Molecular Diversity of Ectomycorrhizal Fungi in Relation to the Diversity of Neighboring Plant Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Sampling
2.2.1. Sampling Plots and Field Investigation
2.2.2. Roots and Rhizosphere Soil Sampling
2.3. EM Fungal DNA Extraction and Sequence Processing
2.4. EM Fungal Diversity and Network Complexity
2.5. Determination of Abiotic and Biotic Factors
2.6. Statistical Analysis
3. Results
3.1. EM Fungal Diversity under Different Forest Types
3.2. Diversity Relationships between EM Fungi and Neighboring Plant Community
3.3. Environmental Variables Influencing Diversity Relationships between EM Fungi and the Neighboring Plant Community
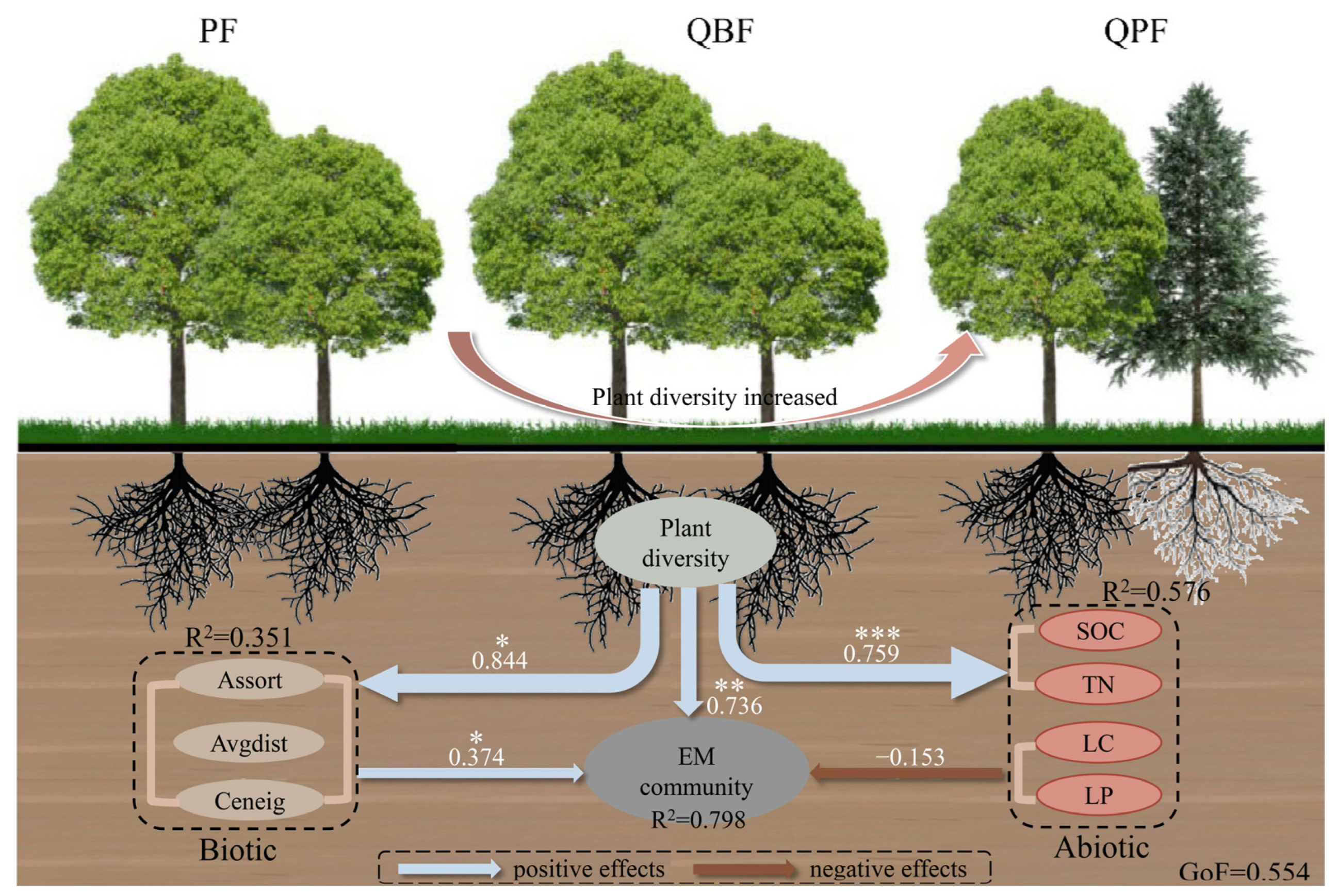
3.4. Keystone Taxa and Relationships wit the Community Diversity of EM Fungi and Neighboring Plants
4. Discussion
4.1. Effects of Forest Types on EM Fungal Diversity
4.2. Effects of Neighboring Plant Community on EM Fungal Diversity
4.3. Environmental Variables Regulate Diversity Relationships between EM Fungi and the Neighboring Plant Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Linde, S.; Suz, L.M.; Orme, C.D.L.; Cox, F.; Andreae, H.; Asi Endla Atkinson, B.; Benham, S.; Carroll, C.; Cools, N.; De Vos, B.; et al. Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 2018, 558, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Bogar, L.M.; Tavasieff, O.S.; Raab, T.K.; Peay, K.G. Does resource exchange in ectomycorrhizal symbiosis vary with competitive context and nitrogen addition? New Phytol. 2022, 233, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.; Valdiviesso, T.; Varela, C.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. Ectomycorrhizal fungal diversity and community structure associated with cork oak in different landscapes. Mycorrhiza 2018, 28, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Otsing, E.; Anslan, S.; Ambrosio, E.; Koricheva, J.; Tedersoo, L. Tree species richness and neighborhood effects on ectomycorrhizal fungal richness and community structure in boreal forest. Front. Microbiol. 2021, 12, 567961. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Bahram, M.; Dickie, I.A. Does host plant richness explain diversity of ectomycorrhizal fungi? Re-evaluation of Gao et al. (2013) data sets reveals sampling effects. Mol. Ecol. 2014, 23, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Mandolini, E.; Bacher, M.; Peintner, U. Ectomycorrhizal fungal communities of Swiss stone pine (Pinus cembra) depend on climate and tree age in natural forests of the Alps. Plant Soil 2022, 1–14. [Google Scholar] [CrossRef]
- Yang, T.; Song, L.Y.; Lin, H.Y.; Dong, K.; Fu, X.; Gao, G.F.; Adams, J.M.; Chu, H.Y. Within-species plant phylogeny drives ectomycorrhizal fungal community composition in tree roots along a timberline. Soil Biol. Biochem. 2023, 176, 108880. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2020, 11, e02999. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Williams, L.J.; Vincent, J.B.; Stefanski, A.; Cavender-Bares, J.; Messier, C.; Paquette, A.; Gravel, D.; Reich, P.B.; Kennedy, P.G. Ectomycorrhizal fungal diversity and saprotrophic fungal diversity are linked to different tree community attributes in a field-based tree experiment. Mol. Ecol. 2016, 25, 4032–4046. [Google Scholar] [CrossRef] [PubMed]
- Arraiano-Castilho, R.; Bidartondoet, M.I.; Niskanen, T.; Zimmermann, S.; Frey, B.; Brunner, I.; Senn-Irlet, B.; Hörandl, E.; Gramlich, S.; Suz, L.M. Plant-fungal interactions in hybrid zones: Ectomycorrhizal communities of willows (Salix) in an alpine glacier forefield. Fungal Ecol. 2020, 45, 100936. [Google Scholar] [CrossRef]
- Qualls, R.G. Comparison of the behavior of soluble organic and inorganic nutrients in forest soils. For. Ecol. Manag. 2000, 138, 29–50. [Google Scholar] [CrossRef]
- Xu, J.W.; Lin, G.; Liu, B.; Mao, R. Linking leaf nutrient resorption and litter decomposition to plant mycorrhizal associations in boreal peatlands. Plant Soil 2020, 448, 413–424. [Google Scholar] [CrossRef]
- Tunlid, A.; Floudas, D.; Koide, R.; Rineau, F. Soil organic matter decomposition mechanisms in ectomycorrhizal fungi. In Molecular Mycorrhizal Symbiosis; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Aponte, C.; García, L.V.; Marañón, T.; Gardes, M. Indirect host effect on ectomycorrhizal fungi: Leaf fall and litter quality explain changes in fungal communities on the roots of co-occurring Mediterranean oaks. Soil Biol. Biochem. 2010, 42, 788–796. [Google Scholar] [CrossRef]
