Calcium Rescues Streptococcus pneumoniae D39 ΔmntE Manganese-Sensitive Growth Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Growth Conditions
2.2. Dilution Drop Test
2.3. Metal Quantification
2.4. Uronic Acid Assay
2.5. Bacteria Adherence Assay
2.6. Biofilm Quantification
2.7. RNA Extraction and Differential Sequence Analysis
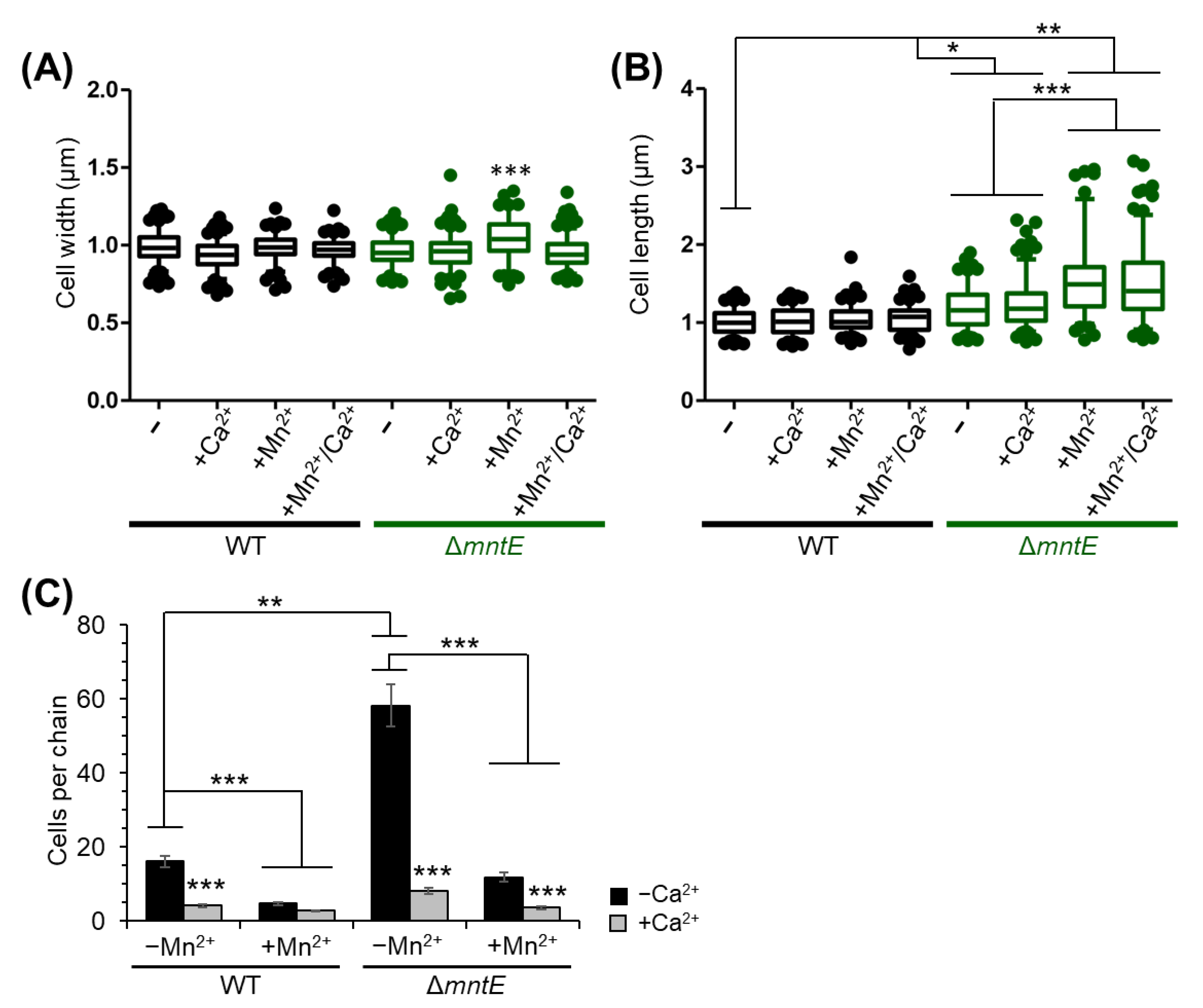
2.8. Cell Morphology Measurements
2.9. Statistical Analysis
3. Results
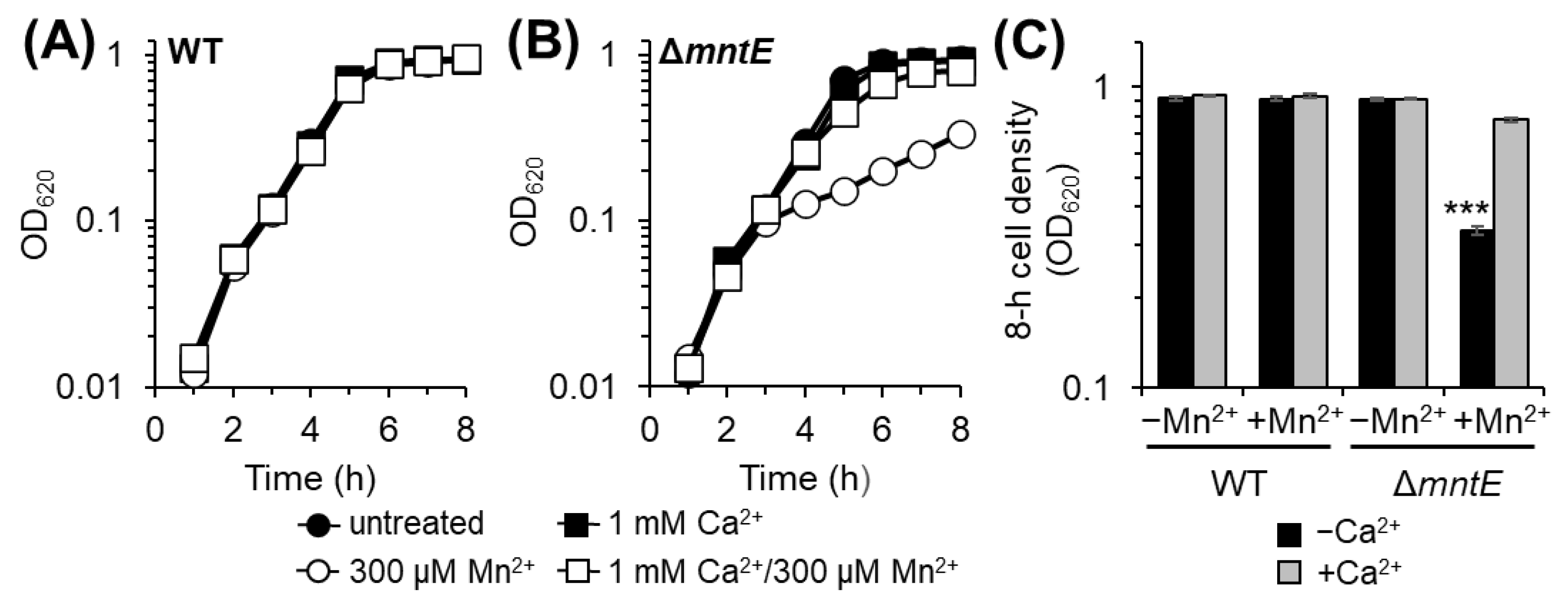
3.1. Ca Rescues Mn2+-Sensitive S. pneumoniae ΔmntE Growth Phenotype
3.2. Ca2+ Rescue of Mn2+ Sensitivity Varies among Bacteria
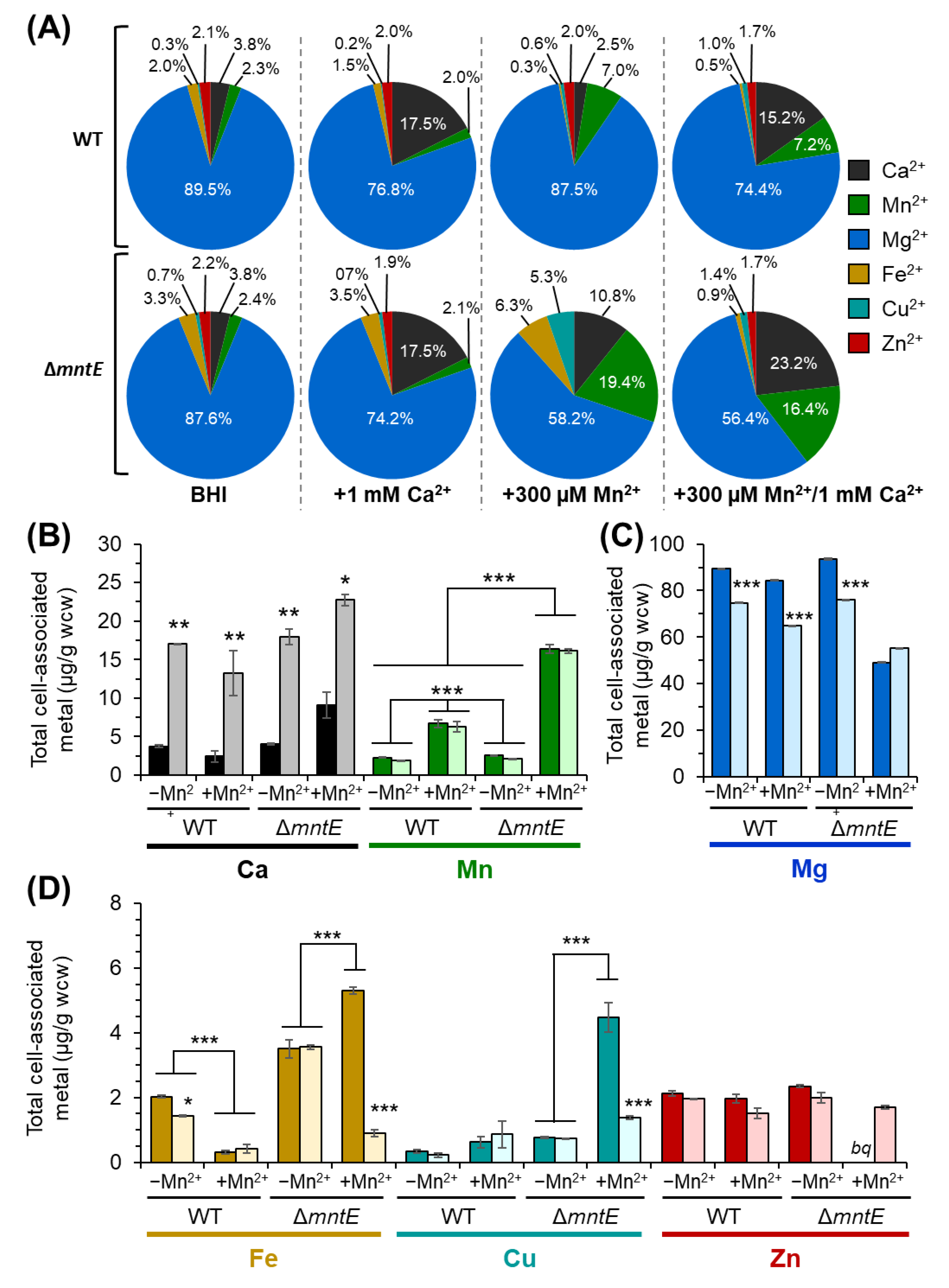
3.3. S. pneumoniae ΔmntE Accumulates High Cellular Mn2+ Despite Growth Restoration by Ca2+
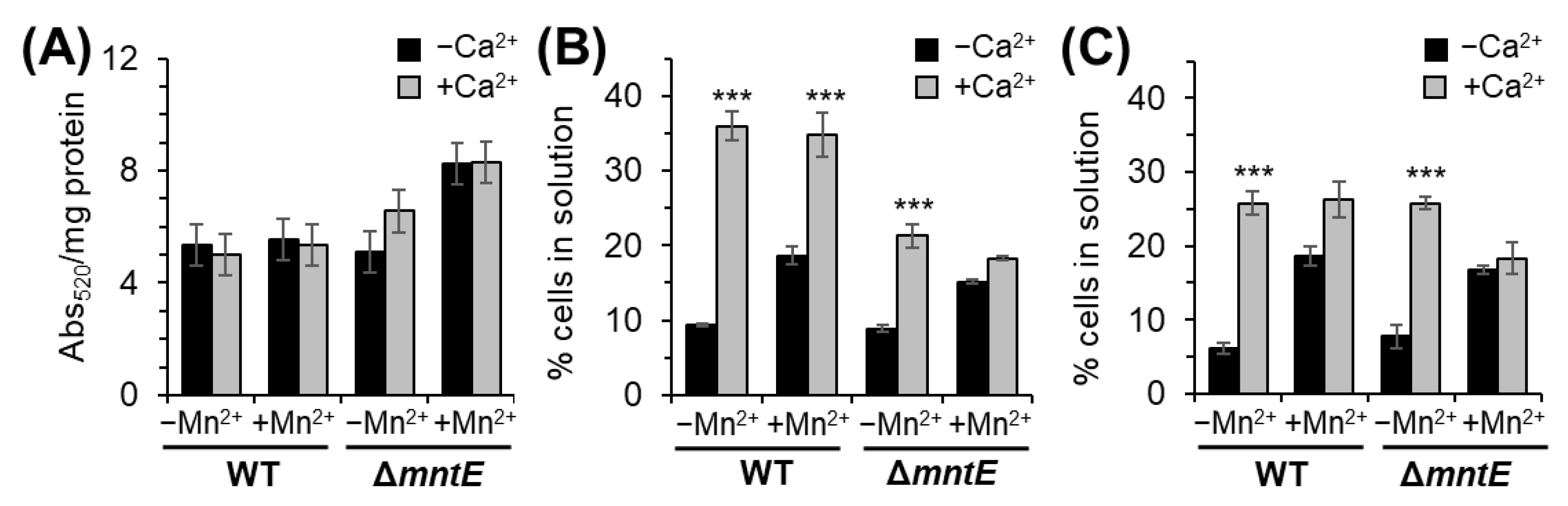
3.4. Ca2+ Does Not Alter S. pneumoniae Capsular Polysaccharide Production
3.5. Mn2+ Stress Inhibits S. pneumoniae Biofilm Formation
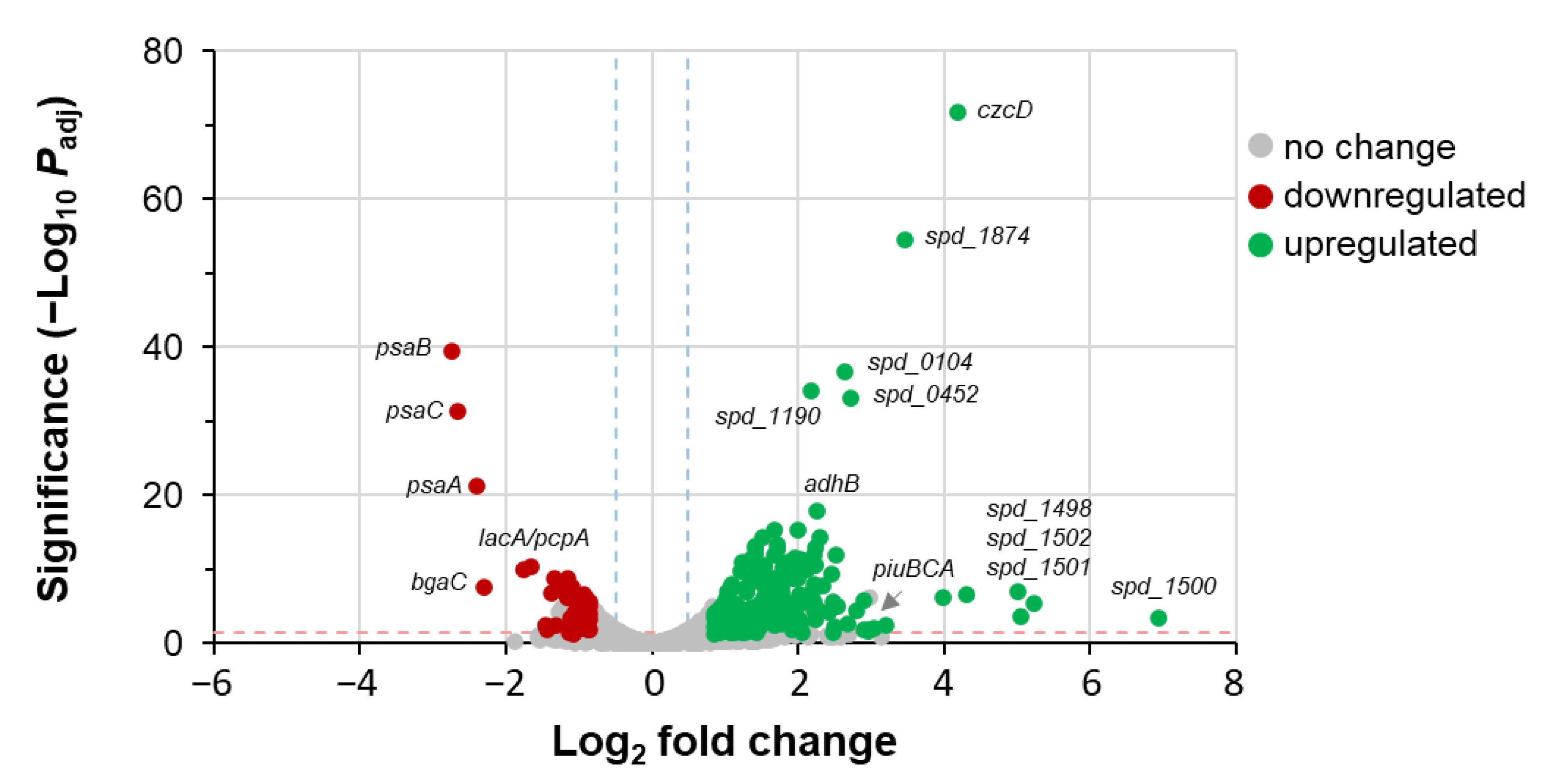
3.6. Ca2+ Does Not Alter S. pneumoniae Gene Expression
3.7. Mn2+ Impacts S. pneumoniae Cell Division, Which Is Rescued by Ca2+
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Permyakov, E.A.; Kretsinger, R.H. Cell signaling, beyond cytosolic calcium in eukaryotes. J. Inorg. Biochem. 2009, 103, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Straley, S.C.; Bowmer, W.S.; Gregor, L.B. Regulation by Ca2+ in the Yersinia low-Ca2+ response. Mol. Microbiol. 1993, 8, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Tisa, L.S.; Adler, J. Cytoplasmic free-Ca2+ level rises with repellents and falls with attractants in Escherichia coli chemotaxis. Proc. Natl. Acad. Sci. USA 1995, 92, 10777–10781. [Google Scholar] [CrossRef]
