Microbiome and Microbial Pure Culture Study Reveal Commensal Microorganisms Alleviate Salmonella enterica Serovar Pullorum Infection in Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. S. Pullorum Challenge and Sampling
2.2. 16S rRNA Gene Amplicon Sequencing
2.3. Species Annotation and Diversity Analysis
2.4. S. Pullorum Infection Related Microorganisms Isolation and Identification
2.5. Antimicrobial Test
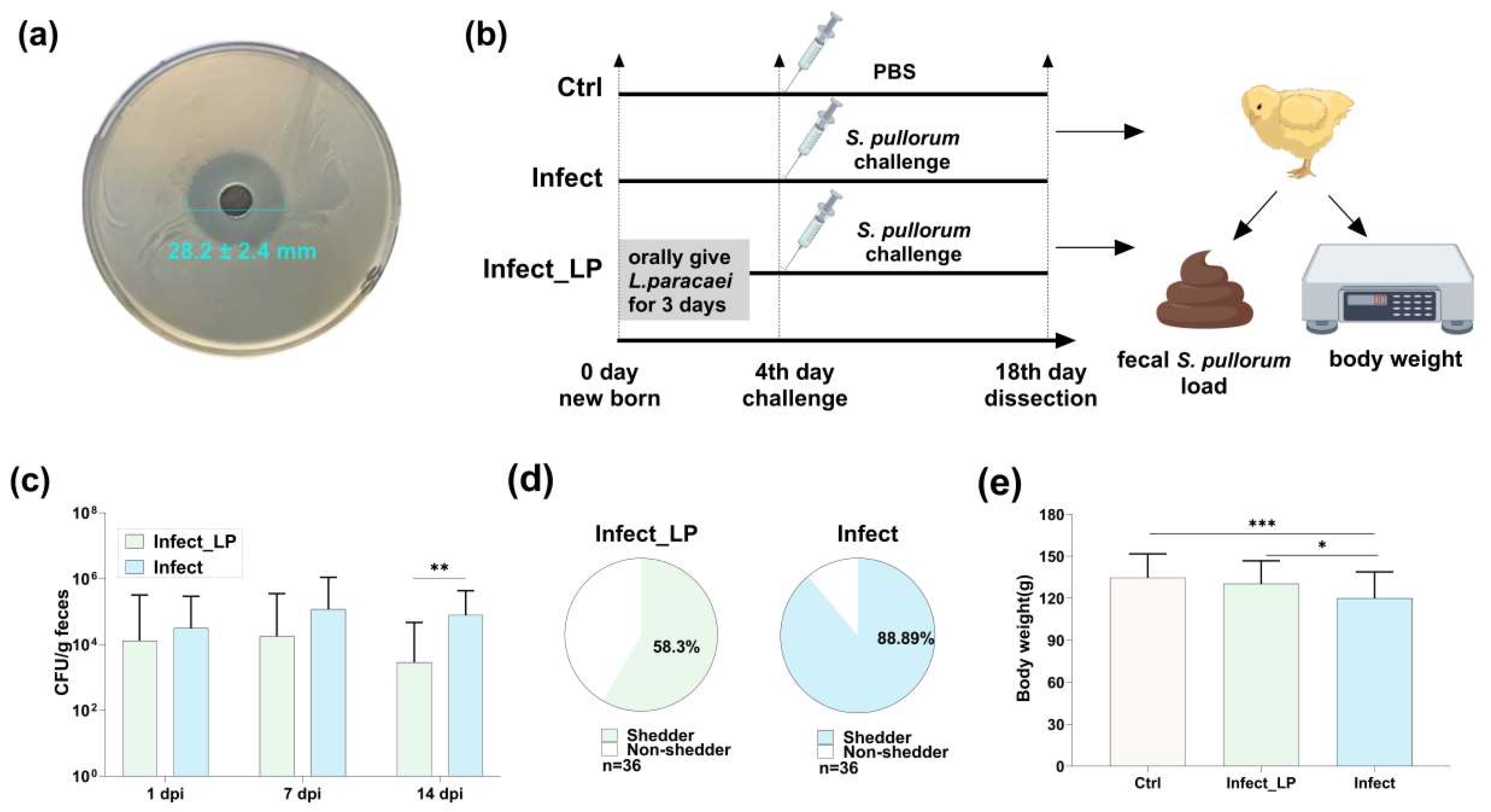
2.6. S. Pullorum Challenge–Intervention Animal Experiment
2.7. Metabolite Detection
2.8. Data Statistics
3. Results
3.1. The Impact of S. Pullorum Challenge on Chicken Performance and Physiology
3.2. S. Pullorum Infection Altered the Richness and Structure of Chicken Gut Microbiota
3.3. Infection Decreased Microorganisms Isolated from Healthy Chicken Feces and Inhibited S. Pullorum Proliferation
3.4. A Lacticaseibacillus paracasei Isolated from Chicken Feces Alleviated S. Pullorum Infection
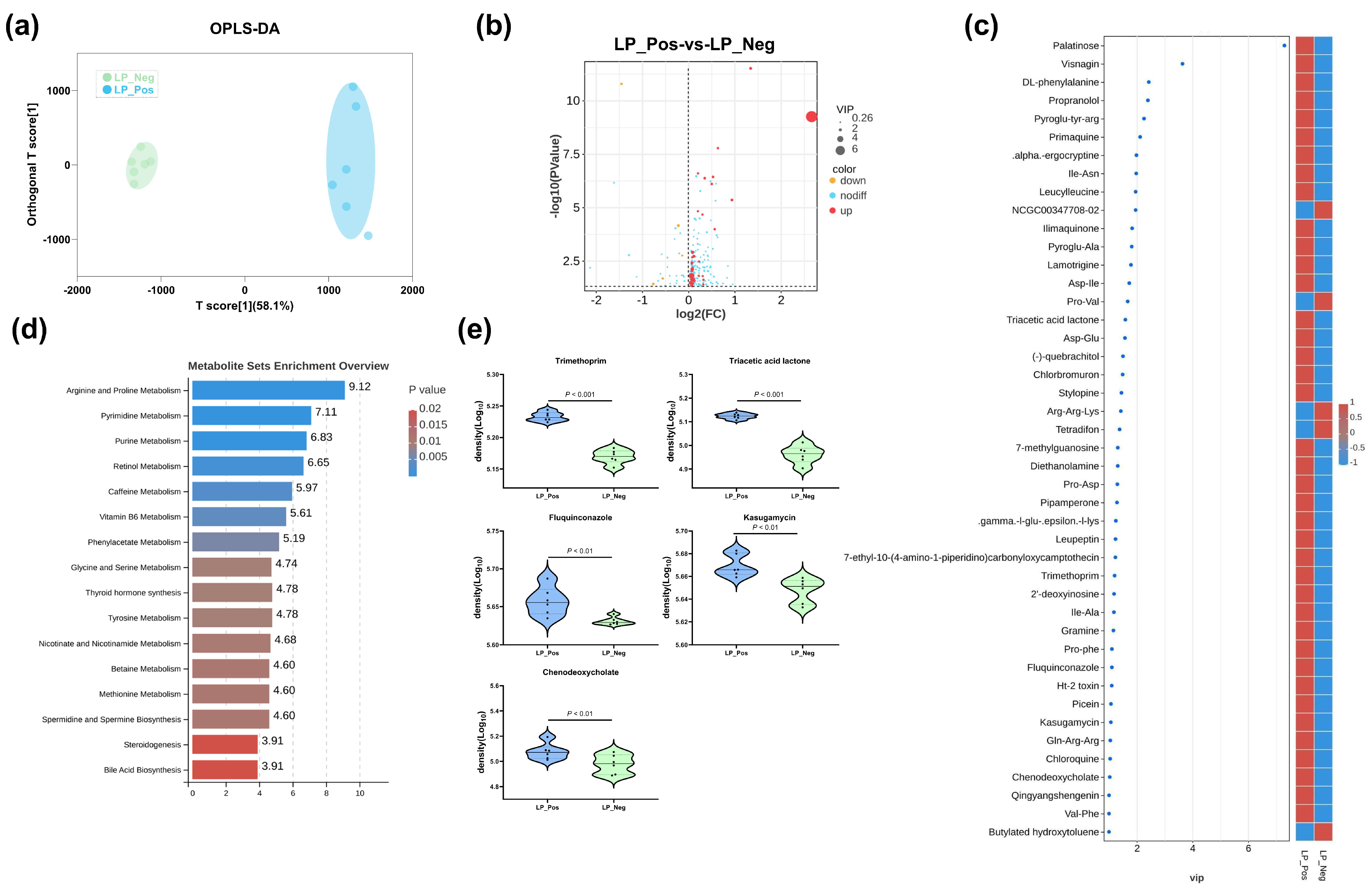
3.5. Comparative Metabolomic Analysis of Sterile Supernatants from L. paracasei Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Farhat, M.; Khayi, S.; Berrada, J.; Mouahid, M.; Ameur, N.; El-Adawy, H.; Fellahi, S. Serovar Gallinarum Biovars Pullorum and Gallinarum in Poultry: Review of Pathogenesis, Antibiotic Resistance, Diagnosis and Control in the Genomic Era. Antibiotics 2024, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singh, V.; Kumar, G.; Gupta, N.K.; Tahlan, A.K. Serovar diversity of Salmonella among poultry. Indian J. Med. Res. 2019, 150, 92–95. [Google Scholar] [CrossRef]
- Da Silva, E.C.; de Oliveira, C.D. Salmonella detection with LAMP and qPCR and identification of serovars of interest by multiplex qPCR in poultry carcasses. Braz. J. Microbiol. 2023, 54, 2173–2182. [Google Scholar] [CrossRef]