- Tu, Q.; Yan, Q.; Deng, Y.; Michaletz, S.T.; Buzzard, V.; Weiser, M.D.; Waide, R.; Ning, D.; Wu, L.; He, Z. Biogeographic patterns of microbial co-occurrence ecological networks in six American forests. Soil Biol. Biochem. 2020, 148, 107897. [Google Scholar] [CrossRef]
- Burke, D.J.; Carrino-Kyker, S.R. The influence of mycorrhizal fungi on rhizosphere bacterial communities in forests. For. Microbiol. 2021, 1, 257–275. [Google Scholar]
- Deveau, A.; Labbé, J. Mycorrhiza helper bacteria. Mol. Mycorrhizal Symbiosis 2016, 437–450. [Google Scholar] [CrossRef]
- Kennedy, P. Ectomycorrhizal fungi and interspecific competition: Species interactions, community structure, coexistence mechanisms, and future research directions. New Phytol. 2010, 187, 895–910. [Google Scholar] [CrossRef]
- Karlsson, I.; Friberg, H.; Steinberg, C.; Persson, P. Fungicide effects on fungal community composition in the wheat phyllosphere. PLoS ONE 2014, 9, e111786. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Põlme, S.; Abarenkov, K.; Nilsson, R.H.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Csardi, G.; Tamas, N. The igraph software package for complex network research. Interj. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Hu, A.; Wang, J.; Sun, H.; Niu, B.; Si, G.; Wang, J.; Yeh, C.F.; Zhu, X.; Lu, X.; Zhou, J.; et al. Mountain biodiversity and ecosystem functions: Interplay between geology and contemporary environments. ISME J. 2020, 14, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Degnan, P.H.; Ochman, H. Illumina-based analysis of microbial community diversity. ISME J. 2012, 6, 183–194. [Google Scholar] [CrossRef]
- Sanchez, G.; Trinchera, L.; Russolillo, G. Package ‘Plspm’; Citeseer: State College, PA, USA, 2013. [Google Scholar]
- Wang, X.; Zhong, M.; Yang, S.; Jiang, J.; Hu, J. Multiple β-diversity patterns and the underlying mechanisms across amphibian communities along a subtropical elevational gradient. Divers. Distrib. 2022, 28, 2489–2502. [Google Scholar] [CrossRef]