- Sarkisova, S.; Patrauchan, M.A.; Berglund, D.; Nivens, D.E.; Franklin, M.J. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa biofilms. J. Bacteriol. 2005, 187, 4327–4337. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, P.; Ye, C.; Zhang, H.; Wang, Y.; Chen, W.; Yang, Z. Structures of Anabaena calcium-binding protein CcbP: Insights into Ca2+ signaling during heterocyst differentiation. J. Biol. Chem. 2011, 286, 12381–12388. [Google Scholar] [CrossRef]
- Rosch, J.W.; Caparon, M.G.; Nasher, H.J. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol. Microbiol. 2008, 70, 435–444. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review on the Recent Use of Phage-Based Strategies with Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef]
- King, M.M.; Yendapally, R.; Asmat, T.M.; Nagaraja, V. Calcium Regulation of Bacterial Virulence. Adv. Exp. Med. Biol. 2020, 1131, 827–855. [Google Scholar]
- Jairaman, A.; Prakriya, M. Calcium Signaling in Airway Epithelial Cells: Current Understanding and Implications for Inflammatory Airway Disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Hajnóczky, G. Intracellular Ca. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Asmat, T.M.; Lin, S.Y.; Richards, D.; Gunderson, K. Streptococcus pneumoniae infection of host epithelial cells via polymeric immunoglobulin receptor transiently induces calcium release from intracellular stores. J. Biol. Chem. 2011, 286, 17861–17869. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Rao, A.S.; Merlin, D.; Madara, J.L.; Jass, J.R. Salmonella typhimurium induces epithelial IL-8 expression via Ca2+-mediated activation of the NF-kappaB pathway. J. Clin. Investig. 2000, 105, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Pace, J.; Hayman, M.J.; Galán, J.E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell 1993, 72, 505–514. [Google Scholar] [CrossRef]
- Rose, R.K. The role of calcium in oral streptococcal aggregation and the implications for biofilm formation and retention. Biochim. Biophys. Acta 2000, 1475, 76–82. [Google Scholar] [CrossRef]
- Jung, C.J.; Zheng, Q.H.; Shieh, H.R.; Lin, C.L.; Hsueh, P.R. AtlA Mediates Extracellular DNA Release, Which Contributes to Streptococcus mutans Biofilm Formation in an Experimental Rat Model of Infective Endocarditis. Infect. Immun. 2017, 85, e00252-17. [Google Scholar] [CrossRef]
- Honsa, E.S.; Johnson, M.D.; Rosch, J.W. The roles of transition metals in the physiology and pathogenesis of Streptococcus pneumoniae. Front. Cell Infect Microbiol 2013, 3, 92. [Google Scholar] [CrossRef]
- Trombe, M.C.; Clavé, C.; Manias, J.M. Calcium regulation of growth and differentiation in Streptococcus pneumoniae. J. Gen. Microbiol. 1992, 138, 77–84. [Google Scholar] [CrossRef]
- Trombe, M.C.; Rieux, V.; Baille, F. Mutations which alter the kinetics of calcium transport alter the regulation of competence in Streptococcus pneumoniae. J. Bacteriol. 1994, 176, 1992–1996. [Google Scholar] [CrossRef]