- Xiong, D.; Yuan, L.; Song, L.; Jiao, X.A.; Pan, Z.M. A new multiplex PCR for the accurate identification and differentiation of serovar Gallinarum biovars Pullorum and Gallinarum. Front. Microbiol. 2022, 13, 983942. [Google Scholar] [CrossRef]
- Kang, X.; An, H.; Wang, B.; Huang, L.; Huang, C.; Huang, Y.; Wang, Z.; He, F.; Li, Y.; Yue, M.; et al. Integrated OMICs approach reveals energy metabolism pathway is vital for Salmonella Pullorum survival within the egg white. mSphere 2024, 9, e0036224. [Google Scholar] [CrossRef]
- Zhou, X.; Kang, X.; Chen, J.; Song, Y.; Jia, C.; Teng, L.; Tang, Y.; Jiang, Z.; Peng, X.; Tao, X.; et al. Genome degradation promotes Salmonella pathoadaptation by remodeling fimbriae-mediated proinflammatory response. Natl. Sci. Rev. 2023, 10, nwad228. [Google Scholar] [CrossRef]
- Li, X.; Nie, C.; Liu, Y.; Chen, Y.; Lv, X.; Wang, L.; Zhang, J.; Li, K.; Jia, Y.; Ban, L.; et al. A genome-wide association study explores the genetic determinism of host resistance to Salmonella pullorum infection in chickens. Genet. Sel. Evol. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Li, X.H.; Nie, C.S.; Zhang, Z.B.; Wang, Q.; Shao, P.P.; Zhao, Q.N.; Chen, Y.; Wang, D.H.; Li, Y.J.; Jiao, W.J.; et al. Evaluation of genetic resistance to Pullorum in three chicken lines. Poult. Sci. 2018, 97, 764–769. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Sun, M.; Wang, Z.; Wang, Y.; Zhao, X.; Jia, F.; Zhang, T.; Ge, C.; Zhang, X.; et al. Protective effects of E. coli Nissle 1917 on chickens infected with Salmonella pullorum. Microb. Pathog. 2022, 172, 105768. [Google Scholar] [CrossRef]
- Guo, R.; Li, Z.; Zhou, X. Induction of arthritis in chickens by infection with novel virulent Salmonella Pul-lorum strains. Vet. Microbiol. 2019, 228, 165–172. [Google Scholar] [CrossRef]