- Wu, K.; Zhao, W.; Li, M.; Picazo, F.; Soininen, J.; Shen, J.; Zhu, L.; Cheng, X.; Wang, J. Taxonomic dependency of beta diversity components in benthic communities of bacteria, diatoms and chironomids along a water-depth gradient. Sci. Total Environ. 2020, 741, 140462. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Soininen, J.; Zhang, Y.; Wang, B.; Yang, X.; Shen, J. Patterns of elevational beta diversity in micro-and macroorganisms. Glob. Ecol. Biogeogr. 2012, 21, 743–750. [Google Scholar] [CrossRef]
- Wagstaff, M.C.; Howell, K.L.; Bett, B.J.; Billett, D.S.M.; Brault, S.; Stuart, C.T.; Rex, M.A. β-diversity of deep-sea holothurians and asteroids along a bathymetric gradient (NE Atlantic). Mar. Ecol. Prog. Ser. 2014, 508, 177–185. [Google Scholar] [CrossRef]
- Huo, X.; Ren, C.; Wang, D.; Wu, R.; Wang, Y.; Li, Z.; Huang, D.; Qi, H. Microbial community assembly and its influencing factors of secondary forests in Qinling Mountains. Soil Biol. Biochem. 2023, 184, 109075. [Google Scholar] [CrossRef]
- Wu, H.; Gao, T.; Hu, A.; Wang, J. Network Complexity and Stability of Microbes Enhanced by Microplastic Diversity. Environ. Sci. Technol. 2024, 58, 4334–4345. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Wang, X.; Wang, J.; Zhang, L.; Liao, L.; Liu, G.; Wang, G.; Song, Z.; Zhang, C. Effect of aridity on the β-diversity of alpine soil potential diazotrophs: Insights into community assembly and co-occurrence patterns. mSystems 2024, 9, e01042-23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Y.; Liu, S.; Fu, S.; Ming, A.; Li, X.; Yao, M.; Li, H.; Tian, C. Mixture of tree species enhances stability of the soil bacterial community through phylogenetic diversity. Eur. J. Soil Sci. 2019, 70, 644–654. [Google Scholar] [CrossRef]
- Johnson, D.; IJdo, M.; Genney, D.R.; Anderson, I.C.; Alexanderet, I.J. How do plants regulate the function, community structure, and diversity of mycorrhizal fungi? J. Exp. Bot. 2005, 56, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Heděnec, P.; Zheng, H.; Siqueira, D.P.; Lin, Q.; Peng, Y.; Schmidt, I.K.; Frøslev, T.D.; Kjøller, R.; Rousk, J.; Vesterdal, L. Tree species traits and mycorrhizal association shape soil microbial communities via litter quality and species mediated soil properties. For. Ecol. Manag. 2023, 527, 120608. [Google Scholar] [CrossRef]