- Gangola, P.; Rosen, B.P. Maintenance of intracellular calcium in Escherichia coli. J. Biol. Chem. 1987, 262, 12570–12574. [Google Scholar] [CrossRef] [PubMed]
- Watkins, N.J.; Knight, M.R.; Trewavas, A.J.; Campbell, A.K. Free calcium transients in chemotactic and non-chemotactic strains of Escherichia coli determined by using recombinant aequorin. Biochem. J. 1995, 306, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Waters, L.S. Regulation of Bacterial Manganese Homeostasis and Usage during Stress Responses and Pathogenesis. Front. Mol. Biosci. 2022, 9, 945724. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, C.A.; Ogunniyi, A.D.; Valkov, E.; Lawrence, M.C.; Kobe, B.; McEwan, A.G.; Paton, J.C. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 2011, 7, e1002357. [Google Scholar] [CrossRef]
- Kloosterman, T.G.; Witwicki, R.M.; van der Kooi-Pol, M.M.; Bijlsma, J.J.; Kuipers, O.P. Opposite effects of Mn2+ and Zn2+ on PsaR-mediated expression of the virulence genes pcpA, prtA, and psaBCA of Streptococcus pneumoniae. J. Bacteriol. 2008, 190, 5382–5393. [Google Scholar] [CrossRef]
- Johnston, J.W.; Briles, D.E.; Myers, L.E.; Hollingshead, S.K. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect. Immun. 2006, 74, 1171–1180. [Google Scholar] [CrossRef]
- Martin, J.E.; Giedroc, D.P. Functional determinants of metal ion transport and selectivity in paralogous cation diffusion facilitator transporters CzcD and MntE in Streptococcus pneumoniae. J. Bacteriol. 2016, 198, 1066–1076. [Google Scholar] [CrossRef]