- Kang, X.; Yang, Y.; Meng, C.; Wang, X.; Liu, B.; Geng, S.; Jiao, X.; Pan, Z. Safety and protective efficacy of Salmonella Pullorum spiC and rfaH deletion rough mutant as a live attenuated DIVA vaccine candidate. Poult. Sci. 2022, 101, 101655. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X. Isolation, whole genome sequencing and application of a broad-spectrum Salmonella phage. Arch. Microbiol. 2024, 206, 335. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, A.; Gu, J.; Zhao, R.; Pan, X.; Dai, Y.; Yin, L.; Zhang, Q.; Hu, X.; Wang, H.; et al. Evaluating Salmonella pullorum dissemination and shedding patterns and antibody production in infected chickens. BMC Vet. Res. 2022, 18, 240. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Huang, J.; Li, P.; Song, M.; Liu, B.; Tang, W.; Sun, S. The Genomic Characteristics of an Arthritis-Causing Salmonella pullorum. Microorganisms 2023, 11, 2986. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Li, X.; Wang, Y.; Wang, F.; Ge, H.; Pan, Z.; Xu, Y.; Wang, Y.; Jiao, X.A.; Chen, X. Epidemic patterns of antimicrobial resistance of Salmonella enterica serovar Gallinarum biovar Pullorum isolates in China during the past half-century. Poult. Sci. 2021, 100, 100894. [Google Scholar] [CrossRef]
- Lucca, V.; Borges, K.A. Phenotypic and molecular characterisation of Salmonella spp. isolates in healthy poultry. Br. Poult. Sci. 2024, 65, 415–423. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S. The Role of the Gut Microbiota in the Relationship Between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liu, X.J.; Hao, J.Y. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J. Dig. Dis. 2020, 21, 147–159. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2018, 4, 293–305. [Google Scholar] [CrossRef]
- Lavelle, A.; Sokol, H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017, 2, 17004. [Google Scholar] [CrossRef]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; de Lima Romão, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Masucci, L.; Ianiro, G.; Bibbò, S.; Dinoi, G.; Costamagna, G.; Sanguinetti, M.; Gasbarrini, A. Randomised clinical trial: Faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2015, 41, 835–843. [Google Scholar] [CrossRef]