- Garrido, J.L.; Alcántara, J.M. Scale dependency of ectomycorrhizal fungal community assembly processes in Mediterranean mixed forests. Mycorrhiza 2022, 32, 315–325. [Google Scholar]
- Tedersoo, L.; Bahram, M. Mycorrhizal types differ in ecophysiology and alter plant nutrition and soil processes. Biol. Rev. 2019, 94, 1857–1880. [Google Scholar] [CrossRef]
- Gillespie, L.M.; Hättenschwiler, S.; Milcu, A.; Wambsganss, J.; Shihan, A.; Fromin, N. Tree species mixing affects soil microbial functioning indirectly via root and litter traits and soil parameters in European forests. Funct. Ecol. 2021, 35, 2190–2204. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V. Soil organic matter properties and C and N cycling processes: Interactions in mixed-species stands of silver birch and conifers. Appl. Soil Ecol. 2021, 160, 103841. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Yu, Y.; Huang, J.; Zhou, Z.; Zeng, J.; Chen, P.; Xiao, F.; He, Z.; Yan, Q. Ecological stability of microbial communities in Lake Donghu regulated by keystone taxa. Ecol. Indic. 2022, 136, 108695. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Agerer, R. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 2010, 327, 71–83. [Google Scholar] [CrossRef]
- Agerer, R. Exploration types of ectomycorrhizae: A proposal to classify ectomycorrhizal mycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 2001, 11, 107–114. [Google Scholar] [CrossRef]
- Jörgensen, K.; Clemmensen, K.E.; Wallander, H.; Lindahl, B.D. Do ectomycorrhizal exploration types reflect mycelial foraging strategies? New Phytol. 2023, 237, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Dawson, W.; Hör, J.; Egert, M.; Kleunen, M.; Pester, M. A small number of low-abundance bacteria dominate plant species-specific responses during rhizosphere colonization. Front. Microbiol. 2017, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xiao, X.; Nuccio, E.E.; Yuan, M.; Zhang, N.; Xue, K.; Cohan, F.M.; Zhou, J.; Sun, B. Differentiation strategies of soil rare and abundant microbial taxa in response to changing climatic regimes. Environ. Microbiol. 2020, 22, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Grossart, H.P.; He, D.; Yuan, W.; Yang, Y. Stronger environmental adaptation of rare rather than abundant bacterioplankton in response to dredging in eutrophic Lake Nanhu (Wuhan, China). Water Res. 2021, 190, 116751. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Kou, Y. Ectomycorrhizal fungi: Participation in nutrient turnover and community assembly pattern in forest ecosystems. Forests 2020, 11, 453. [Google Scholar] [CrossRef]