- Martin, J.E.; Le, M.T.; Bhattarai, N.; Capdevila, D.A.; Shen, J.; Winkler, M.E.; Giedroc, D.P. A Mn-sensing riboswitch activates expression of a Mn2+/Ca2+ ATPase transporter in Streptococcus. Nucleic Acids Res. 2019, 47, 6885–6899. [Google Scholar] [CrossRef]
- McFarland, A.L.; Bhattarai, N.; Joseph, M.; Winkler, M.E.; Martin, J.E. Cellular Mn/Zn ratio influences phosphoglucomutase activity and capsule production in Streptococcus pneumoniae D39. J. Bacteriol. 2021, 203, e0060220. [Google Scholar] [CrossRef]
- Martin, J.E.; Lisher, J.P.; Winkler, M.E.; Giedroc, D.P. Perturbation of manganese metabolism disrupts cell division in Streptococcus pneumoniae. Mol. Microbiol. 2017, 104, 334–348. [Google Scholar] [CrossRef]
- Jacobsen, F.E.; Kazmierczak, K.M.; Lisher, J.P.; Winkler, M.E.; Giedroc, D.P. Interplay between manganese and zinc homeostasis in the human pathogen Streptococcus pneumoniae. Metallomics 2011, 3, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Walker, K.A.; Miner, T.A.; Palacios, M.; Trzilova, D.; Frederick, D.R.; Broberg, C.A.; Sepúlveda, V.E.; Quinn, J.D.; Miller, V.L. A Klebsiella pneumoniae regulatory mutant has reduced capsule expression but retains hypermucoviscosity. mBio 2019, 10, e00089-19. [Google Scholar] [CrossRef]
- Loke, M.F.; Yadav, I.; Lim, T.K.; van der Maarel, J.R.; Sham, L.T.; Chow, V.T. SARS-CoV-2 spike protein and mouse coronavirus inhibit biofilm formation by Streptococcus pneumoniae and Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 3291. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Konishi, T.; Takase, H.; Makino, T. Shin’iseihaito (Xinyiqingfeitang) suppresses the biofilm formation of Streptococcus pneumoniae in vitro. BioMed Res. Int. 2017, 2017, 4575709. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Putri, G.H.; Anders, S.; Pyl, P.T.; E Pimanda, J.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Zheng, J.J.; Tsui, H.-C.T.; Richardson, J.D.; De Lay, N.R.; Winkler, M.E. S1 domain RNA-binding protein CvfD is a new posttranscriptional regulator that mediates cold sensitivity, phosphate transport, and virulence in Streptococcus pneumoniae D39. J. Bacteriol. 2020, 202, e00245-20. [Google Scholar] [CrossRef]
- Rosch, J.W.; Gao, G.; Ridout, G.; Wang, Y.D.; Tuomanen, E.I. Role of the manganese efflux system mntE for signaling and pathogenesis in Streptococcus pneumoniae. Mol. Microbiol. 2009, 72, 12–25. [Google Scholar] [CrossRef]
- Helmann, J.D. Specificity of metal sensing: Iron and manganese homeostasis in Bacillus subtilis. J. Biol. Chem. 2014, 289, 28112–28120. [Google Scholar] [CrossRef]
- Martin, J.E.; Waters, L.S.; Storz, G. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of Manganese. PLoS Genet. 2015, 11, e1004977. [Google Scholar] [CrossRef]
- Lisher, J.P.; Giedroc, D.P. Manganese acquisition and homeostasis at the host-pathogen interface. Front. Cell Infect. Microbiol. 2013, 3, 91. [Google Scholar] [CrossRef] [PubMed]
- Anjem, A.; Varghese, S.; Imlay, J.A. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 2009, 72, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shin, J.-H.; Pinochet-Barros, A.; Helmann, J.D. Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol. Microbiol. 2017, 103, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Lisher, J.P.; Winkler, M.E.; Giedroc, D.P. The zinc efflux activator SczA protects Streptococcus pneumoniae serotype 2 D39 from intracellular zinc toxicity. Mol. Microbiol. 2017, 104, 636–651. [Google Scholar] [CrossRef]