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal Microbiota Transplantation Is Superior to Fidaxomicin for Treatment of Recurrent Clostridium difficile Infection. Gastroenterology 2019, 156, 1324–1332.e3. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal Infusion of Donor Feces for RecurrentClostridium difficile. New Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection. Ann. Intern. Med. 2016, 165, 609–616. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Kelly, C.R.; Kraft, C.S.; Dhere, T.; Henn, M.R.; Lombardo, M.-J.; Vulic, M.; Ohsumi, T.; Winkler, J.; et al. A Novel Microbiome Therapeutic Increases Gut Microbial Diversity and Prevents RecurrentClostridium difficileInfection. J. Infect. Dis. 2016, 214, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.H.; Zhu, H.; Tao, C.L.; Zhang, B.B.; Yao, L.; Zhang, Y.D.; Afayibo, D.J.A.; Li, T.; Tian, M.X.; Qi, J.J.; et al. Rapid Detection and Differentiating of the Predominant Serovars in Chicken Farm by TaqMan Multiplex Real-Time PCR Assay. Front. Cell. Infect. Microbiol. 2021, 11, 759965. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Yi, Z.; Zhang, Y.; Wang, Y.; Zhu, H.; Afayibo, D.J.A.; Li, T.; Tian, M.; Qi, J.; Ding, C.; et al. Characterization of a Broad-Host-Range Lytic Phage SHWT1 Against Multidrug-Resistant Salmonella and Evaluation of Its Therapeutic Efficacy in vitro and in vivo. Front. Vet. Sci. 2021, 8, 683853. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Smidt, H.; Chiodini, R.J.; Dowd, S.E.; Chamberlin, W.M.; Galandiuk, S.; Davis, B.; Glassing, A. Microbial Population Differentials between Mucosal and Submucosal Intestinal Tissues in Advanced Crohn’s Disease of the Ileum. PLoS ONE 2015, 10, e0134382. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.Y.