- Kuyper, T.W.; Suz, L.M. Do ectomycorrhizal trees select ectomycorrhizal fungi that enhance phosphorus uptake under nitrogen enrichment? Forests 2023, 14, 467. [Google Scholar] [CrossRef]
- Becker, J.; Eisenhauer, N.; Scheu, S.; Jousset, A. Increasing antagonistic interactions cause bacterial communities to collapse at high diversity. Ecol. Lett. 2012, 15, 468–474. [Google Scholar] [CrossRef]
- Haq, I.U.; Zhang, M.; Yang, P.; Elsas, J.D. The interactions of bacteria with fungi in soil: Emerging concepts. Adv. Appl. Microbiol. 2014, 89, 185–215. [Google Scholar] [PubMed]

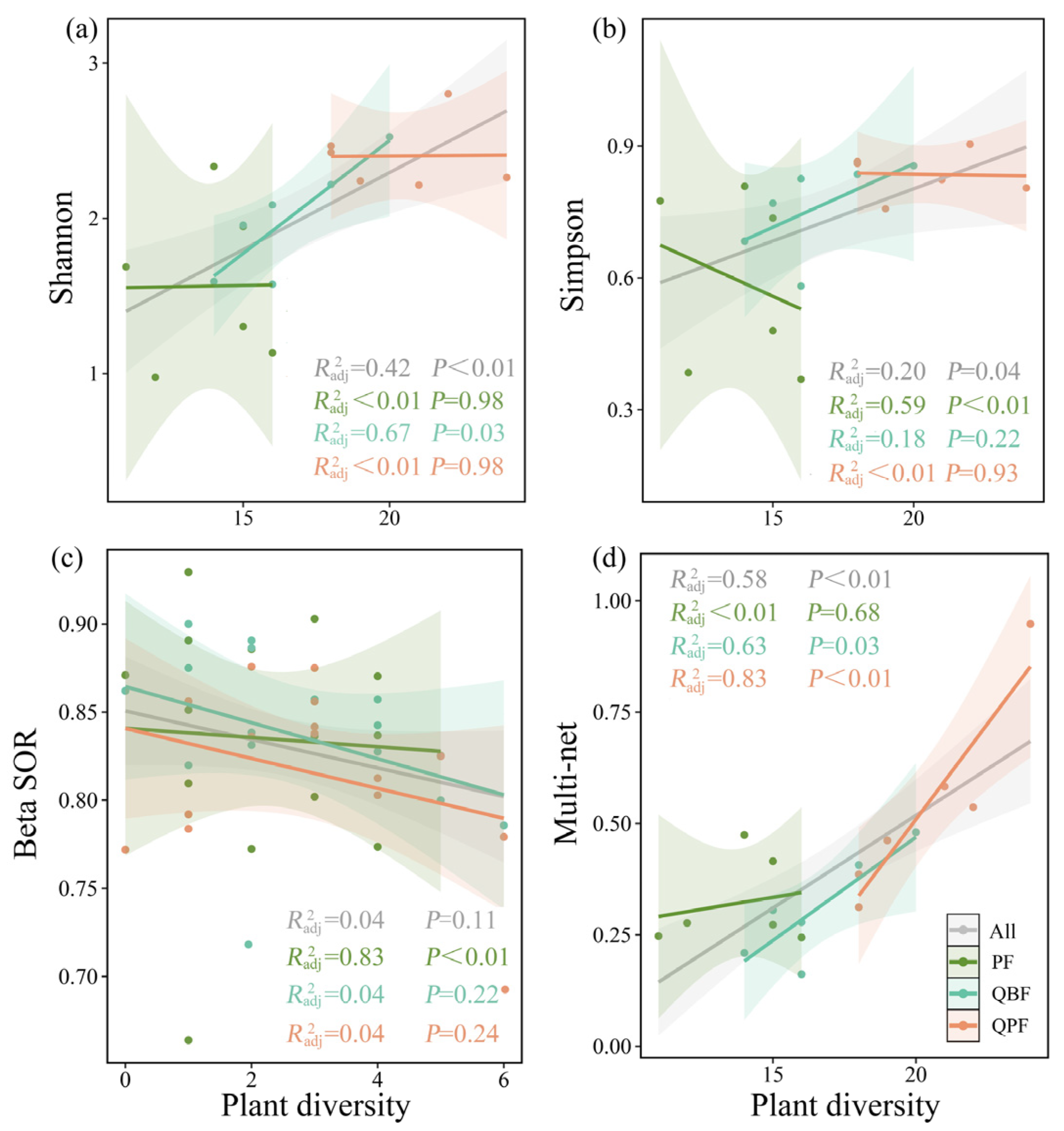
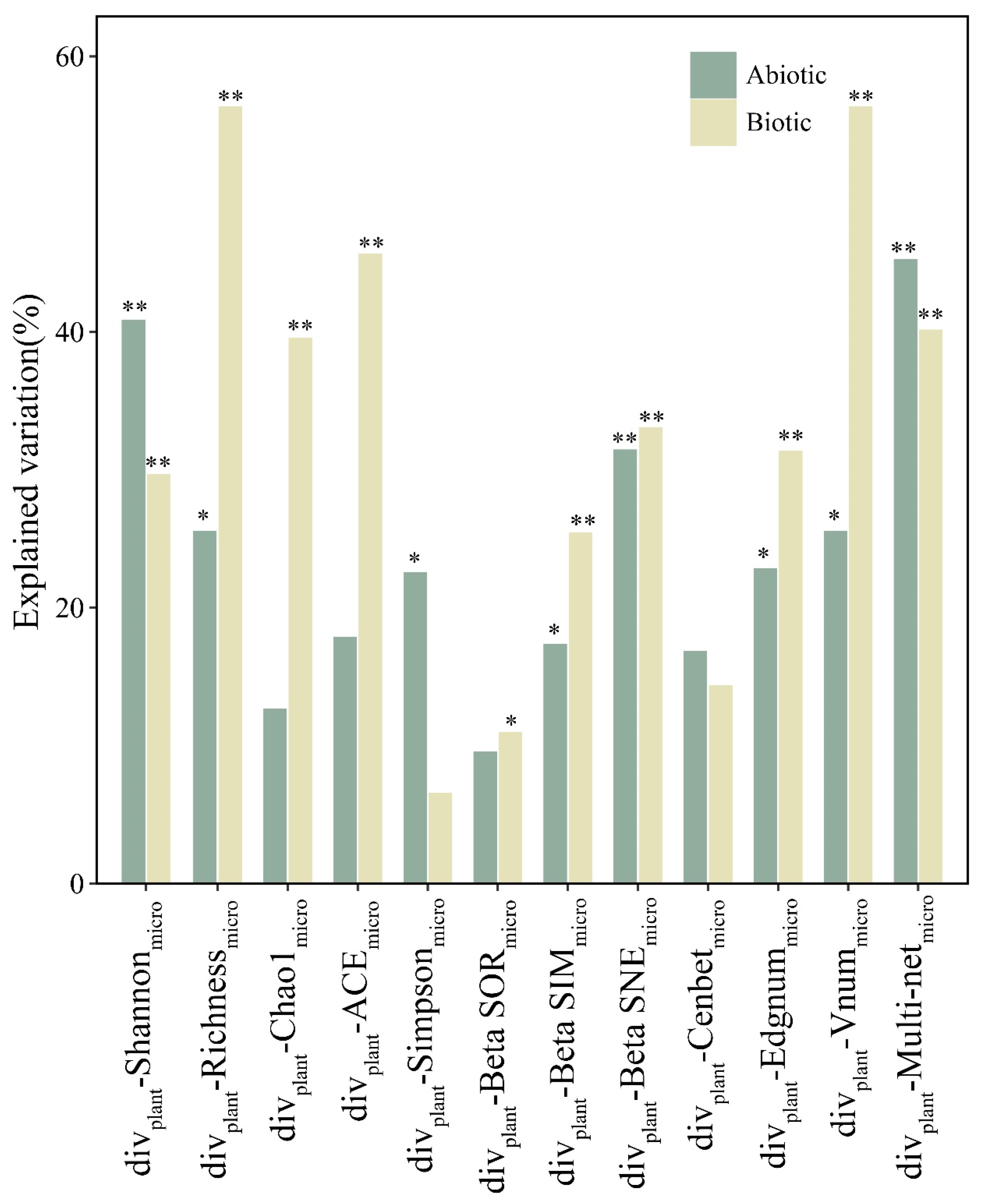

| divplant- Richnessmicro | divplant- Shannonmicro | divplant- Chao1micro | divplant- ACE micro | divplant- Simpsonmicro | divplant- BetaSORmicro | divplant- BetaSIMmicro | divplant- BetaSNEmicro | divplant- Cenbetmicro | divplant- Edgnummicro | divplant- Vnummicro | divplant- Multi-Netmicro | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | Intercept | −3447.84 | −148.60 | 2436.40 | 875.85 | −95.02 | 0.92 | 0.96 | −0.04 | 6.97 | −44,888.20 | −3447.84 | −5.58 |
| Slope | 327.71 | 9.38 | 1.82 | 101.17 | 6.18 | −0.05 | −0.07 | 0.03 | −0.96 | 3024.03 | 327.71 | −0.27 | |
| SOC | Intercept | −17.54 | 1.89 | −32.65 | −35.93 | 0.66 | 0.02 | 0.03 | −0.01 | 0.04 | 73.46 | −17.54 | 0.03 |
| Slope | 1.02 | −0.10 | 1.76 | 2.00 | −0.03 | −0.01 | −0.01 | 0.002 | −0.003 | −7.51 | 1.02 | −0.003 | |
| TN | Intercept | −10.82 | −133.13 | 1612.04 | 1360.21 | −47.59 | 0.87 | 0.70 | 0.17 | −7.96 | −87,257.70 | −10.82 | −31.02 |
| Slope | −15.88 | 8.18 | −103.80 | −95.02 | 2.88 | −0.06 | −0.15 | 0.09 | 0.43 | 4954.48 | −15.88 | 1.67 | |
| LC | Intercept | −0.08 | 0.15 | −1.08 | −1.13 | 0.05 | −0.002 | −0.004 | 0.001 | −0.01 | 32.14 | −0.08 | −0.01 |
| Slope | −0.01 | −0.01 | 0.04 | 0.04 | −0.002 | 0.001 | 0.002 | −0.001 | 0.001 | −1.96 | −0.01 | 0.001 | |