- Weimer, K.E.; Armbruster, C.E.; Juneau, R.A.; Hong, W.; Pang, B.; Swords, W.E. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J. Infect. Dis. 2010, 202, 1068–1075. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Rowe, H.M.; Meliopoulos, V.A.; Iverson, A.R.; Bickford, J.S.; Schrock, D.; Flury, E.; Feng, Y.; Xu, Y.; Albrecht, R.A.; Sands, J.; et al. Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat. Microbiol. 2019, 4, 1328–1336. [Google Scholar] [CrossRef]
- Garcia-Gonzalo, D.; Pagán, R. Influence of environmental factors on bacterial biofilm formation in the food industry: A review. Postdoc J. 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Martin, N.; Patel, J.; Munoz, F.; Sabino, J.; Yeo, T. Regulation and role of calcium in cellular senescence. Cell Calcium 2023, 110, 102701. [Google Scholar] [CrossRef]
- Ravi, B.; Sanyal, S.K.; Pandey, G.K. Calcium decoders and their targets: The holy alliance that regulate cellular responses in stress signaling. Adv. Protein Chem. Struct. Biol. 2023, 134, 371–439. [Google Scholar] [PubMed]
- Domínguez, D.C.; Guragain, M.; Patrauchan, M. Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium 2015, 57, 151–165. [Google Scholar] [CrossRef]
- Smith, D.J.; Yau, P.; Winstanley, C.; Cummings, S.P.; Cooper, R.J.; Knox, A.J.; Singh, M.; Walshaw, M.J.; Chilvers, M.A. Elevated metal concentrations in the CF airway correlate with cellular injury and disease severity. J. Cyst. Fibros. 2014, 13, 289–295. [Google Scholar] [CrossRef]