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Lopez, C.A.; Winter, S.E.; Rivera-Chávez, F.; Xavier, M.N.; Poon, V.; Nuccio, S.P.; Tsolis, R.M.; Bäumler, A.J. Phage-Mediated Acquisition of a Type III Secreted Effector Protein Boosts Growth of by Nitrate Respiration. Mbio 2012, 3, 10–1128. [Google Scholar] [CrossRef]
- Lopez, C.A.; Rivera-Chvez, F.; Byndloss, M.X.; Bäumler, A.J. The Periplasmic Nitrate Reductase NapABC Supports Luminal Growth of Serovar Typhimurium during Colitis. Infect. Immun. 2015, 83, 3470–3478. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-Derived Nitrate Boosts Growth of in the Inflamed Gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef]
- Ng, K.M.; Ferreyra, J.A.; Higginbottom, S.K.; Lynch, J.B.; Kashyap, P.C.; Gopinath, S.; Naidu, N.; Choudhury, B.; Weimer, B.C.; Monack, D.M.; et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 2013, 502, 96–99. [Google Scholar] [CrossRef]
- Crhanova, M.; Karasova, D.; Juricova, H.; Matiasovicova, J.; Jahodarova, E.; Kubasova, T.; Seidlerova, Z.; Cizek, A.; Rychlik, I. Systematic Culturomics Shows that Half of Chicken Caecal Microbiota Members can be Grown in Vitro Except for Two Lineages of Clostridiales and a Single Lineage of Bacteroidetes. Microorganisms 2019, 7, 496. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Khelaifia, S.; Alou, M.T.; Ndongo, S.; Dione, N.; Hugon, P.; Caputo, A.; Cadoret, F.; Traore, S.I.; Seck, E.H.; et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat. Microbiol. 2016, 1, 16203. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.-C.; Dubourg, G.; Million, M.; Cadoret, F.; Bilen, M.; Fenollar, F.; Levasseur, A.; Rolain, J.-M.; Fournier, P.-E.; Raoult, D. Culturing the human microbiota and culturomics. Nat. Rev. Microbiol. 2018, 16, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xue, R.; Wang, Y.; Stirling, E.; Ye, S.; Xu, J.; Ma, B. FACS-iChip: A high-efficiency iChip system for microbial ‘dark matter’ mining. Mar. Life Sci. Technol. 2020, 3, 162–168. [Google Scholar] [CrossRef]
