scholar] [CrossRef]
- Bindal, S.; Gupta, R. Hyperproduction of γ-glutamyl transpeptidase from Bacillus licheniformis ER15 in the presence of high salt concentration. Prep. Biochem. Biotech. 2017, 47, 163–172. [Google Scholar] [CrossRef] [PubMed]
- ParK, M.K.; Hong, C.P.; Kim, B.S.; Lee, D.Y.; Kim, Y.S. Integrated-Omics study on the transcriptomic and metabolic changes of Bacillus licheniformis, a main microorganism of fermented soybeans, according to alkaline pH and osmotic stress. J. Agric. Food Chem. 2023, 71, 14379–14389. [Google Scholar] [CrossRef]
- Hornbæk, T.; Jakobsen, M.; Dynesen, J.; Nielsen, A.K. Global transcription profiles and intracellular pH regulation measured in Bacillus licheniformis upon external pH upshifts. Arch. Microbiol. 2004, 182, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, H.; Wei, X.; Chen, J.; Chen, S. Enhancement of poly-γ-glutamic acid production by alkaline pH stress treatment in Bacillus licheniformis WX-02. J. Chem. Technol. Biot. 2015, 91, 2399–2403. [Google Scholar] [CrossRef]
- Shardendu, K.T. pH modulates arsenic toxicity in Bacillus licheniformis DAS-2. Ecotox. Environ. Saf. 2016, 130, 240–247. [Google Scholar]
- Dong, Z.; Chen, X.; Cai, K.; Chen, Z.; Wang, H.; Jin, P.; Liu, X.; Permaul, K.; Singh, S.; Wang, Z. Exploring the metabolomic responses of Bacillus licheniformis to temperature stress by gas chromatography/mass spectrometry. J. Microbiol. Biotechnol. 2018, 28, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, Z.; Wang, H.; Tian, K.; Jin, P.; Liu, X.; Mchunu, N.P.; Permaul, K.; Singh, S.; Niu, D.; et al. Tandem mass tag-based quantitative proteomics analyses reveal the response of Bacillus licheniformis to high growth temperatures. Ann. Microbiol. 2017, 67, 501–510. [Google Scholar] [CrossRef]
- Wang, N.; Gao, J.; Yuan, L.; Jin, Y.; He, G. Metabolomics profiling during biofilm development of Bacillus licheniformis isolated from milk powder. Int. J. Food Microbiol. 2021, 337, 108939. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.F.; Chen, B.E.; Lin, M.G.; Chi, M.U.; Wang, T.F.; Lin, L.L. Gene expression and molecular characterization of a chaperone protein HtpG from Bacillus licheniformis. Int. J.Biol. Macromol. 2016, 85, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, X.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. ; Engineering of Bacillus promoters based on interacting motifs between UP elements and RNA polymerase (RNAP) alpha-subunit. Int. J. Mol. Sci. 2022, 23, 13480. [Google Scholar] [CrossRef]
- Rao, Y.; Cai, D.; Wang, H.; Xu, Y.; Xiong, S.; Gao, L.; Xiong, M.; Wang, Z.; Chen, S.; Ma, X. Construction and application of a dual promoter system for efficient protein production and metabolic pathway enhancement in Bacillus licheniformis. J. Biotechnol. 2020, 312, 1–10. [Google Scholar] [CrossRef]
- Agaisse, H.; Lereclus, D. STAB-SD: A Shine–Dalgarno sequence in the 5′ untranslated region is a determinant of mRNA stability. Mol. Microbiol. 2003, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Peng, B.; Su, Z.; Liu, A.; Hu, Y.; Nomura, C.T.; Chen, S.; Wang, Q. Facilitating protein expression with portable 5′-UTR secondary structures in Bacillus licheniformis. ACS Syn. Bio. 2020, 9, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, H.; Ventroux, M.; Noirot-Gros, M.F.; Deutscher, J.; Joyet, P. Membrane sequestration by the EIIB domain of the mannitol permease MtlA activates the Bacillus subtilis mtl operon regulator MtlR. Mol. Microbiol. 2013, 87, 789–801. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, Y.; Zhang, L.; Ding, Z.; Shi, G.; Li, Y. Construction of the genetic switches in response to mannitol based on artifical MtlR box. Bioresour. Bioprocess 2023, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Heravi, K.M.; Wenzel, M.; Altenbuchner, J. Regulation of mtl operon promoter of Bacillus subtilis: Requirements of its use in expression vectors. Microb. Cell Fact. 2011, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xiao, F.; Wang, H.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. Engineering of a biosensor in response to malate in Bacillus licheniformis. ACS Synth. Biol. 2021, 10, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Ogurtsov, A.Y.; Shabalina, S.A.; Koonin, E.V. Role of mRNA structure in the control of protein folding. Nucleic Acids Res. 2016, 44, 10898–10911. [Google Scholar] [CrossRef]
- Rao, X.; Li, D.; Su, Z.; Nomura, C.T.; Chen, S.; Wang, Q. A smart RBS library and its prediction model for robust and accurate fine-tuning of gene expression in Bacillus species. Metab. Eng. 2024, 81, 1–9. [Google Scholar] [CrossRef]