- Rosen, T.; Wang, K.A.; Nolan, E.M. Metal sequestration by S100 proteins in chemically diverse environments. Trends Microbiol. 2022, 30, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T.; Magnotti, E.; Spadafora, L.; Snyder, L.M.; Goldfarb, D.; Nolan, E.M. Zinc sequestration by human calprotectin facilitates manganese binding to the bacterial solute-binding proteins PsaA and MntC. Metallomics 2022, 14, mfac001. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Franklin, M.J.; Tang, T.H.; Gilmore, B.F.; Cornelius, V.J.; Song, S.; O’Toole, G.A. Implication of surface properties, bacterial motility, and hydrodynamic conditions on bacterial surface sensing and their initial adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opoku, R.; Carrasco, E.; De Lay, N.R.; Martin, J.E. Calcium Rescues Streptococcus pneumoniae D39 ΔmntE Manganese-Sensitive Growth Phenotype. Microorganisms 2024, 12, 1810. https://doi.org/10.3390/microorganisms12091810
Opoku R, Carrasco E, De Lay NR, Martin JE. Calcium Rescues Streptococcus pneumoniae D39 ΔmntE Manganese-Sensitive Growth Phenotype. Microorganisms. 2024; 12(9):1810. https://doi.org/10.3390/microorganisms12091810
Chicago/Turabian StyleOpoku, Reuben, Edgar Carrasco, Nicholas R. De Lay, and Julia E. Martin. 2024. "Calcium Rescues Streptococcus pneumoniae D39 ΔmntE Manganese-Sensitive Growth Phenotype" Microorganisms 12, no. 9: 1810. https://doi.org/10.3390/microorganisms12091810