- Berdy, B.; Spoering, A.L.; Ling, L.L.; Epstein, S.S. In situ cultivation of previously uncultivable microorganisms using the ichip. Nat. Protoc. 2017, 12, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.C.; Armougom, F.; Million, M.; Hugon, P.; Pagnier, I.; Robert, C.; Bittar, F.; Fournous, G.; Gimenez, G.; Maraninchi, M.; et al. Microbial culturomics: Paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 2012, 18, 1185–1193. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Hugon, P.; Khelaifia, S.; Fournier, P.-E.; La Scola, B.; Raoult, D. The Rebirth of Culture in Microbiology through the Example of Culturomics To Study Human Gut Microbiota. Clin. Microbiol. Rev. 2015, 28, 237–264. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, J.; Huang, W.; Fan, Y.; Li, Z.; Tian, M.; Ma, J.; Lu, X.; Liang, J. Metagenomic and Culturomics Analysis of Microbial Communities within Surface Sediments and the Prevalence of Antibiotic Resistance Genes in a Pristine River: The Zaqu River in the Lancang River Source Region, China. Microorganisms 2024, 12, 911. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Lawley, T.D.; Walker, A.W. Intestinal colonization resistance. Immunology 2012, 138, 1–11. [Google Scholar] [CrossRef]
- Jacobson, A.; Lam, L.; Rajendram, M.; Tamburini, F.; Honeycutt, J.; Pham, T.; Van Treuren, W.; Pruss, K.; Stabler, S.R.; Lugo, K.; et al. A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe 2018, 24, 296–307.e297. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef] [PubMed]
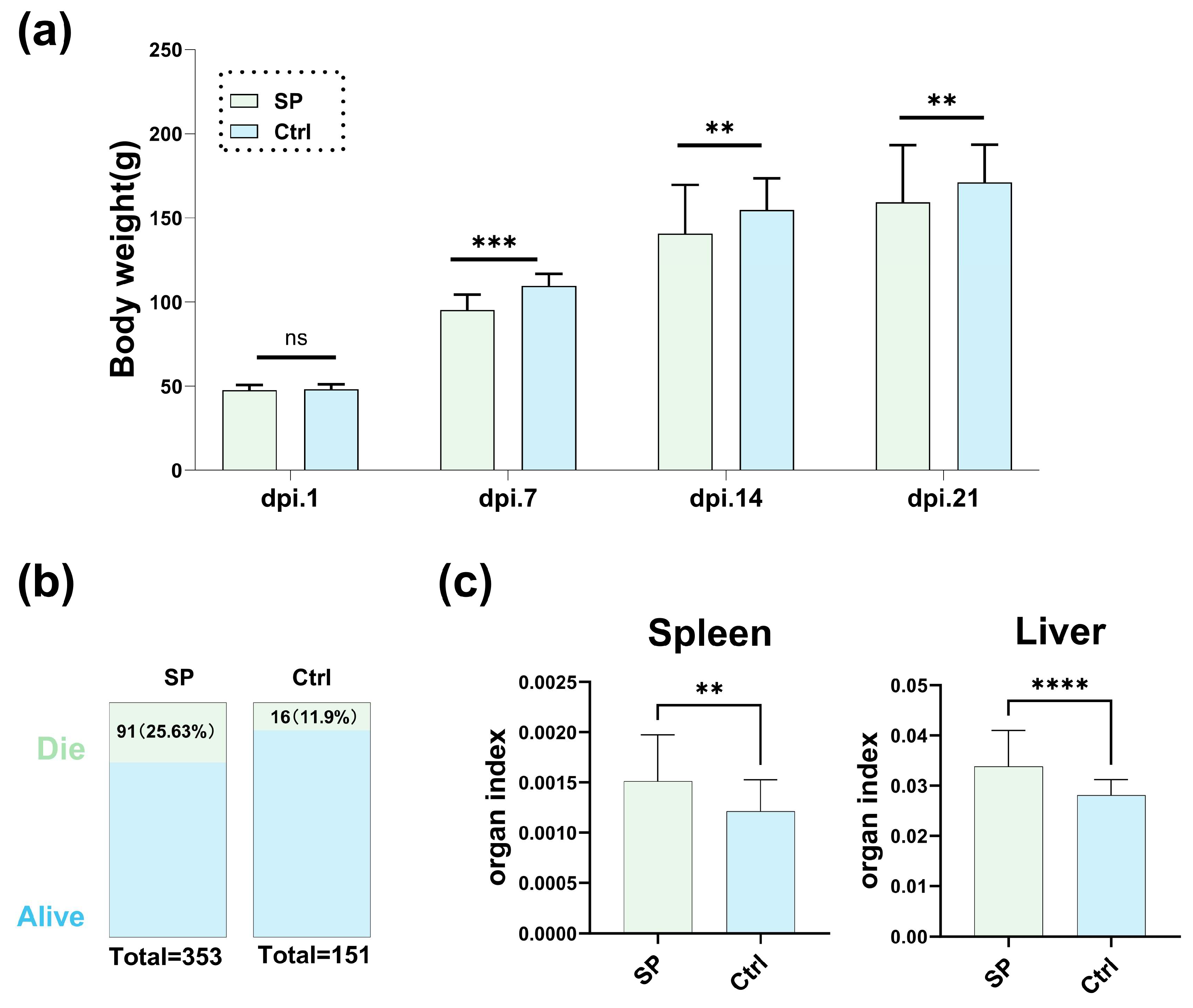

| Mean (%) | |||
|---|---|---|---|
| Genus | SP | Ctrl | FC (SP/Ctrl) |
| Escherichia-Shigella | 65.0 | 40.6 | 1.6 *** ↑ |
| Lactobacillus | 9.86 | 32.1 | 0.31 ** ↓ |
| Enterococcus | 10.8 | 3.70 | 2.92 *** ↑ |