- Zhang, M.; Song, J.; Xiao, J.; Jin, J.; Nomura, C.T.; Chen, S.; Wang, Q. Engineered multiple translation initiation sites: A novel tool to enhance protein production in Bacillus licheniformis and other industrially relevant bacteria. Nucleic Acids Res. 2022, 50, 11979–11990. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Che, H.; Sun, Y.; Wang, S.; Zhan, Y.; Cai, D.; Chen, S. Metabolic engineering of Bacillus licheniformis for the bioproduction of nicotinamide riboside from nicotinamide and glucose. ACS Sustain. Chem. Eng. 2023, 11, 6201–6210. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhou, M.; Wang, H.; Chen, L.; Li, Z.; Cai, D.; Wen, Z.; Ma, X.; Chen, S. Efficient synthesis of 2-phenylethanol from L-phenylalanine by engineered Bacillus licheniformis using molasses as carbon source. Appl Micro. Biotech. 2020, 104, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Lv, X.; Li, J.; Du, G.; Liu, L. CAMERS-B: CRISPR/Cpf1 assisted multiple-genes editing and regulation system for Bacillus subtilis. Biotechnol. Bioeng. 2020, 117, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Cui, W.; Liu, Z.; Suo, F.; Wu, Y.; Han, L.; Zhou, Z. A new-generation base editor with an expanded editing window for microbial cell evolution in vivo based on CRISPR‒Cas12b engineering. Adv. Sci. 2024, 11, e2309767. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xia, Y.; Liang, Z.; Yang, S.; Guo, S.; Sun, L.; Huo, Y. Plasmid-stabilizing strains for antibiotic-free chemical fermentation. Acs. Synth. Biol. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Yue, J.; Li, D.D.; Li, Y.X.; Lee, S.Y.; Zhang, D.W. An operator-based expression toolkjt for Bacillus subtilis enables fine-tuning of gene expression and biosynthetic pathway regulation. Proc. Natl. Acad. Sci. USA 2022, 119, e2119980119. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; David, Y.; Park, S.J.; Choi, J.-i. A chimeric two-component regulatory system-based Escherichia coli biosensor engineered to detect glutamate. Appl. Biochem. Biotech. 2018, 186, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.P.; Detert Oude Weme, R.G.J.; Frenzel, E.; Dirkzwager, M.; Hoffmann, T.; Bremer, E.; Kuipers, O.P. Stress-induced activation of the proline biosynthetic pathway in Bacillus subtilis: A population-wide and single-cell study of the osmotically controlled proHJ promoter. Microb. Biotechnol. 2022, 15, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Y.; Lu, X.; Zhuge, B.; Zong, H. Selection and application of novel high temperature inducible promoters in the tolerant yeast Candida glycerinogenes. J. Biosci. Bioeng. 2020, 130, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arbab, S.; Ullah, H.; Khan, M.I.U.; Khattak, M.N.K.; Zhang, J.; Li, K.; Hassan, I.U. Diversity and distribution of thermophilic microorganisms and their applications in biotechnology. J. Basic Microb. 2021, 62, 95–108. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, X.; Cai, K.; Shen, P.; Tian, K.; Jin, P.; Liu, X.; Wang, Z. Overexpression of the Bacillus licheniformis GroES enhances thermotolerance of Bacillus subtilis WB600. Biotechnol. Biotechnol. Equip. 2018, 32, 1527–1532. [Google Scholar] [CrossRef]
- Wu, L.; Wei, W.; Chen, Z.; Chen, X.; Ni, B.-J. Long-chain alcohol production in open culture anaerobic fermentation. Chem. Eng. J. 2023, 452, 139225. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A. Bacillus licheniformis: The unexplored alternative for the anaerobic production of lipopeptide biosurfactants? Biotechnol. Adv. 2022, 60, 108013. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Hu, S.; Chen, Y.; Liu, L.; Yang, S.; Ma, X.; Chen, S. Enhanced Production of Poly-γ-glutamic acid by overexpression of the global anaerobic regulator Fnr in Bacillus licheniformis WX-02. Appl. Biochem. Biotech. 2018, 185, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Iram, A.; Dong, Y.; Ignea, C. Synthetic biology advances towards a bio-based society in the era of artificial intelligence. Curr. Opin. Biotech. 2024, 87, 130143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, F.; Zhang, Y.; Zhang, L.; Li, S.; Chen, W.; Shi, G.; Li, Y. Advancing Bacillus licheniformis as a Superior Expression Platform through Promoter Engineering. Microorganisms 2024, 12, 1693. https://doi.org/10.3390/microorganisms12081693
Xiao F, Zhang Y, Zhang L, Li S, Chen W, Shi G, Li Y. Advancing Bacillus licheniformis as a Superior Expression Platform through Promoter Engineering. Microorganisms. 2024; 12(8):1693. https://doi.org/10.3390/microorganisms12081693
Chicago/Turabian StyleXiao, Fengxu, Yupeng Zhang, Lihuan Zhang, Siyu Li, Wei Chen, Guiyang Shi, and Youran Li. 2024. "Advancing Bacillus licheniformis as a Superior Expression Platform through Promoter Engineering" Microorganisms 12, no. 8: 1693. https://doi.org/10.3390/microorganisms12081693
APA StyleXiao, F., Zhang, Y., Zhang, L., Li, S., Chen, W., Shi, G., & Li, Y. (2024). Advancing Bacillus licheniformis as a Superior Expression Platform through Promoter Engineering. Microorganisms, 12(8), 1693. https://doi.org/10.3390/microorganisms12081693