| LP | Intercept | −347.77 | 13.39 | −473.94 | −567.94 | 2.95 | −0.56 | −0.69 | 0.13 | 1.14 | −10,337.30 | −347.77 | −0.70 |
| Slope | 21.62 | −0.90 | 29.20 | 35.16 | −0.21 | 0.18 | 0.25 | −0.07 | −0.07 | 614.12 | 21.61 | 0.03 | |
| Avgdist | Intercept | −1.40 | −0.49 | 11.70 | 8.30 | −0.29 | 0.03 | 0.01 | 0.01 | 0.004 | −897.31 | −1.40 | −0.15 |
| Slope | 0.67 | 0.03 | −0.11 | 0.10 | 0.02 | −0.003 | −0.001 | −0.001 | −0.001 | 54.88 | 0.67 | 0.01 | |
| Ceneig | Intercept | 4145.07 | 132.09 | −2046.12 | −158.27 | 92.64 | −8.52 | −6.67 | −1.85 | −1.29 | 89,378.99 | 4145.08 | 26.95 |
| Slope | −358.01 | −8.88 | −10.86 | −128.64 | −6.22 | 2.12 | 1.70 | 0.43 | 0.63 | −5480.61 | −358.01 | −0.92 | |
| Assott | Intercept | 88.19 | −2.70 | 157.25 | 155.07 | −0.74 | 0.001 | −0.02 | 0.02 | −0.41 | 1110.58 | 88.19 | −0.32 |
| Slope | −6.13 | 0.17 | −10.03 | −10.03 | 0.04 | 0.01 | 0.02 | −0.01 | 0.03 | −61.72 | −6.13 | 0.03 | |
| Random effects (variance) | |||||||||||||
| Sites | 1.82 | 0.10 | 5.21 | 4.95 | 0.03 | 0.04 | 0.05 | 0.02 | 0.03 | 196.62 | 1.82 | 0.07 | |
| residual | 0.68 | 0.04 | 1.95 | 1.85 | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 73.73 | 0.68 | 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Xue, W.; Liu, J.; Zhu, H.; Zhao, Z. Molecular Diversity of Ectomycorrhizal Fungi in Relation to the Diversity of Neighboring Plant Species. Microorganisms 2024, 12, 1718. https://doi.org/10.3390/microorganisms12081718
Zhang W, Xue W, Liu J, Zhu H, Zhao Z. Molecular Diversity of Ectomycorrhizal Fungi in Relation to the Diversity of Neighboring Plant Species. Microorganisms. 2024; 12(8):1718. https://doi.org/10.3390/microorganisms12081718
Chicago/Turabian StyleZhang, Weiwei, Wenyan Xue, Jinliang Liu, Hailan Zhu, and Zhong Zhao. 2024. "Molecular Diversity of Ectomycorrhizal Fungi in Relation to the Diversity of Neighboring Plant Species" Microorganisms 12, no. 8: 1718. https://doi.org/10.3390/microorganisms12081718
APA StyleZhang, W., Xue, W., Liu, J., Zhu, H., & Zhao, Z. (2024). Molecular Diversity of Ectomycorrhizal Fungi in Relation to the Diversity of Neighboring Plant Species. Microorganisms, 12(8), 1718. https://doi.org/10.3390/microorganisms12081718