| Ruminococcus | 0.72 | 1.38 | 0.52 * ↓ |
| Streptococcus | 0.16 | 0.83 | 0.19 **↓ |
| Bifidobacterium | 0.02 | 0.66 | 0.03 ***↓ |
| Ralstonia | 0.14 | 0.47 | 0.29 **↓ |
| Clostridium | 0.21 | 0.57 | 0.37 * ↓ |
| Butyricicoccus | 0.15 | 0.43 | 0.35 * ↓ |
| Coprobacillus | 0.05 | 0.41 | 0.12 **↓ |
| Caulobacter | 0.13 | 0.33 | 0.39* ↓ |
| Lactococcus | 0.12 | 0.26 | 0.46 * ↓ |
| Blautia | 0.04 | 0.16 | 0.25 **↓ |
| Veillonella | 0.04 | 0.14 | 0.29 **↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Ding, J.; Yang, K.; Zhou, H.; Yang, W.; Qin, C.; Wang, L.; Xiao, F.; Zhang, B.; Niu, Q.; et al. Microbiome and Microbial Pure Culture Study Reveal Commensal Microorganisms Alleviate Salmonella enterica Serovar Pullorum Infection in Chickens. Microorganisms 2024, 12, 1743. https://doi.org/10.3390/microorganisms12091743
Zhu J, Ding J, Yang K, Zhou H, Yang W, Qin C, Wang L, Xiao F, Zhang B, Niu Q, et al. Microbiome and Microbial Pure Culture Study Reveal Commensal Microorganisms Alleviate Salmonella enterica Serovar Pullorum Infection in Chickens. Microorganisms. 2024; 12(9):1743. https://doi.org/10.3390/microorganisms12091743
Chicago/Turabian StyleZhu, Jianshen, Jinmei Ding, Kaixuan Yang, Hao Zhou, Wenhao Yang, Chao Qin, Liyuan Wang, Fuquan Xiao, Beibei Zhang, Qing Niu, and et al. 2024. "Microbiome and Microbial Pure Culture Study Reveal Commensal Microorganisms Alleviate Salmonella enterica Serovar Pullorum Infection in Chickens" Microorganisms 12, no. 9: 1743. https://doi.org/10.3390/microorganisms12091743
APA StyleZhu, J., Ding, J., Yang, K., Zhou, H., Yang, W., Qin, C., Wang, L., Xiao, F., Zhang, B., Niu, Q., Zhou, Z., Yu, S., Huang, Q., Wang, S., & Meng, H. (2024). Microbiome and Microbial Pure Culture Study Reveal Commensal Microorganisms Alleviate Salmonella enterica Serovar Pullorum Infection in Chickens. Microorganisms, 12(9), 1743. https://doi.org/10.3390/microorganisms12091743








